Abstract
AIM: To study the epidemiology of HCC in Lebanon and prognostic factors predictive of early mortality.
METHODS: An observational follow-up cohort study of HCC cases diagnosed over a five-year period was carried out. Multivariate analysis was conducted to identify prognostic factors in comparison to Cancer of the Liver Italian Program (CLIP) score. Multiple variables including the etiology of underlying liver disease, the demographic characteristics of patients, and the severity of liver disease evaluated by the Child-Pugh score were studied. Tumor parameters included the time of diagnosis of HCC, alpha-fetoprotein level, number and size of nodules, presence of portal vein thrombosis, and treatment modalities. Death or loss of follow-up was considered as an end-point event.
RESULTS: Ninety-two patients (mean 60.5 ± 22.3 years) were included. Etiology of underlying disease was hepatitis B, C, and alcohol in 67%, 20%, and 23.5% respectively. Child-Pugh class at diagnosis was A, B, and C in 34.8%, 39.3% and 25.8% respectively. Overall survival was 44.8%, 32.8% and 17.6% at 1, 2 and 3 years respectively (mean F/U 40.2 ± 23.5 mo). Multivariate analysis identified three predictors of early mortality (< 6 mo): bilirubin > 3.2 mg/dL (P < 0.01), HCC as first presentation of liver disease (P = 0.035), and creatinine > 1 mg/dL (P = 0.017). A score based on these variables outperformed the CLIP score by Cox proportional hazard. ROC curve showed both models to be equivalent and moderately accurate.
CONCLUSION: HBV is the leading cause of HCC in Lebanon. Independent predictors of early mortality are elevated bilirubin, creatinine and HCC as first manifestation of disease. Prospective validation of a score based on these clinical parameters in predicting short-term survival is needed.
Keywords: Liver, Epidemiology, Hepatitis, Neoplasm, Cancer, Cirrhosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is a malignancy occurring most often in the setting of liver cirrhosis. The causes of the underlying liver disease differ according to the geographical distribution. Common risk factors for HCC include infection with hepatitis B virus (HBV), hepatitis C virus (HCV), cirrhosis of any cause, alcoholic liver disease, and inherited metabolic diseases such as hemochromatosis and α1-antitrypsin deficiency. HCC occurs most commonly in sub-Saharan Africa and parts of the Far East such as China, Taiwan, Korea, Japan, and Vietnam. HCV infection with advanced fibrosis or cirrhosis is the main cause of HCC in Japan, South Africa, Egypt, the United States and Southern Europe[1-4]. In Northern and Central European countries, ethanol is still the leading cause of cirrhosis and is responsible for the majority of HCC cases[5]. There has been no report on the epidemiology of HCC in Lebanon. Epidemiologic data from neighboring countries differ widely with the primary risk factor for HCC attributable to HBV in Turkey[6], HBV/HDV in Jordan[7], and HCV in Saudi Arabia[8,9].
The expected survival of patients with HCC is an important parameter in predicting mortality on a liver transplantation waiting list. The prognostic factors for survival in patients with HCC have been identified in previous studies and sub-classified into 3 groups: (1) demographic characteristics including age and gender; (2) factors related to HCC such as tumor size, the number of nodules, vascular invasion, the presence of a tumor capsule or metastasis; and (3) factors related to underlying liver disease severity, including ascites, encephalopathy, or serum bilirubin. The Child-Pugh classification, used in patients with cirrhosis, only considers liver disease and not tumor characteristics. Orthotopic liver transplantation (OLT) is an excellent treatment for early hepatocellular carcinoma. There is, however, a paucity of data on survival according to intention-to-treat analysis and on the rate of dropout from the waiting list for OLT among patients with HCC due to shortage of grafts. This is compounded by the fact that up to 30% of eligible patients develop contraindications to the procedure while waiting for a donor. All of the above result in less than optimal prediction of early mortality in HCC patients, particularly in populations who present with advanced disease and where therapeutic options may be limited. The aim of the present study was to describe the epidemiology of HCC in Lebanon, and to identify factors predictive of early mortality in our patients who could seldom benefit from liver transplantation.
