Abstract
AIM: To evaluate roles of syndecan-1, bcl6 and p53 in diagnosis and prognostication of immunoproliferative small intestinal disease (IPSID) and to study profiles of kappa (κ) and lambda (λ) light chains and IgA heavy chain.
METHODS: The study consisted of 11 cases of IPSID and similar number of controls which included 11 of normal intestinal mucosa and 11 of high grade B cell lymphoma of ileum. The parameters analyzed included clinical profiles, biochemical and other laboratory investigations, radiologic and histological findings including immunohistochemistry.
RESULTS: All IPSID cases had demonstrable serum IgA heavy chain and heavy mucosal plasma cell infiltration. According to Galian’s histological staging, there were 4 patients with stage A and 7 with stage B. κ and λ light chains were over-expressed in 7 patients; 1 stage A patient had H pylori-positive active gastritis and eradication of H pylori led to disease remission. Stage A biopsies had higher expression for syndecan-1, while stage B had higher expression for bcl6 and p53. Syndecan-1, κ and λ light chains and IgA heavy chain showed inverse relationship with bcl6 and p53. All patients were treated with doxycycline. CHOP regime was added in 5 patients who developed frank lymphoma. Three died of the disease due to extensive organ infiltration.
CONCLUSION: Certain immunomarkers like syndecan-1, κ and λ light chains and IgA heavy chain could be of much help in identifying early stage IPSID. Stage B IPSID showed higher expression for bcl6 and p53 than stage A IPSID. bcl6 and p53 expressions correlated with a more advanced disease stage and aggressive tumour behavior.
Keywords: IPSID, Syndecan-1, bcl6, p53 protein, κ and λ light chains, Alpha heavy chain, H pylori
INTRODUCTION
Immunoproliferative small intestinal disease (IPSID) or alpha heavy chain disease is quite frequently seen in Mediterranean countries[1-7] compared to rest of the world[8-12]. It usually affects young individual of low socio-economic status. Reduction in incidence has been observed with improvement of living conditions[13-15]. Plasma cells of IPSID secrete bio-chemically abnormal IgA heavy chain in all body fluids[16-18]. Monoclonality in plasma cell of early stage IPSID has been demonstrated by immunohistochemistry and polymerase chain reaction[19-23]. IPSID is known to have an indolent course. Complete remission could be obtained with long-term antibiotic therapy in stage A IPSID, though the ultimate outcome of the disease is unpredictable[22,23]. Syndecan-1 is a member of cell surface trans-membrane heparan sulphate proteoglycans[24] and is expressed by pre-B, mature plasma cell and myeloma cell line[25-27]. In in vitro studies, syndecan-1 has inhibitory effect on growth of myeloma cell line and B cell lymphoma[27,28]. Reduction in syndecan-1 expression has been observed with dedifferentiation of non-plasma cell malignant tumors[29] and down-regulation of p53 and Ki67 expression[30]. bcl6 gene product, a nuclear phospho-protein, is expressed independently of bcl6 gene rearrangement[31]. bcl6 expression is restricted to germinal center B cell and certain percentage of intra-follicular T cell[32]. It is over-expressed in high grade B-cell lymphoma, marginal zone B cell lymphoma and in some cases of follicular lymphoma and nodular lymphocytic predominance Hodgkin’s lymphoma[31-35]. In good number of gastric MALTomas and intestinal diffuse large B-cell lymphoma, expression patterns of bcl6 have been evaluated[36]. p53 gene plays an important role in cell cycle by checking and controlling apoptotic pathways and abnormal cell proliferation. In absence of a functional p53 gene, apoptotic pathway does not become activated and cell cycle progresses without the repair of the defective DNA. Hence, it is known as “gate-keeper gene”[37]. Mutation in p53 gene results in proliferation of abnormal cell by blocking the apoptotic pathways and is seen in various pre-malignant and malignant conditions[37-39]. H pylori-associated early stage MALToma are known to regress following eradication of H pylori. This observation suggests evolvement of MALToma from benign antigen-driven B cell[40-42]. IPSID, like in H pylori-associated MALTomas, likely represents follicle center and/or marginal zone B-cell response to chronic infection by bacteria or parasites and eventually acquiring uncontrolled proliferation and probably monoclonality. Early staged IPSID usually responds to antibiotic therapy alone[43,44] .
