Abstract
Kupffer cells, the resident liver macrophages have long been considered as mostly scavenger cells responsible for removing particulate material from the portal circulation. However, evidence derived mostly from animal models, indicates that Kupffer cells may be implicated in the pathogenesis of various liver diseases including viral hepatitis, steatohepatitis, alcoholic liver disease, intrahepatic cholestasis, activation or rejection of the liver during liver transplantation and liver fibrosis. There is accumulating evidence, reviewed in this paper, suggesting that Kupffer cells may act both as effector cells in the destruction of hepatocytes by producing harmful soluble mediators as well as antigen presenting cells during viral infections of the liver. Moreover they may represent a significant source of chemoattractant molecules for cytotoxic CD8 and regulatory T cells. Their role in fibrosis is well established as they are one of the main sources of TGFβ1 production, which leads to the transformation of stellate cells into myofibroblasts. Whether all these variable functions in the liver are mediated by different Kupffer cell subpopulations remains to be evaluated. In this review we propose a model that demonstrates the role of Kupffer cells in the pathogenesis of liver disease.
Keywords: Kupffer cells, Liver disease, Hepatic injury, Liver fibrosis, Hepatocellular carcinoma, Hepatitis, Steatohepatitis
INTRODUCTION
The sinusoidal lining of the liver contains the nonparenchymal cell populations which consist of Kupffer cells (KCs), sinusoidal endothelial cells (SEC) and stellate cells (SC). All three cell-types seem to play a crucial role in liver homeostasis and in the pathogenesis of liver disease[1]. KCs constitute 80%-90% of the tissue macrophages in the reticuloendothelial system and account for approximately 15% of the total liver cell population[2]. They are mainly found in the periportal area of the lobule (43%), but KCs also exist in the midzonal (28%) and in the central area (29%)[2]. Despite the view that KCs are fixed tissue macrophages of the liver, there is evidence that they have the ability to migrate along sinusoidal walls with a mean speed of 4.6 ± 2.6 (SD) microns/min[3]. Since the description of these resident liver macrophages in 1876 by von Kupffer various theories have been proposed with regard to their origin and involvement in liver homeostasis and injury. It should be noted that almost all available evidence for the role of Kupffer cells comes from animal models.
KCs are the first cells to be exposed to materials absorbed from the gastrointestinal tract. Their ability to eliminate and detoxify microorganisms, endotoxins, degenerated cells, immune complexes, and toxic agents (e.g. ethanol) is an important physiological function. Due to their key location, KCs might function as antigen-presenting cells[4] and participate in tumour surveillance[5] and the regeneration processes of the liver[6]. They also seem to play a key role in innate immune responses and host defence through the expression and secretion of soluble inflammatory mediators[7]. There is accumulating evidence that the interaction between KC and lipopolysaccharide (LPS) may be the initiating event leading to hepatotoxicity in various types of liver injury including endotoxinaemia, alcoholic liver injury and ischemia/reperfusion injury[8,9] and systemic viral infections[10].
THE ROLE OF KUPFFER CELLS IN HEPATIC INJURY
Kupffer cells are involved in the pathogenesis of liver injury mediated by chemical substances, toxins and pharmacological agents[7,9] such as carbontetrachloride (CCl4)[11], endotoxin[12], galactosamine[13] and acetaminophen[14] through the release of biologically active substances that promote the pathogenic process[9]. In liver injury and hepatocellular necrosis activated Kupffer cells are a major source of inflammatory mediators including cytokines, superoxide, nitric oxide, eicosanoids, chemokines, lysosomal and proteolytic enzymes and demonstrate increased cytotoxicity and chemotaxis[7,14-16].
Reactive oxygen radicals are released by hepatic macrophages after activation with cytokines, LPS and prostaglandins as a defence against bacterial invasion. These molecules have been implicated in the pathogenesis of liver injury induced in a rat model by sequential administration of endotoxin and Corynebacterium parvum[17]. In this model, the products of oxidation of hepatocellular membrane lipids were detected in the systematic circulation and were related with the degree of liver necrosis. Administration of superoxide dismutase, a reactive oxygen radical scavenger, significantly reduced the liver injury and animal mortality[12]. Isolated Kupffer cells from Corynebacterium parvum-treated rats demonstrated significantly increased release of superoxide that was further enhanced following administration of endotoxin[17]. The toxicity of reactive oxygen intermediates on hepatocytes has also been demonstrated in vitro using cultured rat hepatocytes[18]. However, LPS-treated Kupffer cells are cytotoxic to hepatocytes in co-culture experiments only in the presence of L-arginine, probably in response to simultaneous secretion of nitric oxide by Kupffer cells or induction of production by hepatocytes[19].
Nitric oxide is produced in the liver by Kupffer cells and hepatocytes. Its role in the pathogenesis of hepatic injury is controversial. A protective role has been detected in various conditions such as endotoxemia or CCl4-induced damage where it protects hepatocytes via the inhibition of caspases and apoptosis. In other conditions like ischemia/ reperfusion injury, shock, and galactosamine induced liver injury, nitric oxide increases oxidative stress via its interaction with reactive oxygen species leading to the formation of peroxynitrite or it induces the expression of inflammatory mediators such as TNF-α and IL-1[20]. Adiponectin suppresses TNF-α production and induces IL-10 production by Kupffer cells and administration of galactosamine in adiponectin knock-out mice significantly increases mortality rate compared with wild type animals[21]. It has been suggested that the hepato-protective activity of adiponectin is due, at least in part, to a direct anti-inflammatory effect of adiponectin on Kupffer cells[22].
Cytokine and chemokine production by activated Kupffer cells is involved in the pathogenesis of liver damage. It has been reported that alcohol-induced liver injury is accompanied by increases in the portal concentration of endotoxin, leading to activation of Kupffer cells and subsequent TNF-α production[23]. Other studies have shown a role for the increased production of the chemokine MCP-1 by Kupffer cells in the pathogenesis of acute liver injury due to CCl4[24] or acetaminophen[25] administration. Proteolytic enzymes released by recruited and activated liver macrophages were also found to promote hepatic injury in a rat model of hepatic damage[26].
