Abstract
The microvascular supply of the biliary tree, the peribiliary plexus (PBP), stems from the hepatic artery branches and flows into the hepatic sinusoids. A detailed three-dimensional study of the PBP has been performed by using the Scanning Electron Microscopy vascular corrosion casts (SEMvcc) technique. Considering that the PBP plays a fundamental role in supporting the secretory and absorptive functions of the biliary epithelium, their organization in either normalcy and pathology is explored. The normal liver shows the PBP arranged around extra- and intrahepatic biliary tree. In the small portal tract PBP was characterized by a single layer of capillaries which progressively continued with the extrahepatic PBP where it showed a more complex vascular network. After common duct ligation (BDL), progressive modifications of bile duct and PBP proliferation are observed. The PBP presents a three-dimensional network arranged around many bile ducts and appears as bundles of vessels, composed by capillaries of homogeneous diameter with a typical round mesh structure. The PBP network is easily distinguishable from the sinusoidal network which appears normal. Considering the enormous extension of the PBP during BDL, the possible role played by the Vascular Endothelial Growth Factor (VEGF) is evaluated. VEGF-A, VEGF-C and their related receptors appeared highly immunopositive in proliferating cholangiocytes of BDL rats. The administration of anti-VEGF-A or anti-VEGF-C antibodies to BDL rats as well as hepatic artery ligation induced a reduced bile duct mass. The administration of rVEGF-A to BDL hepatic artery ligated rats prevented the decrease of cholangiocyte proliferation and VEGF-A expression as compared to BDL control rats. These data suggest the role of arterial blood supply of the biliary tree in conditions of cholangiocyte proliferation, such as it occurs during chronic cholestasis. On the other hand, the role played by VEGF as a tool of cross-talk between cholangiocytes and PBP endothelial cells suggests that manipulation of VEGF release and function could represent a therapeutic strategy for human pathological conditions characterized by damage of hepatic artery or the biliary tree.
Keywords: Peribiliary plexus, Periportal plexus, Cholangiocytes
INTRODUCTION
Cholangiocytes are currently the object of extensive investigation since recent evidence demonstrates their involvement in different processes (secretion, absorption, proliferation, neoangiogenesis), which are fundamental for the liver pathophysiology[1-4]. The metabolic and functional demands of cholangiocytes are sustained by the hepatic artery which forms a complex network of vessels, the peribiliary plexus, around the biliary tree[5-9].
The central role of liver vascularization in hepatic functions and the importance of microcirculation for liver zonal organization were developed in the 17th century by Marcello Malpighi[10] who first described the "acinus", a hexagonal structure of the liver parenchyma that was comparable with a glandular unit of the liver. This description correlates with the current concept of a liver morpho-functional unit, also termed "acinus", described by Rappaport in 1952[11]. A number of studies on liver vascularization clarified the typical microvascular architecture that supplies the biliary tree. This typical microvascular network runs along the intra- and extrahepatic biliary tree and, as previously mentioned, has been termed peribiliary plexus (PBP)[12,13]. The structural study of either the intrahepatic and extrahepatic PBP in the liver under normal conditions has been the subject of a number of studies using both two-dimensional and three-dimensional approaches[5,12,14,15].
Although it is well known that the liver has a dual blood supply from the hepatic artery (HA) and the portal vein (PV), controversies still remain concerning the distribution of hepatic arterial blood flow. It is generally accepted that terminal branching of the HA opens into sinusoids, as demonstrated by injection of dyes and vascular casting [5,16-24]. The question that remains is if the HA provides arterial blood directly to sinusoids[16,20-22,23,25] or rather runs in the portal tract stroma, thus feeding the PBP and the periportal plexus (PPP) to finally arrive at the sinusoids[26-30]. The presence of a double feeding to the sinusoids from the PV, at a pressure of 6-7 cm H2O, and from the HA, at a pressure of 12-25 cm H2O, has generated many studies in order to explain the prevalence of flow from the PV[31]. Arterio-portal anastomoses are mainly observed in terminal portal tracts[23] but other studies deny the presence of real arterio-portal venous anastomoses[16,27,32,33].