MATERIALS AND METHODS
Patients
The study is a multicenter follow-up cohort study consisting of 92 patients diagnosed with HCC between 1998 and 2003. The study involved three university hospitals in Beirut: Hotel-Dieu de France (Université Saint Joseph), American University of Beirut Medical Center, and Saint-Georges Hospital (Balamand University Medical School). Patients were identified from the medical records and from specialty liver clinics. Information regarding deceased patients was obtained from medical records and prospective follow-up was continued for new and existing patients. Multiple variables including the etiology of underlying liver disease, the demographic characteristics of patients including age and gender, and the severity of liver disease evaluated by the Child-Pugh score were studied. Tumor parameters included the time of diagnosis of HCC, alpha-fetoprotein level, number and size of nodules, presence of portal vein thrombosis, and treatment modalities. Death or loss of follow-up was considered as an end-point event.
HCC was diagnosed according to any one of the following criteria: (1) an abnormal liver morphology with a tumor nodule that is enhanced during the arterial phase following intravenous contrast injection on CT-scan or MRI. (2) an elevated alpha fetoprotein level > 400 ng/mL in the presence of a liver nodule, and (3) biopsy-proven HCC[10]. Patients who did not meet one or more of these diagnostic criteria were not included in the study. Cirrhosis was confirmed according to histology and/or by clinical and biochemical signs of hepatic insufficiency, ascites, esophageal varices, and ultrasonographic signs of portal hypertension. Tumor eligibility-for-treatment criteria included: a single nodule of less than 5 cm without evidence of vascular invasion, or less than 3 nodules of < 3 cm each with the absence of portal vein thrombosis. Tumors more than 5 cm in diameter were considered eligible for surgical resection when there was no evidence of vascular invasion and when surgical resection was technically possible. These patients were not considered eligible for any other curative treatment namely liver transplantation, percutaneous ethanol ablation, or radio frequency ablation. Patients who had tumors eligible for a curative treatment were further selected according to transplantation indication with an upper age limit of 65 years.
Statistical analysis
Biochemical data were collected at the time of diagnosis of hepatocellular carcinoma. Quantitative data were expressed as mean ± standard deviation and range. Group comparisons used the two-sample t-test or the Mann-Whitney test for quantitative variables and the two-tailed Fischer’s exact test was used for qualitative variables. Comparison of survival used the Kaplan-Meier model, the log-rank test and receiver operating characteristic (ROC) curve.
RESULTS
Etiology
Characteristics of the 92 HCC patients are shown in Table 1. The mean age was 60.5 ± 22.3 years and the sex ratio was 5.6:1 (M/F, 78/14). In 77 patients (83.7%), the etiology of the underlying liver disease was viral. HBV infection was found in 62 patients (67.4%) compared to 18 patients (19.6%) with HCV infection. Alcohol abuse was found in 22 patients (23.9%) but was the only cause of liver disease in only 8.2% of our patients. Miscellaneous causes included autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, and tyrosinemia and accounted for 5.9% of cases.
Table 1.
Characteristics of the 92 patients with hepatocellular carcinoma n (%)
| Age(yr) | At diagnosis of cirrhosis before development of HCC | 56.0 ± 26.3 |
| At HCC diagnosis | 60.5 ± 22.3 | |
| Gender | Male | 78 (84.8) |
| Etiology | HBV | 62 (67.4) |
| HCV | 18 (19.6) | |
| HBV + HCV | 77 (83.7) | |
| Alcohol abuse | 22 (23.9) | |
| Liver disease severity at time of diagnosis | Child-Pugh A | 32 (34.8) |
| Platelet count < 75 000 | 16 (17.4) | |
| Bilirubin > 3.2 mg/dL | 25 (27.2) | |
| Albumin < 30 g/L | 46 (50) | |
| Mean MELD score | 9.43 ± 7.58 | |
| Tumor characteristics | Single nodule | 41 (44.6) |
| 2-3 nodules | 19 (20.7) | |
| Diffuse | 33 (35.9) | |
| Portal vein obstruction1 | 31 (34.8) | |
| Follow-up and survival | HCC as first presentation of liver disease | 53 (57.6) |
| Mean F/U prior to HCC in those with known liver disease | 40.2 mo | |
| Mean follow-up after HCC diagnosis | 16.4 mo | |
| Mean survival | 14.9 mo |
Three patients had multiple nodules without details regarding vascular invasion or size of the nodules.