The pivotal role of Kupffer cells in the initiation of hepatocellular damage is supported by experimental models that have demonstrated a correlation between the degree of activation of Kupffer cells and the degree of hepatocellular destruction[14]. Administration of endotoxin to rats with activated Kupffer cells due to liver resection induced damage of endothelium, sinusoidal fibrin deposition, and lethal massive hepatic necrosis[27]. In another rat model, activation with endotoxin enhanced CCl4-induced liver damage, while pretreatment with polymyxin B or administration of endotoxin in low doses induced immune tolerance which protected the liver from CCl4-induced damage[27]. Other studies demonstrated that activated Kupffer cells express CD95L and could induce apoptosis in CD95+ T lymphocytes and hepatocytes[28].
However, Kupffer cells also participate in protective mechanisms via the production of mediators that induce synthesis of the antioxidant agent glutathione[29], or the production of nitric oxide[30,31]. The production of ELR-CXC chemokines such as MIP-2, which induce hepatocyte proliferation also has a protective role in models of hepatotoxicity such as acetaminophen-induced injury[32-34]. This protection is also possibly mediated by the production of IL-10 and IL-18 by Kupffer cells, since depletion of Kupffer cells increases susceptibility of the murine liver to acetaminophen in parallel with a reduction in IL-10 and IL-18[35]. On the other hand, hard evidence for the protective role of Kupffer cells is missing since depletion of Kupffer cells by the traditional method of administration of gadolinium chloride (GdCl3) intraperitoneally might not deplete the liver from Kupffer cells. Instead GdCl3 might change the acinar distribution and phenotype of Kupffer cells promoting the production of TNF-α and IL-6[36-38]. Therefore interpretation of experiments using GdCl3 is difficult. In conclusion, Kupffer cell-induced hepatotoxicity is not only a result of the reaction to hepatotoxins[39], but it might also be a response to an excessive activation or a suppression of hepatoprotective mechanisms[40].
THE ROLE OF KUPFFER CELLS IN LIVER FIBROSIS
Liver fibrosis is a complex process that involves many cells of the hepatic sinusoid and is characterized by disturbance of the architecture and composition of extracellular matrix in the liver[41,42]. The extracellular matrix in the subendothelial space of Disse mainly consists of collagen type IV, laminin, and proteoglycans that are progressively replaced during fibrosis by collagen type Ι and III. This excess deposition disrupts the normal architecture of the hepatic lobule[43,44].
Ito or stellate cells are the main cellular source of extracellular matrix proteins in the liver[45,46]. The initiation and maintenance of fibrogenesis in the liver is characterized by two processes. The former is characterized by the activation and transformation of Ito cells to myofibroblasts resulting in increased production of collagen types I and III[47]. In parallel, there seems to be a disturbance of the homeostatic mechanisms involved in extracellular matrix deposition due to reduced expression of the proteolytic enzymes that degrade the extracellular matrix and increased expression of their inhibitors. Thus, maintaining fibrosis involves decreased production of matrix metalloproteinases (MMPs) and increased production of specific (tissue inhibitors of matrix metalloproteinases, TIMPs) or non specific metalloproteinase inhibitors (alpha1-antitrypsin)[48].
Kupffer cells are involved both in processes via the production of cytokines and growth factors that induce Ito cell myofibroblastic transformation and also via regulation of the production of metalloproteinases and their inhibitors[49]. Kupffer cell-derived TGF-β1 has been suggested to drive Ito cell transformation and to induce production of collagen and proteoglycans by these cells[50]. TGF-β1 is considered as the main cytokine that drives fibrosis in various animal models of hepatic damage, including alcoholic liver fibrogenesis[51], schistosomiasis and CCl4-induced fibrosis[52], and one of the major factors involved in fibrosis in patients with chronic liver disease[53].
In vitro studies have also shown that Kupffer cells can induce expression of platelet-derived growth factor (PDGF) receptors on Ito cells, thus enhancing Ito cell proliferation in response to PDGF[54]. TNF-α, IL-1 and MCP-1, that are produced by activated Kupffer cells, are also mitogenic and chemoattractant for Ito cells[55,56]. In addition, TGF-β1 and IL-6 were found to induce mRNA expression of metalloproteinases (MMPs) and also their specific inhibitors TIMPs (mostly TIMP-1, in hepatocytes, Kupffer cells and Ito cells in rat liver[57].
Finally another mechanism that could lead to the phenotypic change of Ito cells is the production of gelatinases by Kupffer cells. It has been demonstrated that extracellular matrix proteins play a crucial role in the maintenance of normal function of hepatocytes and Ito cells. Culture of Ito cells on type I collagen or plastic resulted in activation of cells and transformation to myofibroblasts. In contrast, culture of Ito cells in collagen type IV did not result in phenotypic change[58]. It has been suggested that activation of Kupffer cells and secretion of gelatinase degrades collagen type IV and therefore triggers the phenotypic change of Ito cells[7,59].
THE ROLE OF KUPFFER CELLS IN LIVER DISEASES
The role of Kupffer cells in liver infections
Kupffer cells are involved in the defence against infections of the liver. Their major role in the host defence and the prognosis of liver infection is indicated by studies in experimental models of sepsis. LPS pre-treatment has been shown to increase Kupffer cell numbers leading to a reduction of bacterial load and improvement of prognosis in a Salmonella septicemia model[60]. Impairment of the phagocytic function and the production of superoxide by Kupffer cells in models of obstructive jaundice leads to increased susceptibility to infection[61].