A number of reports indicate that structural changes of the biliary tree, associated with experimental models of liver damage or human pathologies, are followed by substantial adaptive modifications of the PBP. At the experimental level, the bile duct-ligated (BDL) rat has been widely used as a model of typical and selective cholangiocyte proliferation[9,34,35]. This model is characterized by a dramatic increase of cholangiocyte number which reaches 30% of the total parenchymal hepatic cells compared with 2% in the normal liver [36]. In order to investigate the adaptive changes of hepatic microvascular organization associated with an increased mass of the biliary tree following BDL, our group has used the method of vascular corrosion casts observed with Scanning Electron Microscopy (SEM). This technique provides the ability to follow the tiniest vessels in three dimensions and to distinguish arterial, capillary and venous vessels by surface characteristics[7,13,15,26,37]. As detailed above, this technique demonstrates how the evolution of PBP expansion follows the proliferation of biliary tree suggesting a role of proliferating cholangiocytes in sustaining, through the secretion of angiogenenic factors, and the adaptive proliferation of their vascular supply. To this regard, recent reports demonstrate that cholangiocytes are an important source of vascular endothelial growth factor (VEGF) in the damaged liver and that VEGF secreted by cholangiocytes may play role in driving the adaptive changes of the PBP, which accompany the proliferation of the biliary tree. Therefore, cross talk between cholangiocytes and endothelial cells could be fundamental in determining the response of the liver to cholestatic damage[38].
In this manuscript, we review recent studies related to the physiopathology of hepatic microvasculature in experimental models of liver damage and discuss the biological and clinical implications.
BLOOD SUPPLY
The microvasculature organization was investigated in normal or BDL rats by means of the Scanning Electron Microscopy Vascular Corrosion Casts (SEMvcc) technique[9,15,35]. The casts of normal livers showed an even distribution of sinusoids closely surrounding portal spaces. In larger portal spaces the hepatic artery (HA) branches were always evidently smaller than the portal ones. In smaller portal spaces the HA was often difficult to visualize[12,13,39,40]. The terminal branches of the HA are always represented by either the extra- and intrahepatic Peribiliary Plexus (PBP) or the periportal plexus (PPP). In very small portal spaces a small capillary, representing the terminal branch of a HA, can continue the course of the arteriole and eventually run into the sinusoids. It must be pointed out that it has all the characteristics of a capillary as far as diameter and endothelial imprints are concerned. This typical microvascular organization around the extrahepatic and intrahepatic biliary tree takes origin from the HA[15]. Hepatic artery branches paralleled the divisions of the portal vein up to the interlobular vessels and always showed regular profile and spindle shaped endothelial nuclear imprints. The PBP and the PPP took their origin from both the HA and from short collateral vessels of larger arteries[13,39,42].
In larger portal spaces, the PBP is an anastomotic network between short collateral arterioles from arising from the same arterial branch. The network presents two layers only next to the hilum, being generally mono-layered. In smaller portal spaces the PBP is progressively reduced up to one single capillary[13,39].Capillaries are recognized by the poorly defined endothelial imprints and the irregular profile of the cast[37,43]. Sometimes, capillaries both from the PBP, the PPP as well as single capillaries, were more dilated than normal but always exhibited a smaller diameter than adjacent sinusoids[13,39]. The PPP can be recognized as a true net only in very large portal spaces. However, in other regions the PPP is represented by interstitial, isolated capillaries. In smaller spaces it is not possible to discern periportal from peribiliary capillaries[13,39].
The extrahepatic biliary tree shows a dense vascular network arranged around the circumference of the common bile duct[15]. Arterial and venous trunks are very easily distinguished by observing their different endothelial imprints on the cast surface. Two main layers were recognized in the cast. Closely meshed arterial and venous vessels formed the outer layer of the plexus and a dense capillary network in the inner layer (Figure 1). The arterial network (30-40 μm) ramifies into precapillaries. At the origin of some of precapillary vessels, an indentation produced by perivascular cells or the rearrangement of smooth muscle fibers can be observed[40,44]. The capillaries that originate from precapillary arterioles form a network plane organized in the same plane of the arterial and venous networks and in a deeper, denser and stratified capillary network. This capillary plane, if observed in its internal surface, exhibited well-delineated pits where the capillaries are arranged around a vascular space that, at LM observation, correspond to small acinar glands surrounded by thin capillaries. Large venous vessels can be observed at the same plane of the arterioles; these drained blood arising from both the superficial and inner capillary networks[15].
Figure 1.
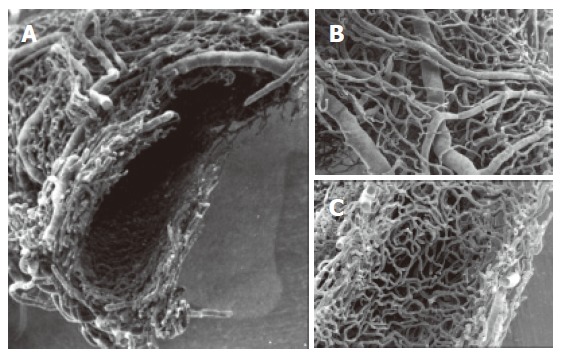
SEMvcc of normal rat liver (orig. magn., 20×). A dense vascular network is arranged around the circumference of the common bile duct. A: transverse section of Extrahepatic peribiliary plexus. Observe the arterial and venous vessels on the outer layer (B) and a dense capillary network in the inner layer of the plexus (C).
