Abstract
Osteoclast-like giant cell tumors (OGCT) are rare abdominal tumors, which mainly occur in the pancreas. The neoplasms are composed of two distinct cell populations and frequently show an inhomogenous appearance with cystic structures. However, due to the rarity of these tumors, only very limited clinical data are available. Imaging features and sonographic appearance have hardly been characterized. Here we report on two cases of osteoclast-like giant cell tumors, one located within the pancreas, the other within the liver, in which OGCTs are extremely rare. Both patients were investigated by contrast sonography, which demonstrated a complex, partly cystic and strongly vascularized tumor within the head of the pancreas in the first patient and a large, hypervascularized neoplasm with calcifications within the liver in the second patient. The liver OGCT responded well to a combination of carboplatin, etoposide and paclitaxel. With a combination of surgical resection, radiofrequency ablation and chemotherapy, the patient’s survival is currently more than 15 mo, making him the longest survivor with an OGCT of the liver to date.
Keywords: Osteoclast-like giant cell tumor, Liver cancer, Pancreatic cancer, Contrast sonography
INTRODUCTION
Osteoclast-like giant cell tumors (OGCTs) have been described in several extraskeletal sites. Within the alimentary tract, these rare neoplasms mainly occur within the pancreas. Since the first description by Rosai in 1968[1] and further classification by Alguacil-Garcia and Weiland in 1977[2], there have been at least 38 publications describing approximately 60 occurrences of OGCTs in the pancreas[1-15], whereas only 12 liver tumors have been reported[16-27].
CASE REPORTS
Case 1
A 54-year-old man regularly visited his urologist because of prostate hyperplasia. After a renal ultrasound was suspicious of a tumor within the left kidney, computed tomography (CT) of the abdomen was performed. CT only showed a parenchyma bridge within the left kidney but surprisingly demonstrated a 7 cm large inhomogenous solid liver tumor involving segments II and III. The patient was then referred to our clinic. He had no clinical symptoms, no history of liver diseases and denied alcohol and tobacco consumption. His further medical history was remarkable for peripheral arterial disease, hypertension, esophageal reflux, hiatal hernia and sleep apnea.
Physical examinations revealed an obese male (173 cm, 92 kg, BMI: 31) with normal vital signs. His abdomen was soft and nontender. Serum chemistries were within the normal range except for a gamma-glutamyl transferase level of 69 IU/L (normal range < 55 IU/L) and normocytic, normochromatous anemia [hematocrit 0.38% (0.4%-0.52%)]. Serum alpha-fetoprotein, carcinoembryonic cancer antigen and cancer antigen 19-9 were normal. Serology for hepatitis A, B, and C was negative.
B-mode sonography (HDI 5000, Philips) demonstrated a well-defined, inhomogenous, cauliflower-like tumor with multiple small calcifications, causing retraction of the liver contour. Within the center of the tumor, a focal nodular hyperplasia (FNH)-like stellar scar was present (Figure 1A). Contrast-enhanced sonography by use of an Acuson Sequoia 512 (Siemens, Germany) with Sonovue® (2 mL; Bracco, Italy) using coded pulse sequences (CPS) and a low mechanic index (MI) demonstrated an inhomogenous perfused tumor with a large feeding artery heading towards the center of the tumor, radially branching to the periphery. During the capillary and arterial perfusion phase, non-perfused tumor areas indicative of necrotic areas were observed, making a focal nodular hyperplasia highly unlikely (Figure 1B). Diagnosis of haemangioma or adenoma was excluded by the spoke-like architecture of tumor arteries with centrifugal filling (Figure 1B). The portal perfusion phase was characterized by a rapid decrease of perfusion within the tumor (Figure 1C), indicating absence of portal vessels and thereby, together with the finding of a strong perfusion within the arterial phase, proving a malignant neoplasm.
Figure 1.