Infection of mice with Listeria monocytogenes is a well studied liver infection model. In this model, the accumulation of bacillus in the liver depends on recognition of bacillus surface sugars and lectins by cognate receptors on Kupffer cells. On the other hand, production of inflammatory mediators such as IL-6, IL-12, IL-1β, TNF-α, and nitric oxide by infected Kupffer cells inhibits proliferation of the microorganism[62,63]. At the same time Kupffer cell derived chemokines such as MIP-1α, MIP-1β, MCP-1, and MIP-2, drive monocyte and neutrophil recruitment into the liver in order to control infection[64-66]. Thus as expected, Kupffer cell inactivation results in impaired infection clearance[67]. Being the first line of defence, Kupffer cells also represent the portal of entry for viruses such as cytomegalovirus[68] and parasites such as Plasmodium bergei[69] and Leishmania[70], which enter and proliferate in Kupffer cells and then infect the rest of the liver cells.
In humans, phenomena like the increased frequency of septicaemia and septic shock from Gram negative bacteria that are observed in patients with acute hepatic failure, have been attributed to the inability of Kupffer cells to clear the portal circulation of micro-organisms and endotoxin[71]. Various studies have shown that a large percentage of patients with chronic hepatic disease present with a systematic endotoxinaemia and high titres of antibodies against intestinal bacteria.In contrast, in normal individuals endotoxin is detected only in the portal circulation[72].
Very recently a direct contribution of Kupffer cells to the pathogenesis of hepatitis has been reported[73]. Influenza hepatitis was associated with absence of virus from the liver and foci of CD8+ virus specific T cells in close contact with Kupffer cells. Moreover, elimination of Kupffer cells abrogated the hepatocellular necrosis, despite persistence of CD8+ reactive cells. It seems that activated T cells are trapped and retained in the liver through an antigen-independent mechanism as a possible interaction between activated integrins like LFA-1 on the T cells and constitutively expressed integrin ligands like VCAM and ICAM-1 on sinusoidal endothelium[74,75]. In this model, Kupffer cells are possibly the effector cells killing hepatocytes in an as yet unidentified manner. Kupffer cells can kill hepatocytes either directly via activation of fas-dependent or CD95-dependent apoptotic pathways[76] or indirectly by interacting with CD8+ (and possibly CD4+) lymphocytes with the stimulation of cytokine secretion[77] and other mediators like phospholipases and nitric oxide, as previously reported. Although such a mechanism as that proposed in the paper by Polakos et al[73] might explain the hepatitis observed in measles, SARS and CMV infection (where the virus is not identified in the liver), a similar mechanism could well operate in the pathogenesis of hepatitis due to hepatotropic viruses like HBV, HCV and HEV. The only difference would be that the generation of CD8+ virus specific cells would take place in either the portal tracts or the sinusoids per se, with Kupffer cells and dendritic cells being the antigen presenters.
Kupffer cells and hepatocellular carcinoma
The liver is a frequent site of hematogenous metastasis particularly for cancers of the gastrointestinal system. Isolated Kupffer cells were found to be cytotoxic against human colon adenocarcinoma cells and this cytotoxicity was increased significantly when the KC were stimulated with INF-γ and endotoxin[78,79]. It has been suggested that this effect is related to TNF-α expression by Kupffer cells as it is inhibited by anti-TNF-α[80,81]. Other studies have demonstrated that Kupffer cells induce Fas expression in colon cancer cells[82] and malignant glioma cells[83] leading to Fas-mediated apoptosis and death in the presence of tumour infiltrating lymphocytes or TNF-α.
Data from in vivo studies show that the degree of activation or repression of Kupffer cells influences the number and the size of hepatic metastases following injection of colon carcinoma cells in portal circulation[84]. Administration of GdCl3, which is reported to deplete and block the function of Kupffer cells, resulted in increased size of metastases, while activation of Kupffer cells with Zymosan and Corynebacterium parvum decreased the size of metastases[85].
In vivo microscopy has shown that Kupffer cells are attracted to tumour cells in the hepatic circulation and have the ability to phagocytose these cells[86]. Nitric oxide produced by Kupffer cells after stimulation with endotoxin, TNF-α and prostaglandin Ε2[16,87] may also be an effective weapon of the Kupffer cell machinery against tumor cells[88]. Moreover, an indirect mechanism of defence by Kupffer cells against hepatic tumours is the induction of natural killer cell (NK-cell) cytotoxicity via the production of IL-12[84] and a possible anti-tumour effect of octreotide in hepatocellular carcinoma[89,90] might, in part, be explained by its antiapoptotic effect on Kupffer cells[91].
Alcohol-related liver disease and Kupffer cells
Alcohol-related liver disease is a chronic inflammatory disease of the liver parenchyma due to chronic ethanol ingestion with the end result being alcoholic fibrosis and cirrhosis. Kupffer cells have been suggested to participate in this process mainly through the increased production of inflammatory mediators. Indeed, increased circulating levels of pro-inflammatory cytokines like TNFα and IL-6, and chemokines like IL-8, MCP-1 and MIP-1α have been detected in patients with alcoholic liver disease, which could potentially be related to Kupffer cell activation[92-95]. Increased numbers of Kupffer cells in the portal tracts have been observed in patients with acute alcoholic hepatitis or chronic alcoholic liver disease[96].
Animal studies have shown that acute or chronic ethanol administration is associated with an increase in numbers of Kupffer cells that exhibit morphologic signs of cell activation[97], up regulation of CD14 expression[98] and increased production of inflammatory mediators such as IL-1, TNF-α[99] and oxygen free radicals[100]. Kupffer cell depletion with GdCl3 has been found to prevent early alcohol-induced liver inflammation and necrosis[101].