Liver disease severity
HCC occurred in the setting of cirrhosis in all but one patient. The Child-Pugh class at time of diagnosis of HCC was A, B, and C in 32 (34.8%), 35 (39.3%), and 23 (25.8%) cases respectively. Model of end stage liver disease (MELD) score was calculated in 79 patients, and the mean was 9.4 ± 7.6. MELD score was < 12, 12-16, 16-24, and >24 in 68.4%, 12.7%, 12.7%, and 6.3% of patients respectively.
Tumor characteristics
The diagnosis of HCC was established during follow-up of liver cirrhosis in 36 patients (42.4%) with a mean follow-up period of 40 mo (median = 24 mo). In 13 patients (36.1%), HCC was diagnosed during the first two years following the diagnosis of cirrhosis, and in 26 patients (72.4%), within 5 years. The size of tumor nodules ranged from 1 cm to 20 cm with a mean of 5.6 cm. The number of nodules was 1, 2, and 3 in 41 (46.1%), 14 (15.7%), and 5 (5.6%) patients respectively; 29 patients (32.6%) had diffuse HCC. Portal vein thrombosis was present in 29 patients (38.2%). Forty patients (43.5%) had a single tumor of less than 5 cm or less than 3 nodules < 3 cm of diameter; ten of those patients had evidence of vascular invasion. The number of patients having a tumor considered eligible for a curative treatment was 32 (34.8%) versus 60 (65.2%) that were ineligible for curative resection on account of diffuse disease or tumor-associated with portal vein thrombosis.
Eligibility for curative treatment
Thirty-two patients had tumors considered eligible for curative treatment, but only 15 (46.9%) patients could benefit from such therapy. This was primarily due to the inability to perform a curative treatment at first intent because of age, tumor location for surgical resection, the lack of regular organ allocation program, or general contraindications other than hepatic disease severity quantified by the Child-Pugh score. When patients older than 70 or 65 were excluded, eligibility for a curative treatment decreased to 25.8% or 20.2% respectively. Other causes of failure to treat were the surveillance of small nodules of 1-2 cm of diameter, or patients awaiting OLT with no other therapeutic possibilities. Fifty-nine patients (64.1%) did not benefit from any treatment because of either decompensated liver disease or advanced tumor cases; in the remaining cases, treatment consisted of percutaneous ethanol injection (7 patients), chemoembolization (17 patients), OLT (8 patients) and surgical resection (7 patients). Eight patients had more than one treatment modality. There was a trend towards a difference in eligibility for treatment between patients presenting with HCC as first manifestation of liver disease (21% eligible for a curative treatment), compared to those discovered on routine screening for HCC (41% eligible for a curative treatment), although this difference did not reach statistical significance (P = 0.13).
Survival and follow-up
Overall survival was 44.8%, 32.8%, and 17.6% at 1, 2, and 3 years respectively. Individual prognostic factors were studied using the Log rank method. Survival was studied with respect to multiple parameters including prothrombin time, platelet count, bilirubin value, albumin, creatinine, alpha-fetoprotein, the diameter of the largest nodule, the presence of portal vein thrombosis, eligibility for curative treatment, Child-Pugh score, MELD score, the presence of ascites, the etiology of liver disease, and the grading of esophageal varices. Prognostic factors identified in univariate analysis included age > 55, bilirubin < 3.2 mg/dL, HCC as the first manifestation of liver disease, eligibility for a curative treatment, International Normalized Ratio (INR) < 2, MELD score > 18, and the presence of portal vein thrombosis. Univariate analysis of overall survival (Table 2) failed to demonstrate a significant difference in survival with respect to Child-Pugh score in HCC patients. The mean survival was 31.4, 18.1, and 24.4 months respectively in Child-Pugh class A, B, and C patients (P = 0.10). In multivariate analysis using Cox regression model; survival in HCC patients was related to age < 55 at diagnosis of HCC (P = 0.004), bilirubin value < 3.2 mg/dL (P = 0.014), and HCC developing in the setting of known liver disease (P = 0.016).
Table 2.
