Abstract
AIM: To evaluate disease-specific quality of life (QOL) in liver cirrhosis patients and to compare it with those of a healthy population. Also an important objective was to assess whether QOL in liver cirrhosis patients differs by age and gender, by type and severity of disease.
METHODS: The case group of 131 liver cirrhosis patients was selected. The control group of 262 was enrolled from a healthy population according to the scheme of case-control study. Clinical, demographic, laboratory data were collected. QOL was measured with a specific chronic liver disease questionnaire (CLDQ), which was translated and validated in Lithuanian. QOL scores were compared between groups by age, gender, type and severity of disease. Cronbach’s alpha statistics calculation was used for evaluation of internal consistency reliability. Student’s t test or ANOVA were used for evaluation hypothesis about probability equation.
RESULTS: QOL was significantly lower in liver cirrhosis patients than in healthy population (59.5 ± 18.3 vs 85.3 ± 12.3, P < 0.001). The significant QOL differences between case and control groups were observed in domains of worry and abdominal symptoms, the smaller differences-in emotional functions and systematic symptom domains. Significantly worse QOL was in observed patients with increased clinical severity of the disease measured by Child-Pugh class. Age, gender and etiology of disease had an insignificant effect on QOL in cirrhotic patients.
CONCLUSION: QOL was significantly impaired in all CLDQ domains in liver cirrhosis patients. Increase in severity of disease was the major factor associated with poorer QOL.
Keywords: Liver cirrhosis, Quality of life, Chronic liver disease questionnaire, Case and control patients
INTRODUCTION
Measurement of quality of life (QOL) becomes increas-ingly important in clinical patient management[1-3]. The World Health Organization has expended and codified health definitions to multidimensional adding mental and social well being[4]. This allowed us, in the last decades of the 20th century, to develop quality of life concepts and adopt different instruments for multidimensional evaluation of health[5-9].
The main reason why the rapid development of QOL measures in health care took place was the growing recognition of the importance of understanding the impact of healthcare interventions on the patients’ every day life, rather than only treatment of their bodies[10]. Also physicians have always intended to find out and better understand how their patients feel. This is particularly important for patients with chronic, disabling or life threatening diseases, in persons, who live with minor expectation to be cured and with conditions that are likely to impact their physical and social well-being. In such patients it may be more relevant than length of life, because they are frequently more concerned about quality and disability than about longevity[11,12].
Health-related QOL is important in measuring the impact or burden of a chronic disease. Liver cirrhosis is an example of such a disease. Patients with chronic liver disease suffer from: fatigue, pruritus, loss of esteem, depression, and other complications of cirrhosis such as hepatic encephalopathy, ascites, spontaneous bacterial peritonitis and recurrent variceal hemorrhages[13,14]. Some of these conditions have obvious clinical manifestation and could be easily measured by the traditional clinical outcome measures (ascites, spontaneous bacterial peritonitis, varicel hemorrhages). Other important conditions (fatigue, loss of esteem, inability to function or work, anxiety, depression, emotional problems) are poorly evaluated by the clinical measures. More evidence that measuring QOL provides a better measurement of these latter conditions is presented in the recent literature[15-17].
The general aim of this study was to evaluate QOL in patients with liver cirrhosis and to compare its features with the control group of persons, selected from the population sample. We aimed also to look for associations between QOL scores and demographical characteristics, type of cirrhosis, severity of disease.
MATERIALS AND METHODS
Patients
The study was conducted during a one-year period in 2001-2002. In the first stage of the investigation the case group was selected (131 patients with cirrhosis). The control group (262 persons) was selected from randomly selected population according to the scheme of case-control epidemiological study. The group of cases was composed from patients with liver cirrhosis of different etiology. These patients have been admitted, diagnosed and treated at the Clinic of Gastroenterology, Hospital of Kaunas University of Medicine. The diagnosis was verified according to the data of anamnesis, clinical, biochemical and instrumental examinations and the results of percutaneous or transjugular liver biopsy data. Only the persons without hepatic encephalopathy, according to psychomotor tests, were included into the study.