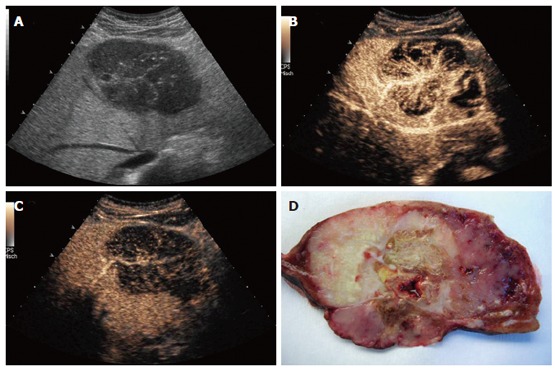
Patient 1: Osteoclastic giant cell tumor of the liver: A: B-mode sonography (HDI 5000, Philips), contrast sonography during the B: arterial and C: portal phase (Siemens); D: resected tumor: cut surface shows distinct tumor areas with necrotic and hemorrhagic regions.
Biopsy of the tumor was performed, demonstrating that the neoplasm was composed of pleomorphic mononuclear cells and scattered non-neoplastic osteoclast-like giant cells (OCGCs) with usually 10-20 uniformly small nuclei. Small mononuclear cells were differently characterized by cytological atypias and showed an increased number of mitoses with a proliferation rate of 70%. Focally there was an adenocarcinomatous component with mucinous inclusions and also areas of squamous cell differentiation (Figure 2). Immunofluorescence demonstrated expression of vimentin within both OCGCs and mononuclear cells, expression of macrophage marker CD 68 within OCGC and negative staining for CK18 (Figure 3). The adenocarcinomatous tumor components demonstrated expression of CK18 but negative staining for CD 68 and vimentin (Figure 3). In conclusion, the histopathological diagnosis revealed an osteoclast-like giant cell tumor of the liver.
Figure 2.
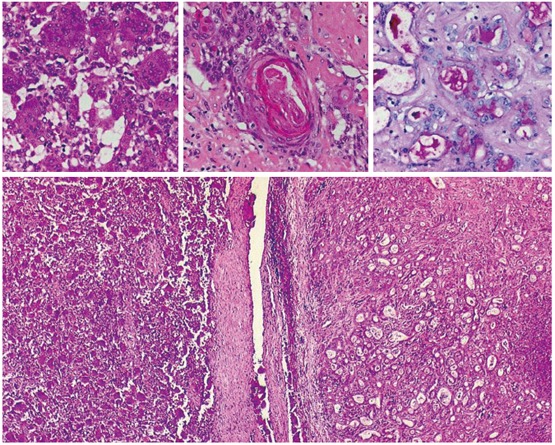
Histology (HE, x 20-40) of OGCT of the liver with a mixed cell population of osteoclastic giant cells and pleomorphic mononuclear cells (left), adenocarcinomatous component with mucinous inclusions (PAS, upper right) and squamous cell differentiation (middle).
Figure 3.
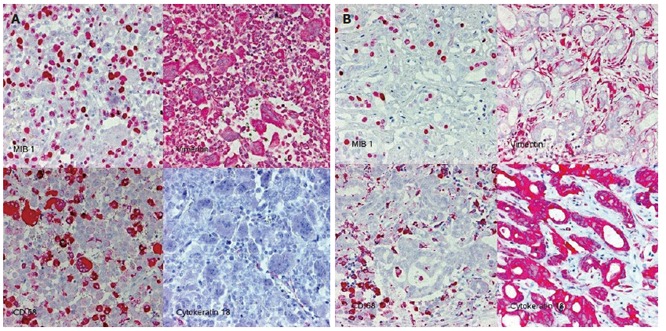
Immunohistology of the OGCT of the liver. Panel A: mixed cell population of osteoclastic giant cells and pleomorphic mononuclear cells. CD68: histio-monocytic differentiation of osteoclastic giant cells, MIB 1: proliferation of mononuclear cells. Panel B: adenocarcinomatous component with mucinous inclusions: CK18+, CD68/vimentin: -.
As extensive further diagnostic procedures including gastroscopy, colonoscopy, endoscopic ultrasound and bone scintigraphy showed no evidence of another tumor or metastases, surgical resection of liver segments II and III with excision of regional lymph nodes was performed. Macroscopic examination of the resected specimen revealed an irregular shaped firm mass measuring 8.5 cm with hemorrhage and necrosis (Figure 1D). All surgical margins were free of tumors. However, two lymph nodes (retroduodenal and at the hepatic artery) showed metastases of an adenocarcinoma.