The extrahepatic PBP progressively continued with the intrahepatic PBP. It was composed of afferent arterial vessels and capillaries and was observed more easily in large portal tracts. In small portal tracts, the PBP was characterized by a single layer of capillaries that typically defined the periphery of the hepatic lobule (Figure 2).
Figure 2.

SEMvcc of normal rat liver (orig. magn., A: 40×; B: 110×). (A) small portal tract: PBP is characterized by a single layer of capillaries. (B) * = terminal hepatic artery, V = vena porta, arrows = peribiliary plexus, S = sinusoids.
PBP supports the bile epithelial function: the extension of the extrahepatic peribiliary tree underlies the ability of the bile duct to absorb water and electrolytes during the passage of bile through this collecting system[45]. Furthermore, the absence of the gallbladder in the rat emphasizes the role of EPBP in water reabsorption during the interdigestive phase[15]. Finally, the connection between the inner capillary venular plexus, portal venules and sinusoids, permits the transport of substances directly to the liver parenchyma where they can exert their actions. The reduction of complexity of the PBP inside the liver is strictly correlated with the progressive decrease of the diameter of the small bile duct, hence in the smaller portal tract the PBP can be reduced to only few capillaries[15].
Cholangiocyte proliferation started after BDL at the edge of the portal tract. During the first week from BDL the hepatic microcirculation did not show any alterations with respect to the normal liver. But after two weeks from BDL SEMvcc showed extention of the PBP from the portal tract. The proliferated PBP presented a three-dimensional net that did not arrange around a single lumen but delimited different troughs with a diameter of 20-30 μm. An empty space separated proliferated PBP and portal tract vessels from sinusoids. The sinusoids showed a normal organization with the typical structure of a classic lobule, in which sinusoids run evidently towards the central vein[9,13,15,24-26].
Three to four weeks after common BDL, a typical well-developed peribiliary microvascular plexus, originating from arterioles derived from hepatic arterial branches, is observed. The plexus runs at the periphery of the lobule and appears hypertrophic, but otherwise normal in its arrangement. The plexus is composed of many layers and shows an intimate meshed network, characterized by round loops, resembling the organization of the inner vascular layer of the extrahepatic peribiliary plexus (Figure 3A). Between the PBP and the sinusoidal network there is an empty space, which corresponds to proliferating connective tissue, digested during the casting procedure (Figure 3A). Infrequent vascular communications between the PBP and sinusoids are also visible. The efferent vessels that arise from the confluence of the capillaries form a small vein that tends to drain into the interlobular vein. In some areas of the liver the PBP does not seem to develop completely. In these areas there is a typical neoangiogenetic organization with interrupted vascular loops and dead-end vessels (Figure 3B). These structures may represent an attempt to increase metabolic exchange within the ductular lumen and PBP[45,46]. In contrast to the profound modification of the PBP, the sinusoidal organization remains quite normal even in BDL rats.
Figure 3.

SEMvcc of BDL rat liver (orig. magn., A: 40×; B: 60×). (A) proliferated PBP run at the periphery of the lobule separated from the sinusoidal network (S) by an empty space (*) which corresponds to proliferating connective tissue. (B) not completely developed PBP with typical neovascular organization with dead end vessels (arrows).
After BDL, the intrahepatic biliary epithelium undergoes cholangiocyte proliferation[48,50], which leads to bile duct mass expansion, followed by an adaptive proliferation of the PBP[9]. Proliferating bile ducts are characterized by enhanced cholangiocyte secretory and proliferative activities[47]. Cholangiocyte proliferation is modulated by a complex system of growth factors, neuropeptides and hormones[51-54]. Most of this information has been achieved in the BDL-rat model, which is characterized by cholangiocyte proliferation[55-57] followed by an adaptive proliferation of the PBP[9] that occurs after that of the intrahepatic biliary epithelium. Therefore, the adaptive proliferation of the PBP (and its circulating factors including VEGF) seems to be adequate for the increased mass of the bile ductal system.