The control group was randomly selected from the list of Kaunas county population. Pair matching method was applied in order to select the control group. The control persons were selected according to gender, age and the education background. Two controls were selected for one case person.
Methods
The routine clinical examination was carried out for the patients with liver cirrhosis: clinical and biochemical blood sample analysis, ultrasound investigation, esophagogastroscopy, percutaneous or transjugular liver biopsy. These persons were classified according to the etiology of disease. Clinical and biochemical analysis, evaluation of failure of liver function, also commonly manifested complications of cirrhosis were recorded and analyzed. Severity of liver cirrhosis was evaluated according to Child-Pugh score[18].
Clinical and epidemiological investigation methods were used. Investigation data of case groups were registered in the Registration Form for Clinical Data. General data about the cases and controls were collected in the General Questionnaire Form. QOL questionnaires were administered for both respondents of case and control groups.
The chronic liver disease questionnaire (CLDQ) was applied as the instrument for measuring QOL. This QOL investigation instrument was developed at the Department of Gastroenterology, The Cleveland Clinic Foundation by Younossi et al in 1999 as the disease specific instrument for evaluating QOL of patients with chronic liver disease[19]. Approval of the authors was received to use this instrument in our study. CLDQ covers 29 items and is designed to measure the six domains of QOL: abdominal symptoms (AB), fatigue (FA), systemic symptoms (SY), activity (AC), emotional functions (EM) and worry (WO). CLDQ has been translated to Lithuanian and passed validation procedures before this study. Evaluation of reliability and validity was carried out. Cronbach’s alpha (measure of internal consistency) of overall scores was 0.93, which was above the acceptable level of 0.70. Approval from Biomedical Ethics Committee was obtained and participants signed a written consent prior filing the questionnaires.
SPSS 10.0 for Windows was used for research analysis. Cronbach’s alpha statistics calculation was applied for evaluation of internal consistency reliability in QOL questionnaire. Standard means for QOL scores with a 95% confidence interval were calculated. For evaluation of continuous variables the statistical mean (m) and standard deviation (SD) were computed. Student’s t test or ANOVA were used for proving hypothesis about probability equation. Mann-Whitney or Kruskall-Wallis tests were used for comparison two or more independent variable groups. P < 0.05 was considered significant in two-tailed tests.
RESULTS
At the baseline survey 131 patients with liver cirrhosis of different etiology were examined. Table 1 summarizes their demographic and clinical characteristics.
Table 1.
Clinical and demographical data of liver cirrhosis patients (n = 131)
| Variable | n (%) |
| Age (yr) | |
| < 40 | 23 (17.6) |
| 40-50 | 34 (26.0) |
| 51-60 | 33 (25.1) |
| > 60 | 41 (31.3) |
| Gender | |
| Male | 68 (51.9) |
| Female | 63 (48.1) |
| Etiology of disease | |
| Viral B and/or C cirrhosis | 53 (40.5) |
| Alcoholic cirrhosis | 50 (38.2) |
| Cholestatic cirrhosis | 11 (8.3) |
| Other cirrhosis | 17 (13.0) |
| Child-Pugh class | |
| Class A | 32 (24.6) |
| Class B | 72 (54.6) |
| Class C | 27 (20.8) |
QOL in liver cirrhosis patients and in the control group
For the researches and clinicians it is important to know, which particular domains of QOL are most affected by liver cirrhosis. Figure 1 presents the distribution of mean scores of six QOL domains measured by CLDQ questionnaire in cases and controls. It was established that in all six domains QOL mean score (SD) were lower in cirrhosis patients than in control group (P < 0.001). The most significant difference in QOL was observed in the domain of worry (56.0 ± 24.2 vs 88.6 ± 14.4, P < 0.001) and in abdominal symptoms (59.7 ± 25.8 vs 88.9 ± 14.2, P < 0.001). Smaller deterioration of QOL was established in the domain of emotional function (58.5 ± 20.9 vs 78.9 ± 16.1, P < 0.001) and in the systemic symptoms domain (68.8 ± 18.1 vs 90.0 ± 10.9, P < 0.001). The overall CLDQ score for patients with liver cirrhosis also was lower than in persons with no cirrhosis (59.5 ± 18.3 vs 85.3 ± 12.3, P < 0.001).