As OGCT’s are typically aggressive tumors with a short life-expectancy, adjuvant chemotherapy consisting of carboplatin 250 mg/m2 (d 1), etoposide (alternating doses of 50 and 100 mg, d 1-10) and paclitaxel (175 mg/m2, d 1) was initiated. However, 6 wk after resection, as the first chemotherapy had just been completed, a single metastasis in the right lobe of the liver was diagnosed. The metastasis was successfully treated by radiofrequency ablation. Chemotherapy was proceeded for 6 mo. After restaging revealed no evidence of active tumor disease, chemotherapy was stopped. However, 3 mo after cessation of chemotherapy, tumor recurrence (liver and peritoneal metastases) was observed and chemotherapy (carboplatin, etoposide, paclitaxel) restarted. Restaging after 6 wk revealed partial remission with size reduction of metastases of 50%. Chemotherapy is currently proceeded. The patient’s survival since diagnosis has been 15 mo.
Case 2
A 49-year-old man suffered from pain in the upper abdomen since 4 mo ago, which was independent of food intake. During this time, his appetite and body weight were unchanged, however, his physical performance was reduced. Abdominal ultrasound revealed a 3 cm cystic mass in the region of the body and tail of the pancreas. The patient was then referred to our clinic for further diagnosis.
At admission he presented in fairly well condition with normal body weight (186 cm, 82 kg, BMI: 21). His abdomen was soft and nontender without evidence of ascites or signs of liver disease. Laboratory work-up showed no pathologic results except for moderate normocytic and normochromatous anemia (Hct 0.36% [0.4%-0.52%]). Neuron-specific enolase was slightly elevated (19.2 μg/L [< 15.2]), carcinoembryogenic antigen (CEA) and cancer antigen (CA) 19-9 were normal. He had stopped smoking 2 years ago and reported only moderate alcohol consumption. His past medical history was unremarkable. His family history revealed prostate carcinoma of his father.
B-mode sonography in our clinic (HDI 5000, Philips) indicated rapid enlargement of the pancreatic neoplasia, which presented as a well-defined complex lesion with several cysts and solid parts and had grown from 3 to 5 cm within 5 wk (Figure 4A). Contrast sonography by use of an Acuson Sequoia 512 with Sonovue® and CPS/low-MI technique demonstrated an intensively perfused tumor with strong vascularized margins and septs (Figure 4B), excluding a ductal pancreatic carcinoma. Strong vascularised pancreatic neoplasms have primarily to be differentiated from neuroendocrine tumors of the pancreas and from cystadenomas, which may also demonstrate strong vascularisation. However, malignant diseases become apparent after liver sonography. B-mode sonography demonstrated two complex nodular structures of 1 and 3 cm. Contrast sonography showed intensively perfused lesions with necrotic areas without portal perfusion, indicating metastases.
Figure 4.
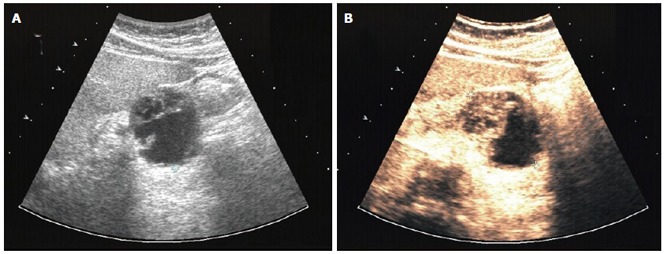
Patient 2: osteoclastic giant cell tumor of the pancreas. A: B-mode (HDI 5000, Philips) and B: contrast sonography (Sonovue®, low MI/CPS, Acuson Sequoia 512).
Biopsy revealed that the neoplasm was composed of pleomorphic mononuclear cells and scattered non-neoplastic osteoclast-like giant cells (OCGCs) with multiple uniformly small nuclei. OCGC’s occasionally contained phagocytosed mononuclear cells. Immunohistochemically, the OCGC’s were negative for cytokeratin but positive for vimentin and CD 68. Immunohistology of the pleomorphic cells demonstrated expression of vimentin, negative staining for cytokeratin and a proliferation rate of 30% (MIB-1). In conclusion, the histopathological diagnosis indicated osteoclast-like giant cell tumor of the pancreas (Figure 5).
Figure 5.