VEGF REGULATION
Considering the enormous extension of the PBP during BDL, it is interesting to evaluate the possible role played by one of the most potent and well known angiogenic factors, the VEGF. VEGF is a member of a family of related growth factors that includes VEGF-A, -B, -C, -D, and -E, and placenta growth factor[58-61]. VEGF’s role in vascular proliferation associated with tumour growth or wound healing has been widely documented in different organs[62]. The expression of VEGF and its receptors is not restricted to vascular endothelial cells, since their expression has been detected in vascular smooth muscle cells, osteoblasts, regenerating myotubes and hematopoietic stem cells[59-61,63]. Moreover, VEGF has also been secreted in a large number of normal epithelial cells, such as keratinocytes, goblet cells in nasal polyps, pulmonary cells, prostate cells, ductal cells derived from normal pancreas and also in normal hepatocytes[66-68]. Because cholangiocyte proliferation, as described above, is regulated by growth hormones, we investigated the expression of VEGF and its receptors in normal and proliferating biliary epithelia.
After immunohistochemistry of liver sections from normal and BDL rats, we found that intrahepatic cholangiocytes from both normal and BDL rats express VEGFR-2 and VEGFR-3 but not VEGFR-1[38]. This data was confirmed by immunoblot analysis in a purified cholangiocyte cell culture. Moreover, we found that intrahepatic cholangiocytes from normal and BDL rats express the protein for VEGF-A and VEGF-C[38]. Immunohistochemistry shows that the expression of VEGF-A and VEGF-C was higher in bile ducts from BDL rats (Figure 4) compared to bile ducts from normal rats[38]. In addition, we found VEGF in the supernatant of primary cultures of normal cholangiocytes, indicating that the intrahepatic cholangiocytes secrete VEGF. Following BDL, the amount of VEGF secreted by cholangiocytes into the supernatant significantly increased compared to the amount of VEGF secreted by normal cholangiocytes. In normal rat liver, pericentral hepatocytes show positivity for VEGF-A (Figure 5).
Figure 4.
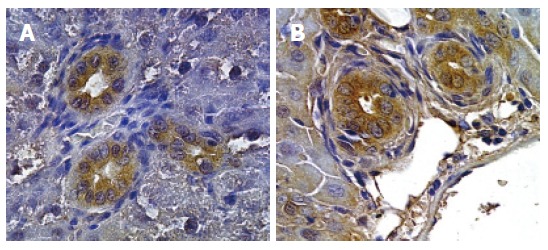
Immunohistochemistry for VEGF-A (A) and VEGF-C (B). Proliferating bile ducts in BDL rat show an intense positivity for both VEGF-A and VEGF-C (Orig. magn., 20×).
Figure 5.
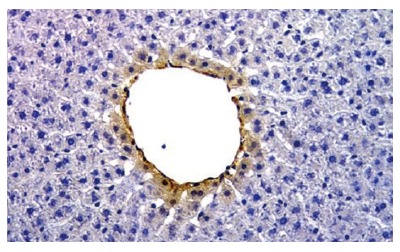
Immunohistochemistry for VEGF-A. Normal rat liver. The hepatocyte of pericentral zone shows positivity for VEGF-A (Orig. magn., 20×).
In order to confirm that VEGF plays an important role in the regulation of cholangiocyte proliferation, we also tested the effect of the administration of an anti-VEGF-A or an anti-VEGFC antibody to BDL rats. The administration of anti-VEGF to BDL rats induced an increased number of apoptotic cholangiocytes compared with BDL and a decrease in the number of PCNA and CK-19 positive cholangiocytes. In order to demonstrate that the proliferation of the PBP following BDL only occurs after cholangiocyte proliferation, we measured the number of PCNA-positive vascular endothelial cells and cholangiocytes in liver sections from rats with BDL for 1 or 2 wk. After BDL for 1 wk, PCNA was expressed only by proliferating cholangiocytes (and in rare hepatocytes), whereas endothelial cells were negative. In contrast, after BDL for 2 wk, both cholangiocytes and endothelial cells became positive for PCNA. This was confirmed by the immunohistochemical expression of Factor VIII-related antigen (a marker of endothelial cells)[69] in liver sections from normal, 1- and 2-wk BDL rats. The number of Factor VIII- related antigen positive cells increase, in comparison with normal rats, only after 2-wk BDL but it was unchanged after 1-wk BDL, thus confirming that the proliferation of PBP occurs after that of the biliary epithelium.
In addition, to better define the role of VEGF-A in the regulation of cholangiocyte and PBP proliferation during BDL we performed in: (1) normal rats and normal rats + hepatic artery ligation (HAL); (2) 1 wk BDL[4,5] rats; and (3) rats that (immediately after BDL or BDI + HAL) were treated by IP implanted with BSA or r-VEGF-A for 1 wk[70-73]. With the aim of evaluating PBP organization and the cholangiocyte VEGF protein expression, SEMvcc were performed. The peribiliary plexus, observed in BDL rats[9], was not demonstrated in BDL + HAL rats by the SEMvcc technique. We did not observe PBP in BDL + HAL rats because the PBP is nourished by the HA. In normal rats, PBP was observed more easily in large portal tracts[9]. In small portal tracts, the PBP was characterized by single layer of capillaries or even by a single capillary; in BDL + HAL we do not observed PBP. Administration of r-VEGF-A to BDL + HAL rats prevented the HAL-induced microvascular modification (absence of PBP). The microvascular pattern observed after administration of r-VEGF-A to BDL + HAL rats demonstrated the presence of a PBP with similar characteristics previously described in BDL rats[9,70].