Figure 1.
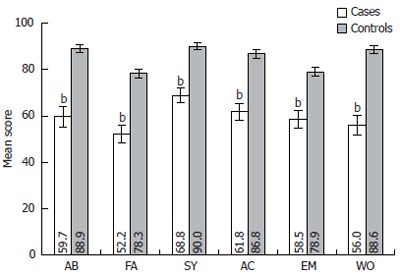
Chronic liver disease questionnaire (CLDQ) scale score differences in six quality of life (QOL) domains in case and control groups. Error bars indicate 95% confidence interval. AB: Abdominal symptoms; FA: Fatigue; SY: Systemic symptoms; AC: Activity; EM: Emotional function; WO: Worry. NS: Not significant. bP < 0.001 vs control group.
Age and QOL
The answers of respondents were analyzed in the four age groups. The distribution of patients by age groups was following: age group < 40 years-23 (17.6%), 40-50-year-old group-34 (26.0%), 51-60-year-old group-33 (25.1%) and > 60-years old group-41 (31.3%). The age structure of the control group was the same according the design of study. Figure 2 presents data on distribution of QOL by age among the respondents in case and control groups. It is evident from this illustration that general QOL was decreasing only insignificantly during period of aging in both case and control groups.
Figure 2.
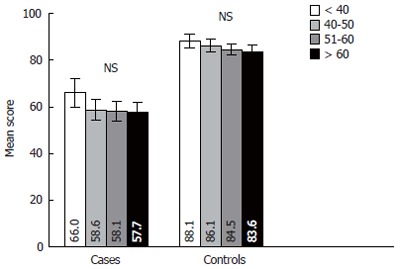
Chronic liver disease questionnaire (CLDQ) scale score comparison between the age groups in case and control groups. Error bars indicate 95% confidence interval. NS: Not significant.
Gender and QOL
The samples of cases and controls were composed of 51.9% of males and 48.1% of females. We have analyzed the effects of gender on QOL. Figure 3 illustrates how gender relates with QOL in both compared groups. It was proved that QOL is higher in control healthy males than in females (P < 0.001). However, no significant difference was established in QOL between genders in liver cirrhosis patients.
Figure 3.
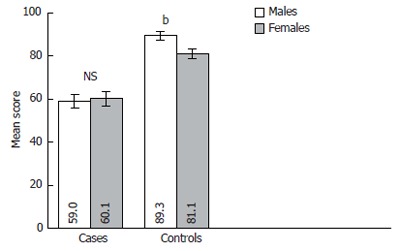
Chronic liver disease questionnaire (CLDQ) scale score comparison between the genders in case and control groups. Error bars indicate 95% confidence interval. NS: Not significant. bP < 0.001 vs females in control group.
Type of liver cirrhosis and QOL
Comparison of QOL in patients with different types of liver cirrhosis was carried out in four groups of patients: alcoholic liver cirrhosis, viral liver cirrhosis, cholestatic liver cirrhosis (primary biliary cirrhosis and primary sclerosing cholangitis), and other forms of liver cirrhosis. It was established by our analysis that in all six CLDQ domains QOL was at the similar score level in all four groups of patients analyzed (Figure 4).
Figure 4.