Univariate analysis of overall survival in HCC patients
| Criteria | Median survival (mo) | Mean survival (mo) | Log rank P | |
| Age at diagnosis of HCC (yr) | < 55 | 18 | 40 | 0.008 |
| > 55 | 8 | 14 | ||
| Child-Pugh score | A | 25 | 31.44 | 0.103 |
| B | 10 | 18.14 | ||
| C | 4 | 24.5 | ||
| Bilirubin | < 3.2 mg/dL | 15 | 23 | 0.015 |
| > 3.2 mg/dL | 3 | 14 | ||
| HCC diagnosis | Known liver disease | 25 | 33 | 0.003 |
| As first manifestation of liver disease | 6 | 12 | ||
| Eligibility for treatment | No | 15 | 6 | 0.005 |
| Yes | 27 | 33 | ||
| INR | < 2 | 12 | 23 | 0.029 |
| > 2 | 2 | 9 | ||
| Portal vein thrombosis | Absent | 14 | 23 | 0.021 |
| Present | 3 | 12 |
Factors associated with six month survival are shown in Table 3. Death occurred within six months in 31 patients (38.3%). Survival beyond six months was associated with INR < 2, bilirubin < 3.2 mg/dL, tumor less than 5 cm of diameter or three nodules of less than 3 cm of diameter without portal vein thrombosis, MELD score < 16, lower Child-Pugh class, and the absence of portal vein thrombosis. Mean creatinine level, INR, and MELD score were lower in patients with > 6 mo survival. In multivariate analysis using Cox regression model, bilirubin > 3.2 mg/dL (P = 0.001), creatinine > 1 mg/dL (P = 0.017), and HCC as the first manifestation of liver disease (P = 0.035) were independent predictors of survival less than six months (P = 0.006). Short-term survival (> 6 mo) was 69.2%, 56%, 45.8%, and 0% when none, one, two, or all of these 3 independent factors were present (P = 0.0002) (Figure 1). In comparison, the CLIP score predicted short-term survival in our patient population was 69.3%, 68%, and 29.6% when the score was respectively 0-1, 2-3, or 4-6 (Log rank P = 0.047) (Figure 1). A score based on the 3 clinical variables identified above (CBT score for creatinine, bilirubin, and tumor) outperformed the CLIP score by Cox proportional hazard. ROC curve showed both prognostic models to be equivalent and of moderate accuracy (Figure 2).
Table 3.
Factors associated with six months survival n (%)
| Survival1 | |||
| < 6 mo | > 6 mo | P | |
| Child-Pugh score A | 4 (14.3) | 14 (35.9) | 0.0322 |
| INR < 2 | 22 (70.9) | 43 (93.5) | 0.0122 |
| Bilirubin < 3.2 mg/dL | 17 (58.6) | 35 (83.3) | 0.0332 |
| MELD score < 16 | 37 (90.2) | 18 (62.1) | 0.0062 |
| Portal vein thrombosis | 17 (54.8) | 13 (30.9) | 0.0352 |
| Tumor < 5 cm or 3 nodules < 3 cm without portal vein thrombosis | 3 (10.3) | 11 (31.4) | 0.0422 |
| Mean creatinine (mg/dL) | 1.57 ± 0.89 | 1.19 ± 0.49 | 0.0213 |
| Mean INR | 1.68 ± 0.47 | 1.47 ± 0.42 | 0.0413 |
| Mean MELD score | 13.26 ± 8.12 | 8.12 ± 6.54 | 0.0053 |
Patients treated curatively by OLT or surgical resection were excluded from this analysis.
Fischer exact test.
Mean comparisons by independent sample t-test.
Figure 1.