Immunohistochemistry in BDL liver sections shows that bile ducts express VEGF-A, VEGFR-2 and VEGFR-3. HAL induced a decrease in the number of cholangiocytes positive for VEGF-A and VEGFR-2 and VEGFR-3 receptors compared to liver sections from 1 wk BDL rats. Following the administration of r-VEGF-A to BDL + HAL rats, the expression of VEGF-A and VEGFR-2 and VEGFR-3 was similar or higher than that of BDL rats. VEGFR-1 was not expressed by cholangiocytes. These indicate that VEGF, predominantly by an autocrine mechanism, plays an important role in modulating cholangiocyte proliferation[70]. Furthermore, if we drastically reduced the HA blood flow in BDL rats by the HAL, we induced the absence of PBP, reduction of both VEGF and VEGF receptors at the cholangiocyte level and a reduction of bile duct mass with no induced effect in the liver of normal rats (Figure 6). Evidently, when the intrahepatic bile duct mass is expanded, as occurs after BDL[47], blood supply through the hepatic artery becomes fundamental in sustaining the enhanced nutritional and functional demands of proliferating intrahepatic biliary epithelium[9]. VEGF has been demonstrated to participate in a complex that sustains cholangiocyte proliferation after BDL[50]; therefore, concerning the effect of HAL on bile duct mass, the fall of synthesis and release of VEGF by cholangiocytes after HAL certainly has a role in compromising cell proliferation.
Figure 6.
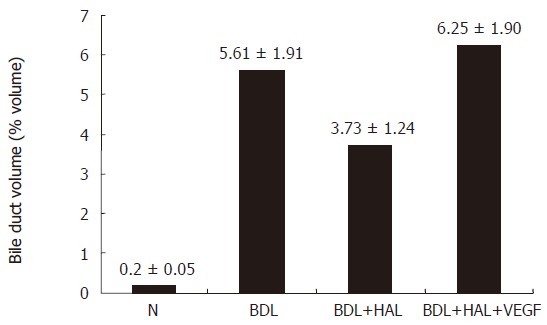
Effect of hepatic artery ligation and chronic administration of VEGF on bile duct volume.Bile Duct volume is measured as a volume fraction of the entire liver tissue specimen. HAL induced in BDL rats a disappearance of PBP and an impaired cholangiocyte proliferation. The effects of HAL on PBP and cholangiocyte functions were prevented by r-VEGF by maintaining the integrity of the peribiliary plexus and cholangiocyte proliferation.
In conclusion, recent data highlights the role of arterial blood supply of biliary tree in conditions of cholangiocyte proliferation, such occurs during chronic cholestasis. On the other hand, the role played by VEGF as a tool of cross-talk between cholangiocytes and PBP endothelial cells suggests that manipulation of VEGF release and function could represent a therapeutic strategy for human pathological conditions characterized by damage of hepatic artery or the biliary tree.