One of the current hypotheses about the pathophysiology of alcohol induced liver damage is that ethanol increases the proportion of Gram negative bacteria in the bowel flora and therefore the intraluminal production of LPS. Concurrently, the increase in the intestinal permeability due to alcohol-induced alterations of the epithelial barrier function results in portal vein endotoxinemia. This activates Kupffer cells leading to production of inflammatory mediators, which in turn activate the endothelium and induce neutrophil and mononuclear cell recruitment and infiltration resulting in liver damage. Furthermore, it has been suggested that ethanol may also have a direct effect on Kupffer cell activation by altering cell membrane calcium channels[102].
A synergistic effect of LPS with ethanol has been described. Recent evidence indicates that chronic ethanol administration decreases the cellular cAMP levels of Kupffer cells and this leads to enhanced NF-κB activation by LPS and TNF-α production[95]. Interestingly an increase in cAMP does not affect NF-κB activation but it decreases its transcription capability.
Kupffer cells and liver transplantation
There is indirect evidence indicating that Kupffer cells may play a role in the process of graft rejection following liver transplantation mainly though their ability to act as antigen presenting cells (APC). Kupffer cells express MHC class II and have been found to be effective APC in vitro[103]. Animal studies have shown that following liver transplantation Kupffer cells up-regulate MHC class II expression and this has been associated with the initiation of the rejection process[104]. In humans the rate of reconstitution of the graft with recipient-derived Kupffer cells has been found to increase during the rejection phase[104]. Finally, graft rejection and the vanishing-bile duct syndrome occur more frequently in cases of MHC class I incompatibility accompanied by a MHC class II partial or complete match, which suggests that presentation of MHC I antigens of the billiary epithelium by donor Kupffer cells may also take place[105].
Ischemia-reperfusion injury during the extracorporal preservation of the graft may often result to primary graft dysfunction[106]. There is accumulating evidence to suggest a major role for Kupffer cells during this process through the activation and production of oxygen free radicals resulting in alteration of the microcirculation of the graft[107]. Kupffer cell inactivation using GdCl3 has been found to prevent ischemia-reperfusion injury, whereas administration of latex particles that induce Kupffer cell activation through phagocytosis, accelerates ischemia-reperfusion injury of the graft[108]. Kupffer cell derived TNF-α, MIP-2 and keratinocyte chemoattractant chemokine have also been found to play a role in the microcirculatory failure that accompanies ischemia-reperfusion. Increased expression of TNFα, MIP-2 and keratinocyte chemoattractant both systemically and in the liver parenchyma have been observed in animal models during the reperfusion phase injury, and they have been associated with endothelial activation and β2-integrin up-regulation[109] and infiltration of the graft by neutrophils[110] respectively.
Kupffer cells and portal hypertension
Kupffer cells have been shown to be the main source of thromboxane A2 production in the liver and this production is mediated by COX-1 and COX-2[111]. Recently it was demonstrated that the infusion of endothelin-1 significantly increased portal pressure in animal models. This increase was mediated by the production of thromboxane A2 by the Kupffer cells[112], since both thromboxane synthase inhibition and thromboxane A2 receptor antagonists blocked the effect of endothelin-1 on portal pressure[113]. Whether this is relevant to the situation in humans remains to be established.
Kupffer cells and non alcoholic steatohepatitis
Recently a connection between Kupffer cells and the progression of non alcoholic steatosis to steatohepatitis and fibrosis was reported[114]. Interestingly, this report is one of the few that are based on human data. The enzyme chitotriosidase (CHIT), a member of the chitinase family, was found exclusively expressed in Kupffer cells in liver biopsies from patients with NASH. The levels of this enzyme were significantly higher in NASH than in simple steatosis and CHIT overexpression influenced hepatic stellate cell activation. A significant correlation was also observed between CHIT, TNF-α and lipid peroxidation in both NASH and simple steatosis. Since CHIT is increased in the liver in other forms of lipid storage disease it is postulated that Kupffer cells are implicated in the pathogenesis of NASH. Another study using an animal model has shown an enhancement of the TNF-α/TNFR mediated signalling pathway via activation of Kupffer cells in an autocrine or paracrine manner which might be critically involved in the pathogenesis of liver fibrosis in this NASH[115].
Kupffer cells and intrahepatic cholestasis
Recently Kupffer cells have been implicated in the pathogenesis of intrahepatic cholestasis following hepatic ischaemia-reperfusion injury. Many hepatic canalicular transporters were reduced in parallel to the production of cytokines by Kupffer cells in an experimental model. Moreover, depletion of Kupffer cells abolished the reduced expression of transporters[116]. However, the role of Kupffer cells in cholestasis remains controversial. Recently, in bile duct ligated rats, selective anti-inflammatory blockade of Kupffer cells increased fibrosis and deposition of collagen I and III[117]. More recently, in a bile duct ligated mouse model, depletion of Kupffer cells by intravenous inoculation of dichloromethylene diphosphonate resulted in high serum alanine transaminase levels and serious histologic portal inflammation and hepatocellular necrosis, indicating that Kupffer cells abrogate cholestatic liver injury in mice[118]. Moreover it seems that the abrogation of liver injury in this model might be cytokine dependent, mostly through the production of IL-6 by Kupffer cells[118].