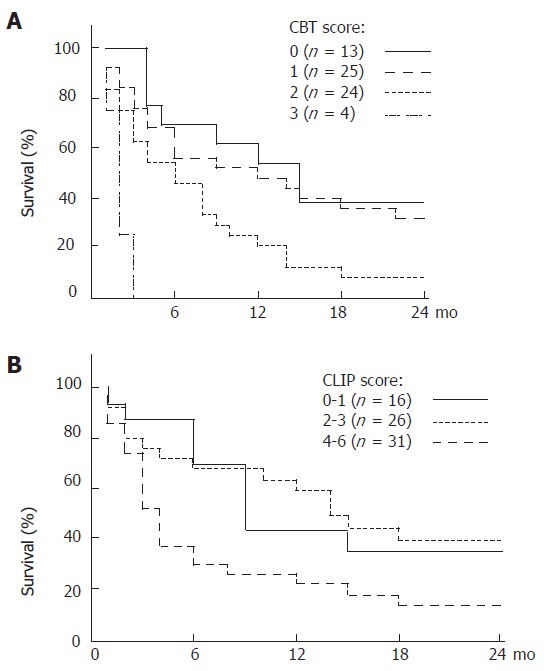
A: Kaplan-Meier survival curves for 68 patients with hepatocellular carcinoma based on 3 clinical criteria CBT (Creatinine, Bilirubin, and Tumor as primary manifestation); B: Kaplan-Meier estimated survival curves according to the Cancer of the Liver Italian Programme (CLIP) classification.
Figure 2.
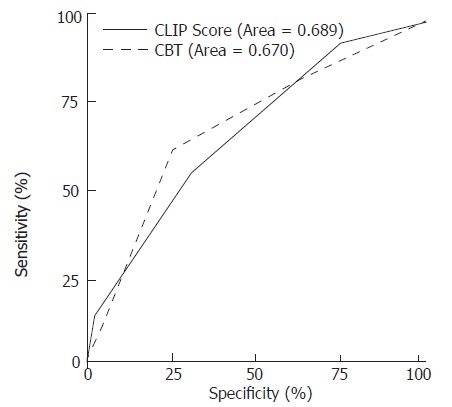
Receiver Operating Characteristic (ROC) curve for early mortality using the CBT (Creatinine, Bilirubin, and Tumor as primary manifestation) and CLIP scores.
DISCUSSION
HCC is a relatively rare cancer in Lebanon, ranking 14th among both males and females, with an age standardized rate of 3.5 and 2.2 per 100 000 respectively[11]. Under-standing the risk factors for HCC and predictors of poor survival are important in prevention and treatment. The results of our study show that most HCC patients in Lebanon have HBV-related liver cirrhosis, accounting for nearly two thirds of our patients. This is concordant with previous findings regarding the etiology of liver cirrhosis in Lebanon where HBV was the most important cause accounting for more than 50% of cirrhosis, followed by HCV, and alcohol abuse[12]. Of note, the HBV and HCV carrier state in Lebanese blood donors is 1%-2% and 0.1%-0.6% respectively[13-15]. Interestingly, there were no cases of non-alcoholic steatohepatitis- or cryptogenic cirrhosis-related HCC in this study population. Studies from neighboring Middle Eastern countries showed wide variation in the etiology of HCC. In Saudi Arabia, HCV plays a major role in the epidemiology of HCC where 39.5% of patients were anti-HCV antibody positive[8]. This is in contrast to an overall HCV seroprevalence of 1.1%-2.9% among Saudi- blood donors[9] versus 0.6% among Lebanese-blood donors[13]. In Jordan, an association between HDV-positive status and HBs-antigen positive primary HCC was found. The prevalence of hepatitis D viral infection in Jordan was 23% in patients with chronic liver disease and in 67% of patients with HCC[7]. In contrast, none of our HCC patients was seropositive for HDV. Lastly, HBV infection was found to be the leading cause of HCC in Turkey, followed by HCV infection, and alcoholic liver disease[6]. Hepatitis B, hepatitis C, excessive alcohol intake were detected in 56%, 23.2%, and 15.9% of Turkish HCC patients respectively.