Footnotes
Supported by MIUR grants PRIN 2005 (prot. 2005067975_001) to E. Gaudio and Biomedicina, Cluster C04, Progetto n. 5 to E.Gaudio-P.Onori; MIUR grants PRIN 2005 (prot.No: 2005067975_002) to D. Alvaro and a VA Research Scholar Award, a VA Merit Award and the NIH grants DK58411 and DK062975 to Gianfranco Alpini
L- Editor Pan BR E- Editor Liu WF
References
- 1.Tietz PS, LaRusso NF. Cholangiocyte biology. Curr Opin Gastroenterol. 2005;21:337–343. doi: 10.1097/01.mog.0000158111.21345.c2. [DOI] [PubMed] [Google Scholar]
- 2.Alvaro D. Biliary epithelium: a new chapter in cell biology. Ital J Gastroenterol Hepatol. 1999;31:78–83. [PubMed] [Google Scholar]
- 3.Demetris AJ. Participation of cytokines and growth factors in biliary cell proliferation and mito-inhibition during ductular reaction. In: Alpini G, Alvaro D, LeSage G, Marzioni M, LaRusso NF, et al., editors. Pathophysiology of the Bile Duct System. Georgetown, Texas, USA: Landes Biosciences; 2004. pp. 167–182. [Google Scholar]
- 4.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 5.Ohtani O, Kikuta A, Ohtsuka A, Taguchi T, Murakami T. Microvasculature as studied by the microvascular corrosion casting/scanning electron microscope method. I. Endocrine and digestive system. Arch Histol Jpn. 1983;46:1–42. doi: 10.1679/aohc.46.1. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Phillips MJ. A hitherto unrecognized bile ductular plexus in normal rat liver. Hepatology. 1984;4:381–385. [PubMed] [Google Scholar]
- 7.Gaudio E, Pannarale L, Ripani M, Onori P, Riggio O. The hepatic microcirculation in experimental cirrhosis. A scanning electron microscopy study of microcorrosion casts. Scanning Microsc. 1991;5:495–502; discussion 502-503. [PubMed] [Google Scholar]
- 8.Gaudio E, Pannarale L, Onori P, Riggio O. A scanning electron microscopic study of liver microcirculation disarrangement in experimental rat cirrhosis. Hepatology. 1993;17:477–485. [PubMed] [Google Scholar]
- 9.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 10.Malpighi M De hepate. Bologna, 1666 [Google Scholar]
- 11.RAPPAPORT AM, BOROWY ZJ, LOUGHEED WM, LOTTO WN. Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec. 1954;119:11–33. doi: 10.1002/ar.1091190103. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani O, Murakami T. Peribiliary portal system in the rat liver as studied by the injection replica scanning electron microscopic method. Scanning electron microscopy. Chicago: SEM; 1978. p. 241–244. [Google Scholar]
- 13.Gaudio E, Pannarale L, Carpino F, Marinozzi G. Microcorrosion casting in normal and pathological biliary tree morphology. Scanning Microsc. 1988;2:471–475. [PubMed] [Google Scholar]
- 14.Haratake J, Hisaoka M, Yamamoto O, Horie A. Morphological changes of hepatic microcirculation in experimental rat cirrhosis: a scanning electron microscopic study. Hepatology. 1991;13:952–956. [PubMed] [Google Scholar]
- 15.Gaudio E, Onori P, Pannarale L, Marinozzi G. Microcirculation of the extrahepatic biliary tree: a scanning electron microscopy study of corrosion casts. J Anat. 1993;182(Pt 1):37–44. [PMC free article] [PubMed] [Google Scholar]
- 16.ELIAS H, PETTY D. Terminal distribution of the hepatic artery. Anat Rec. 1953;116:9–17. doi: 10.1002/ar.1091160103. [DOI] [PubMed] [Google Scholar]
- 17.Hase T, Brim J. Observation on the microcirculatory architecture of the rat liver. Anat Rec. 1966;156:157–173. doi: 10.1002/ar.1091560205. [DOI] [PubMed] [Google Scholar]
- 18.Del rio Lozano I, Andrews WH. A study by means of vascular casts of small vessels related to the mammalian portal vein. J Anat. 1966;100:665–673. [PMC free article] [PubMed] [Google Scholar]
- 19.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 20.Mitra SK. The terminal distribution of the hepatic artery with special reference to arterio-portal anastomosis. J Anat. 1966;100:651–663. [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto T, Komori R, Magara T, Ui T, Kawakami M, Tokuda T, Takasaki S. A study on the normal structure of the human liver, with special reference to angioarchitecture. Jikeikai Med J. 1979;26:1–40. [Google Scholar]
- 22.Kardon RH, Kessel RG. Three-dimensional organization of the hepatic microcirculation in the rodent as observed by scanning electron microscopy of corrosion casts. Gastroenterology. 1980;79:72–81. [PubMed] [Google Scholar]
- 23.Yamamoto K, Sherman I, Phillips MJ, Fisher MM. Three-dimensional observations of the hepatic arterial terminations in rat, hamster and human liver by scanning electron microscopy of microvascular casts. Hepatology. 1985;5:452–456. doi: 10.1002/hep.1840050318. [DOI] [PubMed] [Google Scholar]