A PROPOSED MODEL FOR THE INVOLVEMENT OF KUPFFER CELLS IN THE PATHOGENESIS OF LIVER DISEASE
Based mostly on the presented data from experimental animals, we propose a model to demonstrate the role of Kupffer cells in the pathogenesis of various liver diseases. According to this model Kupffer cells are responsible for six major functions that are vital for the development of liver disease. Kupffer cells are the main effector cells, killing hepatocytes in various forms of hepatitis. This is achieved by the production of proinflammatory cytokines, reactive oxygen species, nitric oxide, phospholipase and lysosomal enzymes. Kupffer cells may harm hepatocytes by initiating their apoptosis through the CD95L-CD95 pathway (1). This effect is possibly accentuated by CD8 positive antigen restricted T cells and is stopped by CD4+CD25+ regulatory T cells. In this respect, Kupffer cells are acting as antigen presenting cells of either extrahepatic viruses like influenza[10,73] or intrahepatic viruses like HBV and HCV (2). Following antigen presentation Kupffer cells attract both CD8+ T cells and regulatory T cells by producing chemokines (3). T cells expressing LFA-1 are trapped as a result of endothelial cell overexpression of adhesion molecules like ICAM-1 and VCAM (4), while CD8 positive cells might be driven to apoptosis by direct contact with Kupffer cells. Moreover, TGF-β1 production by Kupffer cells drives stellate cells to be transformed into myofibroblasts eventually leading to fibrosis (5). Finally, by producing glutathione, IL-6 and MIP-2 Kupffer cells may protect hepatocytes from further damage (6). One vital question remains. Are all these six different functions mediated through the same Kupffer cells or are there different Kupffer cell subpopulations in the liver A schematic presentation of this model is presented in Figure 1.
Figure 1.
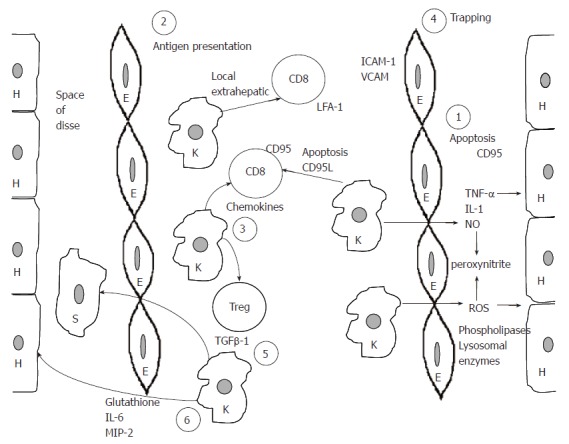
Schematic representation of the proposed model for the role of Kupffer cells in the pathogenesis of liver disease. H: hepatocytes; E: endothelial cells; K: Kupffer cells; S: stellate cells. For numbers (1-6), please see text explanation.
Footnotes
S- Editor Wang GP L- Editor Lakatos PL E- Editor Bi L
References
- 1.Smedsrød B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, Geerts A. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509–1516. doi: 10.1136/gut.35.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- 3.MacPhee PJ, Schmidt EE, Groom AC. Evidence for Kupffer cell migration along liver sinusoids, from high-resolution in vivo microscopy. Am J Physiol. 1992;263:G17–G23. doi: 10.1152/ajpgi.1992.263.1.G17. [DOI] [PubMed] [Google Scholar]
- 4.Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- 5.Bayón LG, Izquierdo MA, Sirovich I, van Rooijen N, Beelen RH, Meijer S. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology. 1996;23:1224–1231. doi: 10.1002/hep.510230542. [DOI] [PubMed] [Google Scholar]
- 6.Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–1536. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- 7.Winwood PJ, Arthur MJ. Kupffer cells: their activation and role in animal models of liver injury and human liver disease. Semin Liver Dis. 1993;13:50–59. doi: 10.1055/s-2007-1007337. [DOI] [PubMed] [Google Scholar]
- 8.Gregory SH, Wing EJ. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J Leukoc Biol. 2002;72:239–248. [PubMed] [Google Scholar]
- 9.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 10.Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226–240. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 12.Arthur MJ, Bentley IS, Tanner AR, Saunders PK, Millward-Sadler GH, Wright R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985;89:1114–1122. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- 13.Shiratori Y, Takikawa H, Kawase T, Sugimoto T. Superoxide anion generating capacity and lysosomal enzyme activities of Kupffer cells in galactosamine induced hepatitis. Gastroenterol Jpn. 1986;21:135–144. doi: 10.1007/BF02774831. [DOI] [PubMed] [Google Scholar]
- 14.Laskin DL. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990;10:293–304. doi: 10.1055/s-2008-1040485. [DOI] [PubMed] [Google Scholar]
- 15.Valatas V, Kolios G, Manousou P, Notas G, Xidakis C, Diamantis I, Kouroumalis E. Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells. Br J Pharmacol. 2004;141:477–487. doi: 10.1038/sj.bjp.0705633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valatas V, Kolios G, Manousou P, Xidakis C, Notas G, Ljumovic D, Kouroumalis EA. Secretion of inflammatory mediators by isolated rat Kupffer cells: the effect of octreotide. Regul Pept. 2004;120:215–225. doi: 10.1016/j.regpep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Arthur MJ, Kowalski-Saunders P, Wright R. Effect of endotoxin on release of reactive oxygen intermediates by rat hepatic macrophages. Gastroenterology. 1988;95:1588–1594. doi: 10.1016/s0016-5085(88)80082-9. [DOI] [PubMed] [Google Scholar]
- 18.Starke PE, Farber JL. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985;260:86–92. [PubMed] [Google Scholar]
- 19.Billiar TR, Curran RD, West MA, Hofmann K, Simmons RL. Kupffer cell cytotoxicity to hepatocytes in coculture requires L-arginine. Arch Surg. 1989;124:1416–1420; discussion 1420-1421. doi: 10.1001/archsurg.1989.01410120062013. [DOI] [PubMed] [Google Scholar]
- 20.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto H, Tamura S, Kamada Y, Kiso S, Fukushima J, Wada A, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, et al. Adiponectin deficiency exacerbates lipopolysaccharide/D-galactosamine-induced liver injury in mice. World J Gastroenterol. 2006;12:3352–3358. doi: 10.3748/wjg.v12.i21.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park PH, Thakur V, Pritchard MT, McMullen MR, Nagy LE. Regulation of Kupffer cell activity during chronic ethanol exposure: role of adiponectin. J Gastroenterol Hepatol. 2006;21 Suppl 3:S30–S33. doi: 10.1111/j.1440-1746.2006.04580.x. [DOI] [PubMed] [Google Scholar]