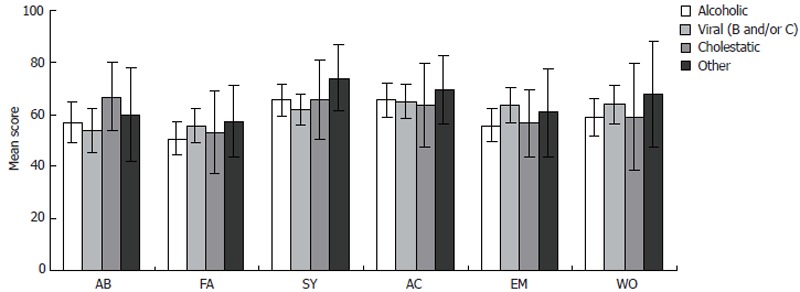
Chronic liver disease questionnaire (CLDQ) scale score comparison in six quality of life (QOL) domains by the etiology in liver cirrhosis patients. Error bars indicate 95% confidence interval. AB: Abdominal symptoms; FA: Fatigue; SY: Systemic symptoms; AC: Activity; EM: Emotional function; WO: Worry.
Severity of the disease according the Child-Pugh scale and QOL
We have analyzed and compared QOL in patients attributed to A, B and C liver cirrhosis severity classes according to Child-Pugh classification (Figure 5). The CLDQ showed significant worsening of QOL in parallel with increase of the clinical severity of disease measured by Child-Pugh scale (QOL mean score [SD] in A and C classes were respectively 65.9 ± 18.6 and 52.6 ± 17.0, P < 0.01).
Figure 5.
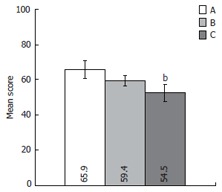
Chronic liver disease questionnaire (CLDQ) scale score comparison according the disease severity in the case group. Error bars indicate 95% confidence interval. A, B and C represent Child-Pugh class A, B and C, respectively. bP < 0.01 vs A Child-Pugh class.
DISCUSSION
Despite the fact that QOL investigations cover more and more diseases and population groups, its application in hepatology is still very scarce. Many recent publications still agree that very limited information is available on the impact of liver cirrhosis on QOL and continue investigating different QOL instruments.
This investigation is important as an example where both population based and clinical approaches in research were applied by selecting patients (from hospital) and controls (from population). Also our study was new for our country and for other countries of Central and Eastern Europe because QOL of liver cirrhosis patients was not investigated before in this European region.
By permission of CLDQ authors the specific questionnaire to measure health related QOL in patients with chronic liver disease was translated, adopted and validated in Lithuania. CLDQ previously have been adapted and validated for German speaking countries and also recently was translated into Farsi, Thais[20-22].
In our study QOL of patients with cirrhosis was compared with a randomly selected population group, because CLDQ is not designed exclusively as the liver disease specific instrument and allows us to answer all the questions for healthy persons also. The extent of impaired QOL of cirrhosis patients differed in the various domains. The most significant decrease in QOL was observed in the domain of worry and in abdominal symptoms. Smaller deterioration of QOL was established in the domain of emotional function and in the systemic symptoms domain.
The author of CLDQ, Younossi, also has established negative impact of different chronic liver disease (primary biliary cirrhosis, viral hepatitis B and C, primary sclerosing cholangitis, hepatocellular disease) for QOL and has established that deterioration of QOL is similar as in patients with chronic obstructive pulmonary disease or congestive heart failure[23,24]. Similar findings, which indicate significantly lower QOL in liver cirrhosis patients, were presented by Italian survey, where short form-36 (SF-36) and Nottingham Health Profile (NHP) questionnaires were used and by Croatian authors, who used SF-36 instrument[25,26].
We have calculated how much QOL is affected in cirrhosis patients in relation to age and gender. It is evident from population-based studies that QOL decreases with age in normal population[2,3,25]. However, in our study QOL both in control and cirrhosis patients groups showed only small and not significant impairment with age (P > 0.05). In an Italian study, where SF-36 was used, a minimum deviation from population norms was established in the oldest group[25]. This could be explained, that in our cirrhosis group proportion of patients with more severe disease (higher Child-Pugh class) was higher than in the Italian study. On the other hand “normative” populations in these two studies could have different age structure and levels of QOL.
Our study has demonstrated that QOL is higher in males from random population than in females. However, gender did not show any effect on QOL of liver cirrhosis patients. Majority or researchers, who analyzed QOL data of cirrhosis patients also, have stated that QOL is not determined by gender[27-29].