Previous studies have identified multiple prognostic factors in HCC[16-19]. Gender differences were studied showing significantly longer median survival in females (14 mo) than in male patients (4 mo)[20]. This does not seem to be the case in our patients. Previous multivariate analysis demonstrated that high serum alpha-fetoprotein levels, venous invasion, extrahepatic metastasis, and lack of therapy were independent factors related to unfavorable prognosis[17]. In another study, only type of estrogen receptors and bilirubin showed independent predictive value for mortality[21]. In Child B and C cirrhosis patients, factors predictive of prolonged survival were albumin level (≥ 30 g/L), absence of esophageal varices, tumor size (≤ 3.0cm), tumor number (solitary), and alpha-fetoprotein (AFP) level (< 400 ng/mL)[18]. In our study, factors predictive of prolonged survival were bilirubin (< 3.2 mg/dL) at diagnosis, age (less than 55), and HCC developing in the setting of known liver disease. Serum creatinine and INR values were not independent factors associated with long-term survival, nor was the etiology of the underlying liver disease. This explains somehow that the MELD score was associated with the outcome in univariate analysis, whereas this score has limited value for HCC since prognosis is not only related to liver failure, but also to tumor evolution which is not taken into account in this score. Furthermore, the etiology of underlying liver disease does not seem to play a role in prognosis either in univariate or multivariate analysis. The finding that HCC developing in the setting of known liver disease is an independent predictor of outcome further supports the role of routine screening for HCC in cirrhosis since some of those patients may be amenable to curative treatment.
Data on short-term survival, particularly from the Middle East, is scarce in the literature. Such data is important for the evaluation of early mortality on liver transplantation waiting lists. In a study from Kuwait involving 145 HCC cases, the CLIP score was found to be superior to the Okuda stage as a prognostic indicator[22]. However, the results from a study by Siddique et al seem to reflect the natural history of the disease (91% did not receive any specific therapy for HCC)[22]. In our study, factors predictive of six month survival were bilirubin, creatinine, and HCC developing in the setting of known liver disease. Even for short-term survival, MELD score does not seem to be fit for the evaluation of early mortality in HCC patients. MELD score did, however, show a significant difference in Kaplan-Meier survival analysis when the chosen cut-off was 16, but no difference in survival was found when the cut-off was 24. Again, the etiology of liver disease does not seem to play a role in the early mortality of HCC neither in univariate nor in multivariate analysis.
Our study is limited by the fact that the number of patients was relatively small which restricts the number of variables that can be examined with confidence by multivariate analysis. HCC is however, an uncommon cancer in Lebanon, ranking 14th among both males and females, with an age standardized rate of 3.5 and 2.2 per 100 000 respectively[11]. With a population of just over 3 million, the number of HCC in the whole of Lebanon over a one-year period is estimated at less than 90 cases. It is estimated that a third to half of these cases are referred to the three major university hospitals involved in this study for evaluation and management. We believe, therefore, that our study cohort is relatively large for Lebanon and is likely to be well representative of the population at large particularly as it relates to etiology and outcome.
In conclusion, our study shows that HCC in Lebanon is primarily related to HBV cirrhosis with HCV and alcohol abuse ranking as distant second and third risk factors. The probability for a curative treatment is still low in Lebanon because of advanced tumor stage at presentation and the lack of a regular liver donation program. Thus determination of independent prognostic factors for early mortality is necessary, which include bilirubin (> 3.2 mg/dL), creatinine (> 1 mg/dL), and HCC as first presentation of liver disease. Further studies are needed to validate the above identified prognostic factors and to evaluate drop-out and mortality on the waiting list taking into account these factors.