- 23.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 24.Marra F, DeFranco R, Grappone C, Parola M, Milani S, Leonarduzzi G, Pastacaldi S, Wenzel UO, Pinzani M, Dianzani MU, et al. Expression of monocyte chemotactic protein-1 precedes monocyte recruitment in a rat model of acute liver injury, and is modulated by vitamin E. J Investig Med. 1999;47:66–75. [PubMed] [Google Scholar]
- 25.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, Simpson KJ, Lukacs NW, Kunkel SL. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–1252. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanner A, Keyhani A, Reiner R, Holdstock G, Wright R. Proteolytic enzymes released by liver macrophages may promote hepatic injury in a rat model of hepatic damage. Gastroenterology. 1981;80:647–654. [PubMed] [Google Scholar]
- 27.Mochida S, Ogata I, Hirata K, Ohta Y, Yamada S, Fujiwara K. Provocation of massive hepatic necrosis by endotoxin after partial hepatectomy in rats. Gastroenterology. 1990;99:771–777. doi: 10.1016/0016-5085(90)90967-6. [DOI] [PubMed] [Google Scholar]
- 28.Müschen M, Warskulat U, Peters-Regehr T, Bode JG, Kubitz R, Häussinger D. Involvement of CD95 (Apo-1/Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterology. 1999;116:666–677. doi: 10.1016/s0016-5085(99)70189-7. [DOI] [PubMed] [Google Scholar]
- 29.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock. 2002;17:280–285. doi: 10.1097/00024382-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Harbrecht BG, Billiar TR. The role of nitric oxide in Kupffer cell-hepatocyte interactions. Shock. 1995;3:79–87. [PubMed] [Google Scholar]
- 32.Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8–20. doi: 10.1034/j.1600-065x.2000.17703.x. [DOI] [PubMed] [Google Scholar]
- 33.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, Kunkel SL. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 1999;13:1565–1574. doi: 10.1096/fasebj.13.12.1565. [DOI] [PubMed] [Google Scholar]
- 34.Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999;6:573–584. doi: 10.1038/sj.gt.3300858. [DOI] [PubMed] [Google Scholar]
- 35.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 36.Rai RM, Zhang JX, Clemens MG, Diehl AM. Gadolinium chloride alters the acinar distribution of phagocytosis and balance between pro- and anti-inflammatory cytokines. Shock. 1996;6:243–247. doi: 10.1097/00024382-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- 38.Rüttinger D, Vollmar B, Wanner GA, Messmer K. In vivo assessment of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J Hepatol. 1996;25:960–967. doi: 10.1016/s0168-8278(96)80302-3. [DOI] [PubMed] [Google Scholar]
- 39.McClain CJ, Price S, Barve S, Devalarja R, Shedlofsky S. Acetaminophen hepatotoxicity: An update. Curr Gastroenterol Rep. 1999;1:42–49. doi: 10.1007/s11894-999-0086-3. [DOI] [PubMed] [Google Scholar]
- 40.Batey RG, Wang J. Molecular pathogenesis of T lymphocyte-induced liver injury in alcoholic hepatitis. Front Biosci. 2002;7:d1662–d1675. doi: 10.2741/A870. [DOI] [PubMed] [Google Scholar]
- 41.Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–193. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- 42.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–S161. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 43.Comporti M, Arezzini B, Signorini C, Sgherri C, Monaco B, Gardi C. F2-isoprostanes stimulate collagen synthesis in activated hepatic stellate cells: a link with liver fibrosis. Lab Invest. 2005;85:1381–1391. doi: 10.1038/labinvest.3700332. [DOI] [PubMed] [Google Scholar]
- 44.Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397–403. doi: 10.3748/wjg.v5.i5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arthur MJ. Role of Ito cells in the degradation of matrix in liver. J Gastroenterol Hepatol. 1995;10 Suppl 1:S57–S62. doi: 10.1111/j.1440-1746.1995.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 46.Gäbele E, Brenner DA, Rippe RA. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Front Biosci. 2003;8:d69–d77. doi: 10.2741/887. [DOI] [PubMed] [Google Scholar]
- 47.Friedman SL. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990;10:20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- 48.Arthur MJ. Fibrosis and altered matrix degradation. Digestion. 1998;59:376–380. doi: 10.1159/000007492. [DOI] [PubMed] [Google Scholar]
- 49.Xidakis C, Ljumovic D, Manousou P, Notas G, Valatas V, Kolios G, Kouroumalis E. Production of pro- and anti-fibrotic agents by rat Kupffer cells; the effect of octreotide. Dig Dis Sci. 2005;50:935–941. doi: 10.1007/s10620-005-2668-8. [DOI] [PubMed] [Google Scholar]
- 50.Meyer DH, Bachem MG, Gressner AM. Modulation of hepatic lipocyte proteoglycan synthesis and proliferation by Kupffer cell-derived transforming growth factors type beta 1 and type alpha. Biochem Biophys Res Commun. 1990;171:1122–1129. doi: 10.1016/0006-291x(90)90801-s. [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka M, Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 52.Czaja MJ, Weiner FR, Flanders KC, Giambrone MA, Wind R, Biempica L, Zern MA. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castilla A, Prieto J, Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med. 1991;324:933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 54.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka M, Pham NT, Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989;9:71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 57.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 58.Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–10762. [PubMed] [Google Scholar]