- 24.Gaudio E, Onori P, Bassi A, Feliciani E, Pannarale L. Liver microcirculation and peribiliary plexus as seen by SEM in normal liver and in bile duct ligated rats. In: Motta PM, et al., editors. Advances in Microscopy of Cells, Tissues and Organs. Roma, Italy: Antonio Delfino Editore; 1997. pp. 449–455. [Google Scholar]
- 25.Ohtani O. The microvascularization of the liver, the bile duct and the gallbladder. In: LJA DiDio, PM Motta, DJ Allen, et al., editors. Biopathology of the liver. Amsterdam, the Netherlands: Elsevier; 1988. pp. 83–88. [Google Scholar]
- 26.Murakami T, Itoshima T, Shimada Y. Peribiliary portal system in the monkey liver as evidenced by the injection replica scanning electron microscope method. Arch Histol Jpn. 1974;37:245–260. doi: 10.1679/aohc1950.37.245. [DOI] [PubMed] [Google Scholar]
- 27.Ohtani O. The peribiliary portal system in the rabbit liver. Arch Histol Jpn. 1979;42:153–167. doi: 10.1679/aohc1950.42.153. [DOI] [PubMed] [Google Scholar]
- 28.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8:139–149. doi: 10.1016/0168-8278(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 29.Kono N, Nakanuma Y. Ultrastructural and immunohistochemical studies of the intrahepatic peribiliary capillary plexus in normal livers and extrahepatic biliary obstruction in human beings. Hepatology. 1992;15:411–418. doi: 10.1002/hep.1840150310. [DOI] [PubMed] [Google Scholar]
- 30.Ekataksin W, Wake K. New concepts in biliary and vascular anatomy of the liver. Prog Liv Dis. 1997;15:1–30. [Google Scholar]
- 31.McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. [DOI] [PubMed] [Google Scholar]
- 32.McCuskey RS. A dynamic and static study of hepatic arterioles and hepatic sphincters. Am J Anat. 1966;119:455–477. doi: 10.1002/aja.1001190307. [DOI] [PubMed] [Google Scholar]
- 33.Nakata K, Kanbe A. The terminal distribution of the hepatic artery and its relationship to the development of focal liver necrosis following interruption of the portal blood supply. Acta Pathol Jpn. 1966;16:313–321. doi: 10.1111/j.1440-1827.1966.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 34.Carpino F, Gaudio E, Marinozzi G, Melis M, Motta PM. A scanning and transmission electron microscopic study of experimental extrahepatic cholestasis in the rat. J Submicrosc Cytol. 1981;13:581–598. [PubMed] [Google Scholar]
- 35.Polimeno L, Azzarone A, Zeng QH, Panella C, Subbotin V, Carr B, Bouzahzah B, Francavilla A, Starzl TE. Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology. 1995;21:1070–1078. [PMC free article] [PubMed] [Google Scholar]
- 36.Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F, et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681–1691. doi: 10.1053/gast.2000.20184. [DOI] [PubMed] [Google Scholar]
- 37.Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts--technique and applications: updated review. Scanning Microsc. 1990;4:889–940; discussion 941. [PubMed] [Google Scholar]
- 38.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Pannarale L, Borghese F, Conte D, Gaudio E. Terminal Branches of the hepatic artery (where does the hepatic artery flow into). A scanning electron microscopic study of vascular corrosion casts of rat liver. Ital J Anat Embryol. 2004;109:157. [PubMed] [Google Scholar]
- 40.Hodde KC, Steeber DA, Albrecht RM. Advances in corrosion casting methods. Scanning Microsc. 1990;4:693–704. [PubMed] [Google Scholar]
- 41.Pannarale L, Onori P, Ripani M, Gaudio E. Precapillary patterns and perivascular cells in the retinal microvasculature. A scanning electron microscope study. J Anat. 1996;188(Pt 3):693–703. [PMC free article] [PubMed] [Google Scholar]
- 42.Ekataksin W. The isolated artery: an intrahepatic arterial pathway that can bypass the lobular parenchyma in mammalian livers. Hepatology. 2000;31:269–279. doi: 10.1002/hep.510310203. [DOI] [PubMed] [Google Scholar]
- 43.Miodonski AJ, Kuss J, Tyrankiewicz R. SEM blood vessels cast analysis. In: LJA DiDio, PM Motta, DJ Allen, et al., editors. Three-dimensional microanatomy of cells and tissue surfaces. Amsterdam, the Netherlands: Elsevier; 1981. pp. 71–87. [Google Scholar]
- 44.Konerding MA. Scanning electron microscopy of corrosion casting in medicine. Scanning Microsc. 1991;5:851–865. [PubMed] [Google Scholar]
- 45.Schrschmidt BF. Bile formation and cholestasis, metabolism and enterohepatic circulation of bile ducts and gallstone formation. In: Zamin D, Boyer TD, eds , et al., editors. Hepatology. A textbook of liver disease. Philadelphia: WB Saunders; 1982. pp. 297–351. [Google Scholar]
- 46.Luciano L, Reale E. The human gallbladder. In: Riva & Motta PM, eds , et al., editors. Ultrastructure of the extraparietal glands of the digestive tract. Boston: Kluwer Academic; 1990. pp. 231–248. [Google Scholar]