Histologically, Galian et al[23] has classified IPSID into 3 stages. Stage A is characterized by mucosal infiltrate by mature plasma cell and lymphocytes. In stage B, mucosa shows presence of atypical or immature plasma cells and lymphocytes, which could extend to deeper portion of the intestinal wall. Stage C is the frank lymphomatous stage with involvement of draining lymph nodes. Stage A is treated by antibiotics alone and stage B requires additional cytotoxic drugs[45,46]. Differentiating between stage A and B is of therapeutic and prognostic importance. With the advancement in imaging techniques, clinical staging is based on observations made on CT and MRI, without subjecting the patient to laparatomy[7]. The protocol was hence planned to study (1) histological profiles of IPSID in endoscopic biopsies, (2) to evaluate roles of syndecan-1, bcl6 and p53 in diagnosis and prognostication of IPSID, and (3) expressions of kappa (κ) and lambda (λ) light chains, and IgA heavy chain.
MATERIALS AND METHODS
Materials
The study included 11 cases of IPSID who presented with features of malabsorption. All had demonstrable serum alpha heavy chain by immuno-electrophoresis. Endoscopic biopsies of 11 patients showed heavy mucosal infiltration predominantly by plasma cell with lymphocyte. According to Galian’s et al[23], histological grading was done as follows: stage A identified by heavy lamina propria infiltration by mature plasma cell and lymphocyte; stage B by lamina propria infiltration with extension into sub-mucosa and/or with few scatter immature plasma cell; and stage C by infiltrating immature plasma cell with variable degree of intestinal wall infiltration and regional lymph node involvement. In all patients studied, 3 to 5 mucosal biopsy fragments were available from involved segments of the intestine. Additional two tissue fragments from pyloric antrum, one each from anterior and posterior wall, were taken only when there was abnormality i.e. either involvement by the ongoing disease or if there was changes of gastritis.
Methods
Baseline assessment of all the biopsies was done on H&E-stained sections for all the eleven patients. Peroxidase anti-peroxidase technique was used for immunohistochemistry. Primary antibodies used were: (1) Syndecan-1 (dilution 1: 50, Dako), (2) bcl6 (pre-diluted, Neomarker), (3) p53 (pre-diluted, DO7, Dako), (4) κ and λ light chains (dilution 1:100, Dako), and (5) IgA specific for heavy chain (dilution 1:800, Dako). Antigen binding epitope site exposure was done by heating in a microwave oven placing the slides in a Coplin jar containing citrate buffer (pH 6) for 12 min in three divided cycles. Detection system used was Envision (Dako). Di-aminobenzedine (DAB) was used for labeling and samples were counterstained lightly with haematoxylene.
The controls included positive control and normal control: (1) CD20-positive non-maltomatous high grade diffuse lymphoma of jejunum served as positive control; and (2) normal intestinal mucosa from margin of surgically resected small intestine served as normal control.
Immunostainings were interpreted with respect to positively stained plasma cells and lymphocytes in percentages of the total cells counted. Mean values were calculated. Light chain over-expression was documented when more than 75% of plasma cells and lymphocytes showed positivity for one of the light chains.
Informed consent was obtained from all patients before subjecting them to endoscopic procedure. Ethic Committee of the Institute approved the protocol.
Statistical analysis
Chi-square test and paired Student’s t test were used for comparison. P value less than 0.05 was considered statistically significant.
RESULTS
The case collection ranged over thirteen years period. The clinical details are depicted in Table 1. Besides anemia, 1 patient had low platelet count (37 000 /mL). Urinary D-xylose excretion test (within 5 h after 5 g of oral D-xylose administration) was abnormal in 10 patients (normal > 1 g). Nine had increased fecal fat (7.67-30 g in 24 h, normal < 6 g/24 h). Abnormal D-xylose excretion test was recorded in 3 stage A and 7 stage B IPSID patients. Fecal fat level was increased in 3 and 4 patients with stage A and stage B IPSID, respectively. Stool examination for ova, cyst and parasite were negative in all patients. All cases had increased level for serum IgA. Pattern of intestinal involvement in radiology and endoscopy are shown in Table 1. Diagnosis of IPSID was based on demonstration of alpha heavy chain in serum and increased IgA levels (range, -340 to 660 mg%). Histology of all the 11 cases showed heavy plasma cell infiltration of the lamina propria admixed with variable amount of lymphocytes and scattered eosinophils. As a result of heavy mucosal cellular infiltration, increased mucosal thickness and wide spacing of the glands were observed (Figure 1). The mucosal infiltrating cell showed extension to sub-mucosa in 7 patients, and scattered immature plasma cells were also observed in 5 cases. One patient had histological evidence of active gastritis with H pylori in the gastric antral biopsy. In remaining cases, antral biopsies showed mild chronic inflammation of superficial mucosa and were negative for H pylori. Histologically (Galian’s staging), stage A was found in 4 cases (1 case with H pylori infection) and stage B in 7 cases. Bone marrow was examined in 7 patients of stage B, but no atypical cells were detected in any biopsies.