- 59.Benyon RC, Hovell CJ, Da Gaça M, Jones EH, Iredale JP, Arthur MJ. Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology. 1999;30:977–986. doi: 10.1002/hep.510300431. [DOI] [PubMed] [Google Scholar]
- 60.Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463–471. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomioka M, Iinuma H, Okinaga K. Impaired Kupffer cell function and effect of immunotherapy in obstructive jaundice. J Surg Res. 2000;92:276–282. doi: 10.1006/jsre.2000.5868. [DOI] [PubMed] [Google Scholar]
- 62.Ehlers S, Mielke ME, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 63.Ofek I, Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988;56:539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barsig J, Flesch IE, Kaufmann SH. Macrophages and hepatocytic cells as chemokine producers in murine listeriosis. Immunobiology. 1998;199:87–104. doi: 10.1016/S0171-2985(98)80066-1. [DOI] [PubMed] [Google Scholar]
- 66.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 67.Cousens LP, Wing EJ. Innate defenses in the liver during Listeria infection. Immunol Rev. 2000;174:150–159. doi: 10.1034/j.1600-0528.2002.017407.x. [DOI] [PubMed] [Google Scholar]
- 68.Henson D, Smith RD, Gehrke J. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am J Pathol. 1966;49:871–888. [PMC free article] [PubMed] [Google Scholar]
- 69.Meis JF, Verhave JP, Jap PH, Meuwissen JH. An ultrastructural study on the role of Kupffer cells in the process of infection by Plasmodium berghei sporozoites in rats. Parasitology. 1983;86(Pt 2):231–242. doi: 10.1017/s003118200005040x. [DOI] [PubMed] [Google Scholar]
- 70.Lepay DA, Nathan CF, Steinman RM, Murray HW, Cohn ZA. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985;161:1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Triger DR, Wright R. Hyperglobulinaemia in liver disease. Lancet. 1973;1:1494–1496. doi: 10.1016/s0140-6736(73)91827-8. [DOI] [PubMed] [Google Scholar]
- 72.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 73.Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169–1178; quiz 1404-1405. doi: 10.2353/ajpath.2006.050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamann A, Klugewitz K, Austrup F, Jablonski-Westrich D. Activation induces rapid and profound alterations in the trafficking of T cells. Eur J Immunol. 2000;30:3207–3218. doi: 10.1002/1521-4141(200011)30:11<3207::AID-IMMU3207>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 75.Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 76.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- 78.Roh MS, Wang L, Oyedeji C, LeRoux ME, Curley SA, Pollock RE, Klostergaard J. Human Kupffer cells are cytotoxic against human colon adenocarcinoma. Surgery. 1990;108:400–404; discussion 404-405. doi: 10.1002/bjs.1800770937. [DOI] [PubMed] [Google Scholar]
- 79.Heuff G, van de Loosdrecht AA, Betjes MG, Beelen RH, Meijer S. Isolation and purification of large quantities of fresh human Kupffer cells, which are cytotoxic against colon carcinoma. Hepatology. 1995;21:740–745. [PubMed] [Google Scholar]
- 80.Curley SA, Roh MS, Feig B, Oyedeji C, Kleinerman ES, Klostergaard J. Mechanisms of Kupffer cell cytotoxicity in vitro against the syngeneic murine colon adenocarcinoma line MCA26. J Leukoc Biol. 1993;53:715–721. doi: 10.1002/jlb.53.6.715. [DOI] [PubMed] [Google Scholar]
- 81.Heuff G, Oldenburg HS, Boutkan H, Visser JJ, Beelen RH, Van Rooijen N, Dijkstra CD, Meyer S. Enhanced tumour growth in the rat liver after selective elimination of Kupffer cells. Cancer Immunol Immunother. 1993;37:125–130. doi: 10.1007/BF01517045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song E, Chen J, Ouyang N, Wang M, Exton MS, Heemann U. Kupffer cells of cirrhotic rat livers sensitize colon cancer cells to Fas-mediated apoptosis. Br J Cancer. 2001;84:1265–1271. doi: 10.1054/bjoc.2000.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lau WY, Chen GG, Lai PB, Chun YS, Leung BC, Chak EC, Lee JF, Chui AK. Induction of Fas and Fas ligand expression on malignant glioma cells by Kupffer cells, a potential pathway of antiliver metastases. J Surg Res. 2001;101:44–51. doi: 10.1006/jsre.2001.6253. [DOI] [PubMed] [Google Scholar]
- 84.Rushfeldt C, Sveinbjørnsson B, Seljelid R, Smedsrød B. Early events of hepatic metastasis formation in mice: role of Kupffer and NK-cells in natural and interferon-gamma-stimulated defense. J Surg Res. 1999;82:209–215. doi: 10.1006/jsre.1998.5532. [DOI] [PubMed] [Google Scholar]
- 85.Pearson HJ, Anderson J, Chamberlain J, Bell PR. The effect of Kupffer cell stimulation or depression on the development of liver metastases in the rat. Cancer Immunol Immunother. 1986;23:214–216. doi: 10.1007/BF00205652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kan Z, Ivancev K, Lunderquist A, McCuskey PA, McCuskey RS, Wallace S. In vivo microscopy of hepatic metastases: dynamic observation of tumor cell invasion and interaction with Kupffer cells. Hepatology. 1995;21:487–494. [PubMed] [Google Scholar]
- 87.Gaillard T, Mülsch A, Busse R, Klein H, Decker K. Regulation of nitric oxide production by stimulated rat Kupffer cells. Pathobiology. 1991;59:280–283. doi: 10.1159/000163663. [DOI] [PubMed] [Google Scholar]
- 88.Aono K, Isobe K, Nakashima I, Kondo S, Miyachi M, Nimura Y. Kupffer cells cytotoxicity against hepatoma cells is related to nitric oxide. Biochem Biophys Res Commun. 1994;201:1175–1181. doi: 10.1006/bbrc.1994.1829. [DOI] [PubMed] [Google Scholar]
- 89.Kouroumalis E, Skordilis P, Thermos K, Vasilaki A, Moschandrea J, Manousos ON. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442–447. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samonakis DN, Moschandreas J, Arnaoutis T, Skordilis P, Leontidis C, Vafiades I, Kouroumalis E. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol Rep. 2002;9:903–907. [PubMed] [Google Scholar]