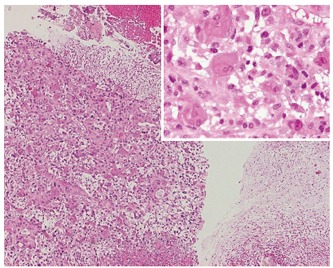
Histology (HE, x 20-40) of OGCT of the pancreas with a mixed cell population of osteoclastic giant cells and pleomorphic mononuclear cells (left) and myxoid tumor areas (right).
As surgical resection was not possible, the patient received palliative chemotherapy with cisplatin 80 mg/m2 (d 1), etoposide (100 mg/m2, d 1, 3, 5) and ifosfamide (2 g/m2, d 1-3) (PEI). After completion of three cycles of PEI, partial remission with significant size reduction of liver metastases was achieved. Thirteen mo after diagnosis chemotherapy is currently proceeded.
DISCUSSION
Osteoclast-like giant cell tumors typically display an inhomogenous macroscopic appearance with cystic-liquid and necrotic areas and parenchymatous and calcified parts. Histologically, this mixed macroscopic structure corresponds to heterogeneous tissue structures of epithelial or undifferentiated tumor cells, which may contain foci of conventional adenocarcinoma, focal cartilagenous differentiation and bone formation[2,6,7,9]. The tumors are composed of two distinct cell types: a mononuclear cell population and in addition, osteoclastic tumor giant cells of uncertain lineage. The exceptional morphology and origin of the different cell types has been a matter of controversy since its first description in 1968. Recent evidence indicates that only the mononuclear cells constitute neoplastic tumor cells and that osteoclastic giant cells develop from secondary infiltrating cells.
Formation of osteoclast-like giant cells (OCGC) is speculated to result from fusion of bone-marrow derived mononuclear histiocytes/macrophages attracted to the tumor by chemotactic factors produced by the neoplastic cells[7,9,12,13]. Indeed, the CD-68/lysozyme reactivity of OCGCs suggests a histiocytic origin. Lack of mitoses and MIB-1 reactivity indicates a terminal stage of differentiation and non-neoplastic nature of the osteoclast-like giant cells, which are also K-ras negative[11]. Moderate staining of OCGCs with epithelial markers[20,25] is now explained by immunoreactivity of phagocytosed epithelial tumor cells[11,27]. The infiltrating mononuclear cells display pleomorphism and neoplastic features and sometimes show features of epithelial tumors. However, other OGCTs lack epithelial differentiation[9,27]. Consequently, the term undifferentiated carcinoma with osteoclast-like giant cells has been proposed to more precisely describe these aggressive tumors.
Probably due to rapid tumor growth, osteoclast-like giant cell tumors of the pancreas only rarely present as small neoplasms. In more than 50 cases, in which tumor size was documented, only three neoplasias measured less than 3 cm. Typically OGCTs present as large tumors with cystic structures and necroses. The cystic component can become predominant, so that lesions may be misdiagnosed as pancreatic pseudocysts[3,4]. At time of diagnosis, more than 80% of tumors were already greater than 5 cm, 50% even greater than 10 cm. Most tumors arise in the head or body of the pancreas[1-15].
The differential diagnosis of pancreatic OGCTs includes cystic lesions like pancreatic cystadenomas, cystadenocarcinomas, serous and mucinous cystic tumors, pancreatic pseudocysts and also solid pancreatic tumors like ductal pancreatic carcinomas or neuroendocrine tumors. Solid tumors may be homogenous on computed tomographic imaging; however, they can also be very inhomogenous, as focal hemorrhage or necrosis is frequently found[9,12,15]. Vascularisation of these neoplasias has been described only in single cases. In one well-characterized patient, the tumor wall was slightly enhanced on contrast-enhanced CT[13]. Selective angiography demonstrated slight tumor staining[13].
In our patient with a pancreatic OGCT, contrast-enhanced sonography demonstrated a strong vascularisation within both the tumor and the liver metastases, making a pancreatic ductal carcinoma, which is typically only poorly vascularised, highly unlikely. A strong vascularised pancreatic neoplasm has to be differentiated from neuroendocrine tumors of the pancreas and from cystadenomas, which may also demonstrate strong vascularisation. In contrast, cystadenocarcinomas are generally poorly vascularised[28]. However, further studies are necessary to evaluate whether intense perfusion is a characteristic feature of OGCTs.