Table 1.
Clinical parameters, histological staging, treatment profiles and disease outcome
| Parameters | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th |
| Sex | M | M | M | M | M | M | F | M | M | F | M |
| Age (yr) | 32 | 20 | 48 | 28 | 51 | 45 | 48 | 21 | 34 | 27 | 39 |
| Clinical features | |||||||||||
| Diarrhea | + | + | + | + | - | + | + | + | + | + | + |
| Weight loss | + | + | + | + | - | + | + | + | + | + | + |
| Pain abd | + | + | - | + | + | + | - | + | + | - | + |
| Clubbing | + | - | + | + | + | - | + | + | - | - | + |
| Anemia | + | + | - | - | + | - | + | + | + | + | - |
| Fever | + | - | - | - | + | + | - | - | - | + | - |
| Endoscopy | |||||||||||
| Stomach | - | - | - | + Infl | - | - | - | - | - | + N | - |
| Duodenum | + | + | +(U) | + | + | +(U) | - | + | +(U) | +(U) | + |
| Jejunum | + | + | + | + | + | + | + | + | + | +(U) | + |
| Ileum | - | - | - | - | - | - | + | - | + | + | - |
| Colon | - | - | - | - | - | - | - | - | - | + | - |
| Radiology | |||||||||||
| Mesenteric LN | - | + | + | + | - | + | - | + | + | + | - |
| Hepatomegaly | - | - | - | - | - | - | - | - | + | + | - |
| Splenomegaly | - | - | - | - | - | - | - | - | - | + | - |
| Histology (S+D+J+C+R) | |||||||||||
| Number of tissue | 4+4+0+0+0 | 4+4+0+0+0 | 5+3+0+0+0 | 2+4+4+0+0 | 3+4+0+0+0 | 4+5+0+0+0 | 4+5+0+0+0 | 3+5+0+0+0 | 3+4+3+0+0 | 2+3+3+3+4 | 4+4+0+0+0 |
| Galian’s stage | A | A | A | A, HP | B | B | B | B | B | B | B |
| Light chain over-expression | λ | κ | κ | -ive | -ive | -ive | κ | -ive | κ | κ | κ |
| Drugs received | Dox | Dox Chm | Dox Chm | Anti-HP | Dox | Dox | Dox Chm | Dox | Dox Chm | Dox Chm | Dox |
| Disease relapse | - | + | + | - | - | - | + | - | + | + | - |
| Follow-up (mo) | 120 | 36 | 29 | 40 | 165 | 93 | No FU | 46 | No FU | No FU | 57 |
Abbreviations: Infl = inflammation, N = nodular, U = ulceration, + = disease present, - = disease absent, HP = H pylori, Dox = doxycycline, Chm = Chemotherapy (CHOP regime), Anti-HP = anti-H pylori, S = stomach, D = duodenum, J = jejunum, C = colon, R = rectum.
Figure 1.
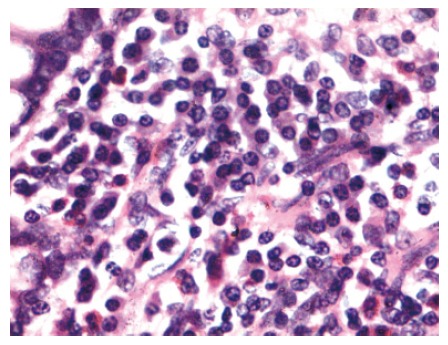
Photomicrograph of mucosal biopsy showing sheets of plasma cells infiltrating the lamina propria. Epithelial cell lining the glands are well preserved (HE, × 240).
Immunohistochemistry
Total number of positively stained cells was expressed in percentages and a total of 1000 cells were counted for each biopsy (Table 2).
Table 2.
Immunoprofiles of the groups studied (mean ± SD)
| Immuno-markers | Stage A | Stage B | Lymphoma | Normal |
| Syndecan-1 | 69 ± 3.40 | 51 ± 10.25 | 10.1 ± 6.354 | 11 ± 7.605 |
| bcl6 | 1.75 ± 1.63 | 3.64 ± 3.541 | 4.25 ± 4.152 | 0.55 ± 0.51 |
| p53 | 3.15 ± 3.257 | 6.5 ± 2.914 | 9.2 ± 4.315 | 0.11 ± 0.901 |
| κ light chain | 67.51 ± 16.952 | 67.42 ± 18.95 | 55 ± 2.399 | 42 ± 13.475 |
| λ light chain | 35 ± 13.857 | 24 ± 1.346 | 25.5 ± 1.087 | 29 ± 12.356 |
| IgA heavy chain | 72 ± 4.570 | 62 ± 24.521 | 22 ± 2.972 | 36 ± 6.513 |
Syndecan-1 expression: As shown in Figure 2, plasma cells had strong cytoplasmic positivity. Epithelial, endothelial and stromal cells showed faint cytoplasmic positivity. Only the plasma cells were counted and expressed in percentages. The mean values were significantly higher in IPSID patients compared to normal and lymphoma controls (P < 0.05). Stage A cases though had higher expression of syndecan-1 compared to stage B IPSID, the difference did not reach statistical significance.