ACKNOWLEDGMENTS
The authors thank the Lebanese National Research Council for partial funding of this project, the staff and patients of the three university hospitals for their excellent co-operation.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
References
- 1.Kew MC, Yu MC, Kedda MA, Coppin A, Sarkin A, Hodkinson J. The relative roles of hepatitis B and C viruses in the etiology of hepatocellular carcinoma in southern African blacks. Gastroenterology. 1997;112:184–187. doi: 10.1016/s0016-5085(97)70233-6. [DOI] [PubMed] [Google Scholar]
- 2.Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, Chappell CL, Beasley RP, Hwang LY. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J Clin Gastroenterol. 2001;33:123–126. doi: 10.1097/00004836-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Caselmann WH, Alt M. Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol. 1996;24:61–66. [PubMed] [Google Scholar]
- 4.Di Bisceglie AM, Order SE, Klein JL, Waggoner JG, Sjogren MH, Kuo G, Houghton M, Choo QL, Hoofnagle JH. The role of chronic viral hepatitis in hepatocellular carcinoma in the United States. Am J Gastroenterol. 1991;86:335–338. [PubMed] [Google Scholar]
- 5.Hellerbrand C, Hartmann A, Richter G, Knöll A, Wiest R, Schölmerich J, Lock G. Hepatocellular carcinoma in southern Germany: epidemiological and clinicopathological characteristics and risk factors. Dig Dis. 2001;19:345–351. doi: 10.1159/000050702. [DOI] [PubMed] [Google Scholar]
- 6.Uzunalimoğlu O, Yurdaydin C, Cetinkaya H, Bozkaya H, Sahin T, Colakoğlu S, Tankurt E, Sarioğlu M, Ozenirler S, Akkiz H, et al. Risk factors for hepatocellular carcinoma in Turkey. Dig Dis Sci. 2001;46:1022–1028. doi: 10.1023/a:1010705910858. [DOI] [PubMed] [Google Scholar]
- 7.Toukan AU, Abu-el-Rub OA, Abu-Laban SA, Tarawneh MS, Kamal MF, Hadler SC, Krawczynski K, Margolis HS, Maynard JE. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology. 1987;7:1340–1345. doi: 10.1002/hep.1840070627. [DOI] [PubMed] [Google Scholar]
- 8.Shobokshi O, Al-Saffi Y, Zaharna J, Mohammad A. The prevalence of hepatitis C virus in patients with established primary hepatocellular carcinoma in the Western region of Saudi Arabia. Saudi Med J. 2003;24 Suppl 2:S130. [Google Scholar]
- 9.Shobokshi OA, Serebour FE, Al-Drees AZ, Mitwalli AH, Qahtani A, Skakni LI. Hepatitis C virus seroprevalence rate among Saudis. Saudi Med J. 2003;24 Suppl 2:S81–S86. [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 11.Shamseddine A, Sibai AM, Gehchan N, Rahal B, El-Saghir N, Ghosn M, Aftimos G, Chamsuddine N, Seoud M. Cancer incidence in postwar Lebanon: findings from the first national population-based registry, 1998. Ann Epidemiol. 2004;14:663–668. doi: 10.1016/j.annepidem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Yaghi C, Sayegh R, Fayad N, Honein K, Bou Jaoude J, Khouri K. [Cirrhosis and renal failure: the influence of creatinine value on prognosis] J Med Liban. 2003;51:15–23. [PubMed] [Google Scholar]
- 13.Irani-Hakime N, Tamim H, Samaha H, Almawi WY. Prevalence of antibodies against hepatitis C virus among blood donors in Lebanon, 1997-2000. Clin Lab Haematol. 2001;23:317–323. doi: 10.1046/j.1365-2257.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- 14.Araj GF, Kfoury-Baz EE, Barada KA, Nassif RE, Alami SY. Hepatitis C virus : prevalence in Lebanese blood donors and brief overview of the disease. J Med Liban. 1995;43:11–16. [PubMed] [Google Scholar]
- 15.Nabulsi MM, El Saleeby CM, Araj GF. The current status of hepatitis B in Lebanon. J Med Liban. 2003;51:64–70. [PubMed] [Google Scholar]
- 16.Sangro B, Herráiz M, Martínez-González MA, Bilbao I, Herrero I, Beloqui O, Betés M, de-la-Peña A, Cienfuegos JA, Quiroga J, et al. Prognosis of hepatocellular carcinoma in relation to treatment: a multivariate analysis of 178 patients from a single European institution. Surgery. 1998;124:575–583. [PubMed] [Google Scholar]
- 17.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ueno S, Tanabe G, Nuruki K, Oketani M, Komorizono Y, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469–477. doi: 10.1007/s005340200058. [DOI] [PubMed] [Google Scholar]
- 19.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 20.Tangkijvanich P, Mahachai V, Suwangool P, Poovorawan Y. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:1547–1550. doi: 10.3748/wjg.v10.i11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors' status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]
- 22.Siddique I, El-Naga HA, Memon A, Thalib L, Hasan F, Al-Nakib B. CLIP score as a prognostic indicator for hepatocellular carcinoma: experience with patients in the Middle East. Eur J Gastroenterol Hepatol. 2004;16:675–680. doi: 10.1097/01.meg.0000108338.41221.ec. [DOI] [PubMed] [Google Scholar]