We have classified cirrhosis patients according to the disease etiology into four groups: viral, alcoholic, cholestatic and other origin. It was established by our analysis that QOL do not differ significantly in all four groups. This fact could indicate that etiology has minor impact for QOL. Our results are in accordance with other surveys, which established the similar patterns[23,24].
However, literature indicates on significant effects of disease severity and worsening of QOL across the disease stages[23-25,30]. In our study chronic liver disease stages were classified into three groups (A, B and C) according to Child-Pugh score. We also established that higher severity of disease (higher Child-Pugh class) was associated with a lower CLDQ score.
Lithuania is a relatively small country and the number of liver cirrhosis patients that could be accessed at the university hospital during one year, is not big. We made an attempt to diminish this possible limitation by selecting two-fold larger control group from randomly selected “healthy” population and by matching case and controls. Groups of patients with liver cirrhosis represents clinical group of cases, which can not be considered as completely representing the whole population of patents with liver cirrhosis in the country. It is evident that in primary stages of disease the patients with liver cirrhosis have less probability to be referred to the hospital. Also severe patients with Child-Pugh class C could have encephalopathy and mental disorders-these were excluded from the study. On the other hand we should take into account the possibility of selection bias in the “healthy” group of controls-non-respondents, who are completely healthy, tend to refuse to fill in questionnaires. This could result in selection of the control group with lower QOL. These circumstances allow extrapolating the research inference for the whole population of cirrhosis patients with caution.
In summary, our data obtained by this survey have shown that general QOL and QOL in all health domains were lower in patients with cirrhosis than in controls selected from the normal population. The most significant QOL differences between case and control groups were observed in domains of worry and abdominal symptoms, the smaller differences-in domains of emotional functions and systematic symptoms. Disease severity (higher Child-Pugh class) was associated with lower Chronic Liver Disease Questionnaire score. Etiological type of liver cirrhosis had minor and insignificant effect on QOL.
ACKNOWLEDGMENTS
The authors would like to thank the administration of Hospital of Kaunas University of Medicine (head: Professor J Pundzius) for organizational support and input in providing facilities to conduct the study. Research Laboratory for Population-Based Studies (head: Professor S Domarkiene) of Institute for Cardiology of Kaunas University of Medicine has provided possibility to access random samples of normal population.
Footnotes
S- Editor Wang GP L- Editor Alpini GD E- Editor Bai SH
References
- 1.Higgins IJ, Carr AJ. The clinical utility of quality of life measures. In: Higgins IJ, Carr AJ, Robbins PG, eds , editors. Quality of life. London: BMJ Books; 2003. pp. 63–78. [Google Scholar]
- 2.Bowling A. Measuring disease. A review of disease specific quality of life measurement scales. 2nd ed. Buckingham: Open University Press; 2001. pp. 2–12. [Google Scholar]
- 3.Fayers PM, Machin D. Quality of life. Assessment, Analysis and Interpretation. Chichester: John Wiley and Sons Ltd; 2000. pp. 3–27. [Google Scholar]
- 4.World Health Organization. Basic documents. 44th ed. Geneva: WHO; 2003. pp. 1–18. [Google Scholar]
- 5.Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. J Epidemiol Community Health. 1980;34:281–286. doi: 10.1136/jech.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lee CW, Chi KN. The standard of reporting of health-related quality of life in clinical cancer trials. J Clin Epidemiol. 2000;53:451–458. doi: 10.1016/s0895-4356(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 8.Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL) Qual Life Res. 1993;2:153–159. [PubMed] [Google Scholar]