Figure 2.
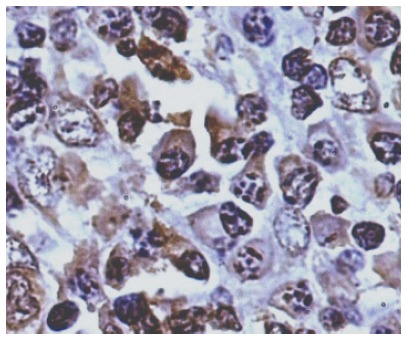
Photomicrograph showing strong syndecan-1-positive cells (PAP, × 440).
bcl6 expression: As shown in Figure 3, bcl6 expression was low on the whole; higher number of positively stained cells were seen in stage B IPSID and lymphoma control than stage A IPSID and normal control. Four of normal controls failed to stain. The differences between the mean values were not significant.
Figure 3.
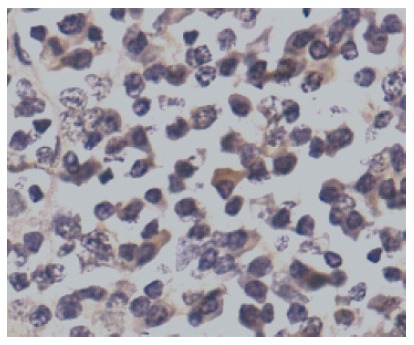
Photomicrograph showing bcl6-positive cells (PAP, × 440).
p53 expression: As shown in Figure 4, p53 expression was low and comparable amongst the different groups. Lymphoma had maximum number of positive cells, while lowest or negligible positive cells were observed in normal control. Stage A IPSID and normal control showed significantly lower mean values compared to lymphoma control (P < 0.05). However, no significant difference was observed between stage A, stage B and normal control.
Figure 4.
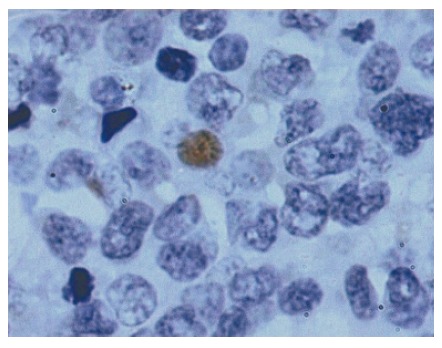
Photomicrograph showing p53 positivity in nuclei (PAP, × 1000).
Light chains
κ light chain: The expression of κ light chain was seen both in plasma cell and lymphocyte. Highest value was detected in stage A patients ranging from 12% to 80%; two patients had 78% and 80%, respectively, one patient had 12%, and the 4th patient had 58%. Stage B patients had the values ranging from 46% to 87%, and 4 patients showed more than 75%. Lymphoma had a range of 18% to 82% and 5 had more than 75%. Normal control had a range of 39% to 53%. Stage A and B IPSID patients had significantly higher values compared to normal and lymphoma controls (P < 0.05). κ light chain over-expression (> 75%) was seen in 2 of stage A and 4 of stage B patients. Few lymphocytes and plasma cells failed to stain.
λ light chain: The expression of λ light chain was found to be inversely correlated with κ light chain expression. There was a wide range for λ light chain expression. One stage A patient with λ light chain over-expression had a value of 78%. Those patients with κ light chain over-expression had values less than 25%. Two of stage A patients with κ light chain over-expression had 9% and 15% for λ light chain. The 4th stage A patient had 38%. Stage B patients had values ranging from 9% to 40%. The four patients with κ light chain over-expression had 9%, 15%, 20% and 18% of λ light chain, respectively. In the rest 3 patients, no over-expression of any of the two light chains had 30%, 36% to 40% for λ light chain. Lymphoma control had a range of 12% to 29% and one had 69%. Normal control had a range of 26% to 37%. Seven IPSID patients (3 stage A and 4 stage B patients) showed light chain over-expression (i.e. κ light chain in 6 and λ light chain in 1).