Osteoclast-like giant cell tumors of the liver are also generally large and inhomogenous neoplasias, ranging from 5 to 12 cm in size. Tumors typically feature necrotic or hemorrhagic regions and may also show cystic structures[16-27]. As published reports mainly focused on histopathology, radiological findings have only been documented in single cases. Magnetic resonance imaging in one case demonstrated a 10 cm large, fairly well circumscribed heterogenous solid mass with multiple fluid-like regions representing cystic components or necrosis on T1-weighted images[25]. Positron emission tomography scan in the same patient showed fluorine-18 fluorodeoxyglucose-uptake within the tumor[25]. In another patient, computed tomography described a 6 cm homogenous and hypervascularised tumor, which after resection presented as an inhomogenous tumor with hemorrhagic and necrotic areas[26].
In our patient, the liver tumor presented as an inhomogenous, cauliflower-like tumor with multiple small calcifications and a central stellar scar. However, contrast sonography revealed non-perfused tumor areas during the capillary and arterial perfusion phase, indicating necroses, thereby making a focal nodular hyperplasia highly unlikely[29,30]. Diagnosis of hemangioma or adenoma could be excluded by the spoke-like architecture of tumor arteries with centrifugal filling. The portal perfusion phase was characterized by a rapid decrease of perfusion within the tumor, indicating absence of portal vessels and thereby, together with the finding of a strong perfusion within the arterial phase, proving a malignant neoplasm[29,30].
A hepatocellular carcinoma was very unlikely, as the tumor demonstrated a very unusual vascular architecture and because there were no signs of cirrhosis. According to sonographic morphology and vascularisation pattern, the differential diagnosis included malignant primary liver tumors like peripheral cholangiocarcinoma (which, however, typically are poorly vascularized and do not show cysts or necroses), fibrolamellar carcinoma, epithelial hemangioendothelioma (which generally shows a multinodular structure) and a metastasis. However, a definitive sonographic diagnosis was impossible.
The average age of patients with osteoclast-like giant cell tumors of both pancreas and liver is around 60 years, ranging from 28 to 88 years. In pancreatic OGCTs, males and females appear to be affected in a fairly equal ratio[9,13]. The main symptoms are abdominal pain or discomfort and weight loss. Jaundice also frequently occurs if the tumor is located in the head. In cases of great cystic tumors, a palpable mass may be found. Invasion into adjacent structures is common. Nodal or intra-abdominal metastases are found in approximately 50% of patients at the time of diagnosis[1-15]. Overall, the prognosis of pancreatic OGCTs is unfavorable. As patients usually present with advanced diseases, complete resection can only rarely be performed. Leighton and Shiozawa reviewed 20 and 32 cases of pancreatic tumors, respectively, and determined the median survival rates to be less than 1 year. Interval to death or disease progression ranged from 4 mo to 5 years in these series[12,13]. In one patient, long-term survival of 15 years was documented; however, tumor recurrence within the pancreas after 10 years has also been reported[8]. Overall, the prognosis of pancreatic OGCTs is comparable to that of common ductal pancreatic carcinomas[12].
In contrast to pancreatic manifestations, OGCTs of the liver have been mostly described in male patients. Ten of 12 tumors (in one publication no data on patient’s age and sex were reported)[27] occurred in men. The overall prognosis of OGCTs of the liver seems to be even worse than for pancreatic cases. Previous reports have demonstrated that these tumors are uniformly very aggressive and that survival ranges from 1 to 10 mo[16-26]. To date, there is only one report of chemotherapy and radiotherapy in the management of osteoclast-like giant cell tumors. Hood et al treated a patient with recurrent liver tumor with a combination of chemotherapy (5-fluorouracil and adriamycin), external beam radiation and radioimmunotherapy (I131-labeled antiferritin immunoglobulin [IgG]), but could only achieve a partial response for several months[20]. In our case, the liver OGCT responded well to a combination of carboplatin, etoposide and paclitaxel. With a combination of surgery, local ablative therapy and chemotherapy, our patient is currently the longest survivor reported. Due to epithelial features of the mononuclear neoplastic cells in some patients, agents like gemcitabine have been suggested as adjuvant or palliative therapy. As histology in our patients demonstrated undifferentiated tumors in both cases, we chose a polychemotherapy with cisplatin, etoposide and ifosfamide (PEI) and carboplatin, etoposide and paclitaxel, respectively. After three cycles of PEI, partial remission was observed in the patient with pancreatic OGCT. In the other patient with an OGCT of the liver, early tumor recurrence within the right lobe of the liver was observed 6 wk after surgical resection.