- 91.Xidakis C, Kolios G, Valatas V, Notas G, Mouzas I, Kouroumalis E. Effect of octreotide on apoptosis-related proteins in rat Kupffer cells: a possible anti-tumour mechanism. Anticancer Res. 2004;24:833–841. [PubMed] [Google Scholar]
- 92.Pennington HL, Wilce PA, Worrall S. Chemokine and cell adhesion molecule mRNA expression and neutrophil infiltration in lipopolysaccharide-induced hepatitis in ethanol-fed rats. Alcohol Clin Exp Res. 1998;22:1713–1718. [PubMed] [Google Scholar]
- 93.Huang YS, Chan CY, Wu JC, Pai CH, Chao Y, Lee SD. Serum levels of interleukin-8 in alcoholic liver disease: relationship with disease stage, biochemical parameters and survival. J Hepatol. 1996;24:377–384. doi: 10.1016/s0168-8278(96)80156-5. [DOI] [PubMed] [Google Scholar]
- 94.Fisher NC, Neil DA, Williams A, Adams DH. Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut. 1999;45:416–420. doi: 10.1136/gut.45.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G681–G688. doi: 10.1152/ajpgi.00098.2006. [DOI] [PubMed] [Google Scholar]
- 96.Karakucuk I, Dilly SA, Maxwell JD. Portal tract macrophages are increased in alcoholic liver disease. Histopathology. 1989;14:245–253. doi: 10.1111/j.1365-2559.1989.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 97.Eguchi H, McCuskey PA, McCuskey RS. Kupffer cell activity and hepatic microvascular events after acute ethanol ingestion in mice. Hepatology. 1991;13:751–757. [PubMed] [Google Scholar]
- 98.Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, Thurman RG. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 99.Earnest DL, Abril ER, Jolley CS, Martinez F. Ethanol and diet-induced alterations in Kupffer cell function. Alcohol Alcohol. 1993;28:73–83. [PubMed] [Google Scholar]
- 100.Yamada S, Mochida S, Ohno A, Hirata K, Ogata I, Ohta Y, Fujiwara K. Evidence for enhanced secretory function of hepatic macrophages after long-term ethanol feeding in rats. Liver. 1991;11:220–224. doi: 10.1111/j.1600-0676.1991.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 101.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 102.Iimuro Y, Ikejima K, Rose ML, Bradford BU, Thurman RG. Nimodipine, a dihydropyridine-type calcium channel blocker, prevents alcoholic hepatitis caused by chronic intragastric ethanol exposure in the rat. Hepatology. 1996;24:391–397. doi: 10.1002/hep.510240217. [DOI] [PubMed] [Google Scholar]
- 103.Rogoff TM, Lipsky PE. Role of the Kupffer cells in local and systemic immune responses. Gastroenterology. 1981;80:854–860. [PubMed] [Google Scholar]
- 104.Gouw AS, Houthoff HJ, Huitema S, Beelen JM, Gips CH, Poppema S. Expression of major histocompatibility complex antigens and replacement of donor cells by recipient ones in human liver grafts. Transplantation. 1987;43:291–296. doi: 10.1097/00007890-198702000-00025. [DOI] [PubMed] [Google Scholar]
- 105.Donaldson PT, Alexander GJ, O'Grady J, Neuberger J, Portmann B, Thick M, Davis H, Calne RY, Williams R. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987;1:945–951. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 106.Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 107.Brass CA, Roberts TG. Hepatic free radical production after cold storage: Kupffer cell-dependent and -independent mechanisms in rats. Gastroenterology. 1995;108:1167–1175. doi: 10.1016/0016-5085(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 108.Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39:1265–1272. doi: 10.1007/BF02093792. [DOI] [PubMed] [Google Scholar]
- 109.Schauer RJ, Bilzer M, Kalmuk S, Gerbes AL, Leiderer R, Schildberg FW, Messmer K. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72:1692–1699. doi: 10.1097/00007890-200111270-00022. [DOI] [PubMed] [Google Scholar]
- 110.Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res. 2001;99:201–210. doi: 10.1006/jsre.2001.6217. [DOI] [PubMed] [Google Scholar]
- 111.Bezugla Y, Kolada A, Kamionka S, Bernard B, Scheibe R, Dieter P. COX-1 and COX-2 contribute differentially to the LPS-induced release of PGE2 and TxA2 in liver macrophages. Prostaglandins Other Lipid Mediat. 2006;79:93–100. doi: 10.1016/j.prostaglandins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Yokoyama Y, Xu H, Kresge N, Keller S, Sarmadi AH, Baveja R, Clemens MG, Zhang JX. Role of thromboxane A2 in early BDL-induced portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2003;284:G453–G460. doi: 10.1152/ajpgi.00315.2002. [DOI] [PubMed] [Google Scholar]
- 113.Xu H, Korneszczuk K, Karaa A, Lin T, Clemens MG, Zhang JX. Thromboxane A2 from Kupffer cells contributes to the hyperresponsiveness of hepatic portal circulation to endothelin-1 in endotoxemic rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G277–G283. doi: 10.1152/ajpgi.00256.2004. [DOI] [PubMed] [Google Scholar]
- 114.Malaguarnera L, Di Rosa M, Zambito AM, dell'Ombra N, Di Marco R, Malaguarnera M. Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am J Gastroenterol. 2006;101:2060–2069. doi: 10.1111/j.1572-0241.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 115.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanaka Y, Chen C, Maher JM, Klaassen CD. Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischemia-reperfusion. Transplantation. 2006;82:258–266. doi: 10.1097/01.tp.0000226243.69023.54. [DOI] [PubMed] [Google Scholar]
- 117.Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719–728. doi: 10.1053/jhep.2001.27805. [DOI] [PubMed] [Google Scholar]
- 118.Gehring S, Dickson EM, San Martin ME, van Rooijen N, Papa EF, Harty MW, Tracy TF Jr, Gregory SH. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]