Heavy chain
IgA heavy chain expression was higher in IPSID patients compared to other groups. Stage A patients had values ranging from 58% to 81% and stage B had 40% to 91%. No significant differences were observed between stage A and B IPSID patients. Similarly, no significant differences were found between the patients of stage A and B with light chain over-expression and without light chain over-expression. Lymphoma control had values ranging from 13% to 30% and 5 of the control cases failed to express heavy chain. Normal control had values ranging from 32% to 45%. Stage A and B patients had significantly higher values than the two controls (P < 0.05).
Treatment and outcome
All cases were treated either with doxycycline (100 mg BD for 4 wk, followed by 100 mg OD for the next 6 to 12 mo) or tetracycline (500 mg TDS for 6 to 12 mo). Three patients initially received anti-tubercular drugs for a month with no clinical response. Subsequently, all three were diagnosed as IPSID (1 stage A and 2 stage B) based on demonstration of serum alpha heavy chain and heavy plasma cell infiltration in the endoscopic biopsies.
Stage A IPSID: The patient with λ light chain over-expression received 8 mo of doxycycline and remained disease-free at 120 mo of follow-up. Two patients with κ light chain over-expression responded initially to 9 mo of doxycycline and developed sub-acute intestinal obstruction at 18 and 11 mo of follow-up. Laparotomy revealed patches of proximal jejunal loops thickening. Resected intestinal segments showed trans-mural plasma cell infiltration mixed with lymphocyte and scattered immature plasma cell. Both patients received CHOP regime for six cycles and remained disease-free at 36 and 29 mo of follow-up, respectively. One patient with duodenal and proximal jejunal involvement had associated H pylori-positive active gastritis. Following treatment for H pylori (Omeprazole 20 mg BD, amoxycilin 500 mg BD and clarithromycin 250 mg TDS for 2 wk), he showed marked clinical improvement. Two weeks latter, repeat radiology and endoscopy showed no residual disease and no evidence of gastritis on repeat follow-up biopsy. He remained disease-free at 40 mo of follow-up. This particular patient did not have positivity for bcl6. The other 2 stage A patients failed show positive staining for bcl6. The 2 patients who developed frank lymphoma on follow-up had higher p53 and low syndecan-1 expression.
Stage B IPSID: Three patients received doxycycline for 9, 9 and 12 mo, respectively; all remained disease-free at 165, 93 and 46 mo of follow-up, respectively. One patient with κ light chain over-expression with hepatosplenomegaly and involvement of splenic flexure and stomach responded to 6 courses of CHOP and remained in remission for 6 mo. She went into relapsed with no response to treatment. At autopsy, terminal part of small intestine and proximal half of colon showed diffuse thickening with mucosal nodularity. Histology showed features of immunoblastic lymphoma with hepatic and splenic infiltration. One case with κ light chain over-expression was disease-free at 57 mo of follow-up. Three patients with κ light chain over-expression responded to doxycycline therapy and remained in remission at 35, 42, and 57 mo of follow-up. The first two patients had recurrence of the symptoms after 35 and 42 mo and biopsies from proximal jejunum revealed high-grade lymphoma. Subsequently, both were put on CHOP regime but failed to respond and died of the disease. Syndecan-1 expression was higher in four patients (57%, 61%, 65%, and 69%) and all remained disease-free at follow-up compared to the patients who had disease recurrence or died of the disease (36%, 40%, and 42%) (P < 0.05). Those 3 patients with unfavorable prognosis also had higher values for bcl6 but the differences were not significant (P > 0.05) (8%, 7%, and 5%) than those in remission (0.5%, 2%, 3%, and 3%). Expression of p53 was also obviously higher in those who succumbed to their illness (i.e. 11.5%, 9%, and 7%) than the ones who were in remission (1%, 4%, 5%, and 6%) (P < 0.05).
DISCUSSION
Syndecan-1 expression was found to be higher in all cases of IPSID compared to other groups. This would be in relation to number of plasma cell present. Higher mean values observed in stage A than stage B would suggest a phenotypic alteration of the cells i.e. benign to malignant clone. Syndecan-1-positive cell was significantly lower in lymphoma control cases (P < 0.01). Patients of stage A and B IPSID with unfavorable disease outcome also had low syndecan-1 expression compared to the ones with favorable outcome (P > 0.05). A higher syndecan-1 expression (> 50% positive) would infer a better prognosis. Similarly, such distinctive difference in the number of positive cell has been recorded in certain other types of tumors[26-30]. bcl6 expression was weak and low. Stage B IPSID patients and control lymphoma had comparatively higher values than stage A IPSID patients and other groups (P > 0.05). These three stage B cases subsequently developed high grade tumor had higher bcl6 expression. In our study, bcl6 appears to be a poor prognostic indicator. Similar observations have been made in diffuse large and marginal zone B-cell lymphomas and nodular variant of Hodgkin’s lymphoma[31,34-36]. Besides, bcl6 expression would infer IPSID cells originating from germinal center B cell. Ariatti et al[33] made such suggestion with respect to bcl6-positive lymphoma. Mutant form of p53 is over-expressed by many malignant tumors including lymphomas. Similar to bcl6 expression, higher p53 values were recorded in those cases with poor outcome, whereas lower value was seen in those cases who were in remission at follow-up (P < 0.05). Minimum value was expressed by normal control followed by stage A and B IPSID and highest by lymphoma control.