In conclusion, the histopathological features of these rare tumors have been precisely described in recent years, providing the basis for correct histological classification. OGCTs should also be included in the sonographic differential diagnosis of tumors of the pancreas and liver. B-mode sonography, as well as arterial perfusion of a liver OGCT resembles those of an FNH, reflecting its hepatic origin. However, demarcation of hemorrhagic necroses during the arterial and capillary phase together with a missing increase of signal intensity in comparison to the surrounding liver tissue during the portal and late phases allows exclusion of an FNH and diagnosis of a malignant neoplasm. For optimization of chemotherapy and other treatment strategies of osteoclast-like giant cell tumors, future studies should not only focus on histopathologic features, but also on diagnostic and therapeutic approaches.
Footnotes
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
References
- 1.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22:333–344. doi: 10.1002/1097-0142(196808)22:2<333::aid-cncr2820220210>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977;39:1181–1189. doi: 10.1002/1097-0142(197703)39:3<1181::aid-cncr2820390325>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Scott R, Jersky J, Hariparsad G. Case report: malignant giant cell tumour of the pancreas presenting as a large pancreatic cyst. Br J Radiol. 1993;66:1055–1057. doi: 10.1259/0007-1285-66-791-1055. [DOI] [PubMed] [Google Scholar]
- 4.Oehler U, Jürs M, Klöppel G, Helpap B. Osteoclast-like giant cell tumour of the pancreas presenting as a pseudocyst-like lesion. Virchows Arch. 1997;431:215–218. doi: 10.1007/s004280050091. [DOI] [PubMed] [Google Scholar]
- 5.Dworak O, Wittekind C, Koerfgen HP, Gall FP. Osteoclastic giant cell tumor of the pancreas. An immunohistological study and review of the literature. Pathol Res Pract. 1993;189:228–31; discussion 232-4. doi: 10.1016/S0344-0338(11)80101-9. [DOI] [PubMed] [Google Scholar]
- 6.Fischer HP, Altmannsberger M, Kracht J. Osteoclast-type giant cell tumour of the pancreas. Virchows Arch A Pathol Anat Histopathol. 1988;412:247–253. doi: 10.1007/BF00737149. [DOI] [PubMed] [Google Scholar]
- 7.Newbould MJ, Benbow EW, Sene A, Young M, Taylor TV. Adenocarcinoma of the pancreas with osteoclast-like giant cells: a case report with immunocytochemistry. Pancreas. 1992;7:611–615. doi: 10.1097/00006676-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Mercer PM, McCabe MM, Murphy JJ. Recurrence of osteoclast-like giant cell carcinoma of the pancreas after 10 years. Aust N Z J Surg. 1996;66:334–335. doi: 10.1111/j.1445-2197.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 9.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Imai Y, Morishita S, Ikeda Y, Toyoda M, Ashizawa T, Yamamoto K, Inoue T, Ishikawa T. Immunohistochemical and molecular analysis of giant cell carcinoma of the pancreas: a report of three cases. Pancreas. 1999;18:308–315. doi: 10.1097/00006676-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Sakai Y, Kupelioglu AA, Yanagisawa A, Yamaguchi K, Hidaka E, Matsuya S, Ohbuchi T, Tada Y, Saisho H, Kato Y. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol. 2000;31:1223–1229. doi: 10.1053/hupa.2000.18491. [DOI] [PubMed] [Google Scholar]
- 12.Leighton CC, Shum DT. Osteoclastic giant cell tumor of the pancreas: case report and literature review. Am J Clin Oncol. 2001;24:77–80. doi: 10.1097/00000421-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, Nakatani Y, Inayama Y. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002;7:376–380. doi: 10.1007/s101470200059. [DOI] [PubMed] [Google Scholar]
- 14.Nai GA, Amico E, Gimenez VR, Guilmar M. Osteoclast-like giant cell tumor of the pancreas associated with mucus-secreting adenocarcinoma. Case report and discussion of the histogenesis. Pancreatology. 2005;5:279–284. doi: 10.1159/000085283. [DOI] [PubMed] [Google Scholar]