- 47.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 49.Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdury U, Papa E, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 50.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, et al., editors. The Liver; Biology & Pathobiology, 4th Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–435I. [Google Scholar]
- 51.Siegel EG, Schmidt WE, Fölsch UR. Severe ischemic-type biliary strictures due to hepatic artery occlusion seven years after liver transplantation--a rare cause of late cholestatic graft failure. Z Gastroenterol. 1998;36:509–513. [PubMed] [Google Scholar]
- 52.Alvaro D, Gigliozzi A, Attili AF. Regulation and deregulation of cholangiocyte proliferation. J Hepatol. 2000;33:333–340. doi: 10.1016/s0168-8278(00)80377-3. [DOI] [PubMed] [Google Scholar]
- 53.Yamagiwa Y, Patel T. Cytokine regulation of cholangiocyte growth. In: Alpini G, Alvaro D, LeSage G, Marzioni M, LaRusso NF, et al., editors. Pathophysiology of the Bile Duct System. Georgetown, Texas, USA: Landes Biosciences; 2004. pp. 227–234. [Google Scholar]
- 54.Nozaki I, Lunz JG 3rd, Specht S, Stolz DB, Taguchi K, Subbotin VM, Murase N, Demetris AJ. Small proline-rich proteins 2 are noncoordinately upregulated by IL-6/STAT3 signaling after bile duct ligation. Lab Invest. 2005;85:109–123. doi: 10.1038/labinvest.3700213. [DOI] [PubMed] [Google Scholar]
- 55.Castaing D, Houssin D, Bismuth H. Anatomy of the liver and portal system. In: Castaing D, Houssin D, Bismuth H, eds , et al., editors. Hepatic and portal surgery in the Rat. Paris: Masson; 1980. pp. 27–45. [Google Scholar]
- 56.Davis BH, Madri JA. Type I and type III procollagen peptides during hepatic fibrogenesis. An immunohistochemical and ELISA serum study in the CCl4 rat model. Am J Pathol. 1987;126:137–147. [PMC free article] [PubMed] [Google Scholar]
- 57.Alpini G, Ulrich CD 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 58.Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000;26:561–569. doi: 10.1055/s-2000-13213. [DOI] [PubMed] [Google Scholar]
- 59.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 60.Larrivée B, Karsan A. Signaling pathways induced by vascular endothelial growth factor (review) Int J Mol Med. 2000;5:447–456. doi: 10.3892/ijmm.5.5.447. [DOI] [PubMed] [Google Scholar]
- 61.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 62.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 63.Uchida S, Sakai A, Kudo H, Otomo H, Watanuki M, Tanaka M, Nagashima M, Nakamura T. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone. 2003;32:491–501. doi: 10.1016/s8756-3282(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 64.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- 65.Coste A, Brugel L, Maître B, Boussat S, Papon JF, Wingerstmann L, Peynègre R, Escudier E. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J. 2000;15:367–372. doi: 10.1034/j.1399-3003.2000.15b24.x. [DOI] [PubMed] [Google Scholar]
- 66.Marszalek A, Daa T, Kashima K, Nakayama I, Yokoyama S. Expression of vascular endothelial growth factor and its receptors in the developing rat lung. Jpn J Physiol. 2001;51:313–318. doi: 10.2170/jjphysiol.51.313. [DOI] [PubMed] [Google Scholar]
- 67.Köllermann J, Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am J Clin Pathol. 2001;116:115–121. doi: 10.1309/1LBM-6X32-JH6W-ENUD. [DOI] [PubMed] [Google Scholar]
- 68.Teraoka H, Sawada T, Nishihara T, Yashiro M, Ohira M, Ishikawa T, Nishino H, Hirakawa K. Enhanced VEGF production and decreased immunogenicity induced by TGF-beta 1 promote liver metastasis of pancreatic cancer. Br J Cancer. 2001;85:612–617. doi: 10.1054/bjoc.2001.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harach HR, Jasani B, Williams ED. Factor VIII as a marker of endothelial cells in follicular carcinoma of the thyroid. J Clin Pathol. 1983;36:1050–1054. doi: 10.1136/jcp.36.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaudio E, Onori P, Franchitto A, Pannarale L, Alpini G, Alvaro D. Hepatic microcirculation and cholangiocyte physiopathology. Ital J Anat Embryol. 2005;110(2 Suppl 1):71–75. [PubMed] [Google Scholar]
- 71.Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296–300. doi: 10.3748/wjg.v5.i4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin R, Feng J, Yao Z. Dynamic changes of serum vascular endothelial growth factor levels in a rat myocardial infarction model. Chin Med Sci J. 2000;15:154–156. [PubMed] [Google Scholar]
- 73.LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver. 2001;21:73–80. doi: 10.1034/j.1600-0676.2001.021002073.x. [DOI] [PubMed] [Google Scholar]