Over-expression of one type of light chain was exhibited by our cases, as had been observed in few other studies[19-21,23]. Number of cases with κ light chain over-expression was more than that of λ light chain. Over-expression of λ light chain had also been reported in one case by Isaacson et al[19,20]. Monoclonality, exhibited by our patients, further strengthens the view expressed in previous studies, as an inherent character of IPSID as in lymphoma. However, monoclonality appeared not to influence the ultimate outcome of disease process. But study in more number of cases would be required before drawing such conclusion. IgA heavy chain over-expression was diffuse and uniformly strong in IPSID cases. This character of IPSID could be interpreted with important clinical implication. And this would help to identify and differentiate patients of IPSID, especially stage A, from other similar clinical conditions and investigative findings including histology.
Similar to our cases of H pylori-positive stage A IPSID, Fishbach et al[44] reported one patient in whom the underlying disease was controlled following eradication of H pylori. As stage A IPSID could be successfully treated with antibiotics, this effect of anti-H pylori could be more generalized rather than specifically related to H pylori eradication. Exposure to a long-standing cause and effect relationship between H pylori infection and IPSID localizing to foregut requires critical analysis and documentation in more number of cases. Gastric biopsy in the rest of cases did not show evidence of H pylori infection, though 1 case had evidence of infiltration by underlying disease process.
Colonic and hepatosplenic involvement in IPSID is usually seen only with overt lymphomatous conversion or in recurrent disease[47-49]. Three of our patients had gastric and colonic involvement at presentation with no overt lymphomatous conversion. But within one-year period, all the three cases developed overt lymphoma and two of the cases succumbed to their illness in spite of the treatment
In conclusion, over-expression of syndecan-1, IgA and light chain would help in differentiating stage A IPSID from normal and stage B IPSID. Expression of bcl6 and p53 would indicate an aggressive behavior and a more advanced disease stage. bcl6 and p53 exhibited an inverse relationship with syndecan-1 expression. Gastric H pylori infection, though was seen only in one case with the active gastritis, would be advisable to look for in all cases of IPSID by carrying out gastric or pyloric antral biopsies.
ACKNOWLEDGMENTS
We would like to thank Ms Anu Khuller and Monica Ahuja for carrying out the immunostainings, Ms Surekha Sharma for typographical help and Ms Tranum Kaur (Research Scholar) for statistical calculation.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
References
- 1.Seligmann M, Danon F, Hurez D, Mihaesco E, Preud'homme JL. Alpha-chain disease: a new immunoglobulin abnormality. Science. 1968;162:1396–1397. doi: 10.1126/science.162.3860.1396. [DOI] [PubMed] [Google Scholar]
- 2.Doe WF, Henry K, Hobbs JR, Jones FA, Dent CE, Booth CC. Five cases of alpha chain disease. Gut. 1972;13:947–957. doi: 10.1136/gut.13.12.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seligmann M. Alpha chain disease: immunoglobulin abnormalities, pathogenesis and current concepts. Br J Cancer Suppl. 1975;2:356–361. [PMC free article] [PubMed] [Google Scholar]
- 4.Eidelman S. Abdominal lymphoma with malabsorption. JAMA. 1974;229:1103–1104. [PubMed] [Google Scholar]
- 5.Selzer G, Sherman G, Callihan TR, Schwartz Y. Primary small intestinal lymphomas and alpha-heavy-chain disease. A study of 43 cases from a pathology department in Israel. Isr J Med Sci. 1979;15:111–123. [PubMed] [Google Scholar]
- 6.Seligmann M, Mihaesco E, Frangione B. Alpha chain disease. Ann N Y Acad Sci. 1971;190:487–500. doi: 10.1111/j.1749-6632.1971.tb13558.x. [DOI] [PubMed] [Google Scholar]