- 9.Muldoon MF, Barger SD, Flory JD, Manuck SB. What are quality of life measurements measuring. BMJ. 1998;316:542–545. doi: 10.1136/bmj.316.7130.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addington-Hall J, Kalra L. Who should measure quality of life. BMJ. 2001;322:1417–1420. doi: 10.1136/bmj.322.7299.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chochinov HM, Tataryn D, Clinch JJ, Dudgeon D. Will to live in the terminally ill. Lancet. 1999;354:816–819. doi: 10.1016/S0140-6736(99)80011-7. [DOI] [PubMed] [Google Scholar]
- 12.El-Dika S, Guyatt GH, Armstrong D, Degl'innocenti A, Wiklund I, Fallone CA, Tanser L, Veldhuyzen van Zanten S, Heels-Ansdell D, Wahlqvist P, et al. The impact of illness in patients with moderate to severe gastro-esophageal reflux disease. BMC Gastroenterol. 2005;5:23. doi: 10.1186/1471-230X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson KJ, Finlayson ND. Clinical evaluation of liver disease. Baillieres Clin Gastroenterol. 1995;9:639–659. doi: 10.1016/0950-3528(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 14.McGuire BM, Bloomer JR. Complications of cirrhosis. Why they occur and what to do about them. Postgrad Med. 1998;103:209–12, 217-8, 223-4. doi: 10.3810/pgm.1998.02.361. [DOI] [PubMed] [Google Scholar]
- 15.Moore KA, McL Jones R, Burrows GD. Quality of life and cognitive function of liver transplant patients: a prospective study. Liver Transpl. 2000;6:633–642. doi: 10.1053/jlts.2000.9743. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, Hays RD, Kilbourne AM, Dulai GS, Gralnek IM. Are physician-derived disease severity indices associated with health-related quality of life in patients with end-stage liver disease. Am J Gastroenterol. 2004;99:1726–1732. doi: 10.1111/j.1572-0241.2004.30300.x. [DOI] [PubMed] [Google Scholar]
- 17.Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, Artinian L, Kim S, Lazarovici D, Jensen DM, Busuttil RW, et al. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease--the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 18.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Häuser W, Schnur M, Steder-Neukamm U, Muthny FA, Grandt D. Validation of the German version of the Chronic Liver Disease Questionnaire. Eur J Gastroenterol Hepatol. 2004;16:599–606. doi: 10.1097/00042737-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Zandi M, Adib-Hajbagheri M, Memarian R, Nejhad AK, Alavian SM. Effects of a self-care program on quality of life of cirrhotic patients referring to Tehran Hepatitis Center. Health Qual Life Outcomes. 2005;3:35. doi: 10.1186/1477-7525-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobhonslidsuk A, Silpakit C, Kongsakon R, Satitpornkul P, Sripetch C. Chronic liver disease questionnaire: translation and validation in Thais. World J Gastroenterol. 2004;10:1954–1957. doi: 10.3748/wjg.v10.i13.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younossi ZM, Kiwi ML, Boparai N, Price LL, Guyatt G. Cholestatic liver diseases and health-related quality of life. Am J Gastroenterol. 2000;95:497–502. doi: 10.1111/j.1572-0241.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 24.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 25.Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 26.Lacević N, Vanis N, Bratović I. [Reduced quality of life in liver cirrhosis] Med Arh. 2000;54:93–96. [PubMed] [Google Scholar]
- 27.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622–1626. doi: 10.1023/a:1024784327783. [DOI] [PubMed] [Google Scholar]
- 28.Park CK, Park SY, Kim ES, Park JH, Hyun DW, Yun YM, Jo CM, Tak WY, Kweon YO, Kim SK, et al. [Assessment of quality of life and associated factors in patients with chronic viral liver disease] Taehan Kan Hakhoe Chi. 2003;9:212–221. [PubMed] [Google Scholar]
- 29.Häuser W, Holtmann G, Grandt D. Determinants of health-related quality of life in patients with chronic liver diseases. Clin Gastroenterol Hepatol. 2004;2:157–163. doi: 10.1016/s1542-3565(03)00315-x. [DOI] [PubMed] [Google Scholar]
- 30.van der Plas SM, Hansen BE, de Boer JB, Stijnen T, Passchier J, de Man RA, Schalm SW. Generic and disease-specific health related quality of life in non-cirrhotic, cirrhotic and transplanted liver patients: a cross-sectional study. BMC Gastroenterol. 2003;3:33. doi: 10.1186/1471-230X-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]