- 15.Joo YE, Heo T, Park CH, Lee WS, Kim HS, Kim JC, Koh YS, Choi SK, Cho CK, Rew JS, et al. A case of osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma: histopathological, immunohistochemical, ultrastructural and molecular biological studies. J Korean Med Sci. 2005;20:516–520. doi: 10.3346/jkms.2005.20.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz PA, Rao MS, Reddy JK. Osteoclastoma-like giant cell tumor of the liver. Cancer. 1980;46:771–779. doi: 10.1002/1097-0142(19800815)46:4<771::aid-cncr2820460422>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano H, Sonoda T, Hashimoto H, Enjoji M. Hepatocellular carcinoma with osteoclast-like giant cells. Cancer. 1984;54:837–842. doi: 10.1002/1097-0142(19840901)54:5<837::aid-cncr2820540513>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Andreola S, Lombardi L, Scurelli A, Bersiga A. Osteoclastoma-like giant-cell tumor of the liver. Case report. Tumori. 1985;71:615–620. doi: 10.1177/030089168507100616. [DOI] [PubMed] [Google Scholar]
- 19.Horie Y, Hori T, Hirayama C, Hashimoto K, Yumoto T, Tanikawa K. Osteoclast-like giant cell tumor of the liver. Acta Pathol Jpn. 1987;37:1327–1335. doi: 10.1111/j.1440-1827.1987.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 20.Hood DL, Bauer TW, Leibel SA, McMahon JT. Hepatic giant cell carcinoma. An ultrastructural and immunohistochemical study. Am J Clin Pathol. 1990;93:111–116. doi: 10.1093/ajcp/93.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Haratake J, Yamada H, Horie A, Inokuma T. Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer. 1992;69:2444–2448. doi: 10.1002/1097-0142(19920515)69:10<2444::aid-cncr2820691010>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.McCluggage WG, Toner PG. Hepatocellular carcinoma with osteoclast-like giant cells. Histopathology. 1993;23:187–189. doi: 10.1111/j.1365-2559.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki A, Yokoyama S, Nakayama I, Nakashima K, Kim YI, Kitano S. Sarcomatoid hepatocellular carcinoma with osteoclast-like giant cells: case report and immunohistochemical observations. Pathol Int. 1997;47:318–324. doi: 10.1111/j.1440-1827.1997.tb04500.x. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T, Seki S, Maki M, Noguchi N, Kawamura T, Arii S, Igari T, Koike M, Hirokawa K. Hepatocellular carcinoma with osteoclast-like giant cells: possibility of osteoclastogenesis by hepatocyte-derived cells. Pathol Int. 2003;53:450–456. doi: 10.1046/j.1440-1827.2003.01503.x. [DOI] [PubMed] [Google Scholar]
- 25.Rudloff U, Gao ZQ, Fields S, Gecelter GR. Osteoclast-like giant cell tumor of the liver: a rare neoplasm with an aggressive clinical course. J Gastrointest Surg. 2005;9:207–214. doi: 10.1016/j.gassur.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Ahaouche M, Cazals-Hatem D, Sommacale D, Cadranel JF, Belghiti J, Degott C. A malignant hepatic tumour with osteoclast-like giant cells. Histopathology. 2005;46:590–592. doi: 10.1111/j.1365-2559.2005.02018.x. [DOI] [PubMed] [Google Scholar]
- 27.Westra WH, Sturm P, Drillenburg P, Choti MA, Klimstra DS, Albores-Saavedra J, Montag A, Offerhaus GJ, Hruban RH. K-ras oncogene mutations in osteoclast-like giant cell tumors of the pancreas and liver: genetic evidence to support origin from the duct epithelium. Am J Surg Pathol. 1998;22:1247–1254. doi: 10.1097/00000478-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Rickes S, Wermke W. Differentiation of cystic pancreatic neoplasms and pseudocysts by conventional and echo-enhanced ultrasound. J Gastroenterol Hepatol. 2004;19:761–766. doi: 10.1111/j.1440-1746.2004.03406.x. [DOI] [PubMed] [Google Scholar]
- 29.Wermke W, Gassmann B. Tumor diagnostics of the liver with echo enhancers. Berlin/New York: Springer; 1998. [Google Scholar]
- 30.Wermke W. Sonographische Differenzialdiagnose Leberkrankheiten. Köln: Deutscher Ärzte-Verlag; 2005. [Google Scholar]