- 7.Tabbane F, Mourali N, Cammoun M, Najjar T. Results of laparotomy in immunoproliferative small intestinal disease. Cancer. 1988;61:1699–1706. doi: 10.1002/1097-0142(19880415)61:8<1699::aid-cncr2820610831>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Ghoshal UC, Chetri K, Banerjee PK, Choudhuri G, Pal BB, Dabadghao S, Dhar K, Naik S, Naik SR. Is immunoproliferative small intestinal disease uncommon in India. Trop Gastroenterol. 2001;22:14–17. [PubMed] [Google Scholar]
- 9.Puri AS, Kumar M, Khan EM, Pandey R, Aggarwal R, Naik S, Choudhuri G, Naik SR. Immunoproliferative small intestinal disease: a frequently missed diagnosis. Indian J Gastroenterol. 1996;15:46–48. [PubMed] [Google Scholar]
- 10.Dhir V, Mohandas KM. Immunoproliferative small intestinal disease (IPSID): the Indian scenario. Indian J Gastroenterol. 1997;16:38. [PubMed] [Google Scholar]
- 11.Nair S, Mathan M, Ramakrishna BS, Mathan VI. Immunoproliferative small intestinal disease in South India: a clinical and immunomorphological study. J Gastroenterol Hepatol. 1998;13:1207–1211. [PubMed] [Google Scholar]
- 12.Mohandas KM, Nagral A. Epidemiology of digestive tract cancers in India. II. Stomach, and gastrointestinal lymphomas. Indian J Gastroenterol. 1998;17:24–27. [PubMed] [Google Scholar]
- 13.Ramot B, Many A. Primary intestinal lymphoma: clinical manifestations and possible effect of environmental factors. Recent Results Cancer Res. 1972;39:193–199. [PubMed] [Google Scholar]
- 14.Alpha-chain disease and related small-intestinal lymphoma: a memorandum. Bull World Health Organ. 1976;54:615–624. [PMC free article] [PubMed] [Google Scholar]
- 15.Cammoun M, Jaafoura H, Tabbane F, Halphen M. Immunoproliferative small intestinal disease without alpha-chain disease: a pathological study. Gastroenterology. 1989;96:750–763. [PubMed] [Google Scholar]
- 16.Seligmann M, Rambaud JC. IgA abnormalities in abdominal lymphoma (alpha-chain disease) Isr J Med Sci. 1969;5:151–157. [PubMed] [Google Scholar]
- 17.Seligmann M, Mihaesco E, Hurez D, Mihaesco C, Preud'homme JL, Rambaud JC. Immunochemical studies in four cases of alpha chain disease. J Clin Invest. 1969;48:2374–2389. doi: 10.1172/JCI106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seligmann M, Rambaud JC. Alpha-chain disease: an immunoproliferative disease of the secretory immune system. Ann N Y Acad Sci. 1983;409:478–485. doi: 10.1111/j.1749-6632.1983.tb26892.x. [DOI] [PubMed] [Google Scholar]
- 19.Isaacson PG, Price SK. Light chains in Mediterranean lymphoma. J Clin Pathol. 1985;38:601–607. doi: 10.1136/jcp.38.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacson PG, Dogan A, Price SK, Spencer J. Immunoproliferative small-intestinal disease. An immunohistochemical study. Am J Surg Pathol. 1989;13:1023–1033. doi: 10.1097/00000478-198912000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Smith WJ, Price SK, Isaacson PG. Immunoglobulin gene rearrangement in immunoproliferative small intestinal disease (IPSID) J Clin Pathol. 1987;40:1291–1297. doi: 10.1136/jcp.40.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khojasteh A, Haghshenass M, Haghighi P. Current concepts immunoproliferative small intestinal disease. A "Third-World lesion". N Engl J Med. 1983;308:1401–1405. doi: 10.1056/NEJM198306093082309. [DOI] [PubMed] [Google Scholar]
- 23.Galian A, Lecestre MJ, Scotto J, Bognel C, Matuchansky C, Rambaud JC. Pathological study of alpha-chain disease, with special emphasis on evolution. Cancer. 1977;39:2081–2101. doi: 10.1002/1097-0142(197705)39:5<2081::aid-cncr2820390526>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327(Pt 1):1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley RC, Xiao H, Hata H, Woodliff J, Epstein J, Sanderson RD. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–774. [PubMed] [Google Scholar]
- 27.Dhodapkar MV, Abe E, Theus A, Lacy M, Langford JK, Barlogie B, Sanderson RD. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679–2688. [PubMed] [Google Scholar]
- 28.Sánchez-Beato M, Sánchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101:1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 29.Millet VG, Casado Pérez S, Alvarez Grande J, Hernando Avendaño L. Cataracts after renal transplantation. Br Med J. 1972;4:47. doi: 10.1136/bmj.4.5831.47-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurokawa H, Matsumoto S, Murata T, Yamashita Y, Tomoyose T, Zhang M, Fukuyama H, Takahashi T. Immunohistochemical study of syndecan-1 down-regulation and the expression of p53 protein or Ki-67 antigen in oral leukoplakia with or without epithelial dysplasia. J Oral Pathol Med. 2003;32:513–521. doi: 10.1034/j.1600-0714.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 31.Dierlamm J, Wlodarska I, Michaux L, Stefanova M, Hinz K, Van Den Berghe H, Hagemeijer A, Hossfeld DK. Genetic abnormalities in marginal zone B-cell lymphoma. Hematol Oncol. 2000;18:1–13. doi: 10.1002/(sici)1099-1069(200003)18:1<1::aid-hon647>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Ree HJ, Kadin ME, Kikuchi M, Ko YH, Suzumiya J, Go JH. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30:403–411. doi: 10.1016/s0046-8177(99)90115-6. [DOI] [PubMed] [Google Scholar]
- 33.Ariatti C, Vivenza D, Capello D, Migliazza A, Parvis G, Fassone L, Buonaiuto D, Savinelli F, Rossi D, Saglio G, et al. Common-variable immunodeficiency-related lymphomas associate with mutations and rearrangements of BCL-6: pathogenetic and histogenetic implications. Hum Pathol. 2000;31:871–873. doi: 10.1053/hupa.2000.7626. [DOI] [PubMed] [Google Scholar]
- 34.Akasaka T, Lossos IS, Levy R. BCL6 gene translocation in follicular lymphoma: a harbinger of eventual transformation to diffuse aggressive lymphoma. Blood. 2003;102:1443–1448. doi: 10.1182/blood-2002-08-2482. [DOI] [PubMed] [Google Scholar]
- 35.Wlodarska I, Nooyen P, Maes B, Martin-Subero JI, Siebert R, Pauwels P, De Wolf-Peeters C, Hagemeijer A. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101:706–710. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- 36.Kwon MS, Go JH, Choi JS, Lee SS, Ko YH, Rhee JC, Ree HJ. Critical evaluation of Bcl-6 protein expression in diffuse large B-cell lymphoma of the stomach and small intestine. Am J Surg Pathol. 2003;27:790–798. doi: 10.1097/00000478-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 38.Nelson WG, Kastan MB. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.el-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 40.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 41.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 42.Isaacson PG, Spencer J. Is gastric lymphoma an infectious disease. Hum Pathol. 1993;24:569–570. doi: 10.1016/0046-8177(93)90233-7. [DOI] [PubMed] [Google Scholar]
- 43.Fine KD, Stone MJ. Alpha-heavy chain disease, Mediterranean lymphoma, and immunoproliferative small intestinal disease: a review of clinicopathological features, pathogenesis, and differential diagnosis. Am J Gastroenterol. 1999;94:1139–1152. doi: 10.1111/j.1572-0241.1999.01057.x. [DOI] [PubMed] [Google Scholar]
- 44.Fischbach W, Tacke W, Greiner A, Konrad H. Regression of immunoproliferative small intestinal disease after eradication of Helicobacter pylori. Lancet. 1997;349:31–32. doi: 10.1016/s0140-6736(05)62165-4. [DOI] [PubMed] [Google Scholar]
- 45.el Saghir NS, Jessen K, Mass RE, al-Mofleh I, Santhosh-Kumar CR, Hall AD, Stuart AE. Combination chemotherapy for primary small intestinal lymphoma in the Middle East. Eur J Cancer Clin Oncol. 1989;25:851–856. doi: 10.1016/0277-5379(89)90131-4. [DOI] [PubMed] [Google Scholar]
- 46.Ramakrishna BS. Malabsorption syndrome in India. Indian J Gastroenterol. 1996;15:135–141. [PubMed] [Google Scholar]
- 47.Colombel JF, Rambaud JC, Vaerman JP, Galian A, Delacroix DL, Nemeth J, Duprey F, Halphen M, Godeau P, Dive C. Massive plasma cell infiltration of the digestive tract. Secretory component as the rate-limiting factor of immunoglobulin secretion in external fluids. Gastroenterology. 1988;95:1106–1113. doi: 10.1016/0016-5085(88)90189-8. [DOI] [PubMed] [Google Scholar]
- 48.Tashiro T, Sato H, Takahashi T, Genda T, Sugitani S, Yoshida T, Funakoshi K, Tukada Y, Narisawa R, Nomoto M. Non-secretory alpha chain disease involving stomach, small intestine and colon. Intern Med. 1995;34:255–260. doi: 10.2169/internalmedicine.34.255. [DOI] [PubMed] [Google Scholar]
- 49.Rhodes JM, Jewell DP, Janossy G. Alpha-chain disease diagnosed by rectal biopsy. Br Med J. 1980;280:1043–1044. doi: 10.1136/bmj.280.6220.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]


