Abstract
AIM: To determine the inhibitory effect of the vector-generated small interfering RNAs (siRNAs) on the expression of the Bcl-XL gene in established human esophageal cancer cells, and to investigate the effect of the Bcl-XL siRNAs on cell growth and apoptosis in esophageal cancer cells.
METHODS: Three siRNA-expressing vectors targeting different sites of the Bcl-XL gene were constructed from pTZ-U6+1 vector. Cultured esophageal cancer cells were transfected with the siRNA-expressing vector (or the control vector) using lipofectamine 2000. Bcl-XL gene expression was determined with semiquantitative RT-PCR assay and Western blotting. Among the three siRNA-expressing vectors, the most highly functional vector and its effect on cell growth and apoptosis in esophageal cancer cells was further analyzed.
RESULTS: Of the three siRNA-expressing vectors, siRNA-expressing vector No.1 was the most potent one which suppressed Bcl-XL mRNA production to 32.5% of that in the untreated esophageal cancer cells. Western blotting analysis showed that siRNA-expressing vector No.1 markedly down-regulated the expression of Bcl-XL in human esophageal cancer cells. Treatment of esophageal cancer cells with siRNA-expressing vector No.1 resulted in inhibition of cell growth and induction of apoptosis.
CONCLUSION: Down-regulation of Bcl-XL by vector-generated small interfering RNAs can suppress cell growth and induce apoptosis in human esophageal cancer cells.
Keywords: Esophageal cancer, Bcl-XL, RNA Interference, Apoptosis
INTRODUCTION
Esophageal cancer is one of the most common malignant tumors of mankind. About 300 000 people died of esophageal cancer each year in the world. The incidence and mortality of esophageal cancer are unusually high in China, especially in the areas of Henan, Shanxi, Hebei and Sichuan provinces[1]. Even though a small number of esophageal cancer patients survive longer than 5 years after initial surgical treatment, over 60% of patients still die of metastasis and local recurrence[2]. It is therefore imperative to investigate new therapeutic strategies in the treatment of esophageal cancer. Due to genetic abnormalities observed in esophageal cancer cells, the application of gene therapy has attracted the attention of many researchers. Activation of the cellular apoptotic program is a current strategy for the treatment of human cancer. It has been demonstrated that radiation and standard chemotherapeutic drugs kill some tumor cells through induction of apoptosis[3,4]. Upon apoptosis stimulation, several key events occur in mitochondria, including the release of cytochrome c. The mitochondrial cytochrome c release can be inhibited by expression of an antiapoptotic Bcl-2 family member (such as Bcl-2 or Bcl-XL) and induced by expression of a proapoptotic member of the Bcl-2 family (such as Bax or Bid)[5]. In fact, an increased expression of Bcl-XL has been found in a variety of cancers[6-9]. In many neoplastic cells, high expression of Bcl-XL also correlates with resistance to conventional chemotherapy[10-13]. Bcl-XL is considered to be a highly promising molecular target to design new molecular targeted anticancer therapies.
Small interfering RNAs (siRNAs) are double-stranded RNA molecules that induce sequence-specific degradation of homologous single-stranded RNA. It has been verified as a powerful tool to knock down the expression of a target protein in mammalian cells. siRNA technology has several major advantages over other posttranscriptional gene silencing techniques, such as antisense and gene knockout technology. It is easier to deliver, requires only small doses of siRNA to produce its silencing effect, and can inactivate a gene at almost any stage in development[14]. siRNA can be synthesized in vitro, but a specific gene silencing induced by synthetic siRNA might not be maintained long enough to achieve a phenotypic change[15]. In order to solve this problem some investigators have developed several vector-based expression systems to produce endogenous, functional siRNA molecules in vivo[16,17].
In this study, we present data showing that RNAi technology can be used to down-regulate Bcl-XL expression, resulting in suppression of cell growth and induction of apoptosis in esophageal cancer cells Eca109. It can be concluded that Bcl-XL is an alternative target in developing new therapeutic strategies for the treatment of esophageal cancers.
MATERIALS AND METHODS
Preparation of siRNA-expressing vector
siRNA-expressing vector was constructed from pTZ-U6+1 vector (Provided by Rossi JJ, Division of Molecular Biology, Beckman Research Institute of the City of Hope, Duarte, California 91010, USA) according to the instructions of the manufacturer. Briefly, a pair of oligonucleotides were synthesized in vitro. Each oligonucleotide contained a 21 nucleotide target sequence followed by a short loop sequence, the reverse complement of the target sequence, and five thymidines as a RNA polymerase III transcriptional stop signal. The oligonucleotides were annealed in a buffer (potassium acetate 100 mmol/L, 30 mmol/L HEPES-KOH pH 7.4, and magnesium acetate 2 mmol/L) and the mixture was incubated at 95°C for 5 min and then at 37°C for 1 h. The double stranded oligos were cloned into the SalI and XbaI sites of the pTZ-U6+1 vector where short hairpin RNAs were expressed under the control of the U6 promoter. The target sequences, corresponding oligonucleotides and resulting siRNA-expressing vectors in this study are shown in Table 1.
Table 1.
Target sequences, corresponding oligonucleotides and resulting siRNA-expressing vectors in this study
| Target sequences and corresponding oligonucleotides | siRNA-expressing vector |
| Target 1: 5´-ggaagagaacaggactgaggc-3´ (Target site: 90-110) | siRNA- expressing vector No.1 |
| Oligo 1 F: 5´-tcgaggaagagaacaggactgaggcttcaagagagcctcagtcctgttctcttccttttt-3´ | |
| Oligo 1 R: 5´-ctagaaaaaggaagagaacaggactgaggctctcttgaagcctcagtcctgttctcttcc-3´ | |
| Target 2 :5´- gaacaggtagtgaatgaactc-3´(Target site: 370-390) | siRNA- expressing vector No.2 |
| Oligo 2 F: 5´-tcgagaacaggtagtgaatgaactcttcaagagagagttcattcactacctgttcttttt-3´ | |
| Oligo 2 R: 5´-ctagaaaaagaacaggtagtgaatgaactctctcttgaagagttcattcactacctgttc-3´ | |
| Target 3: 5´- gaacgcttcaaccgctggttc-3´(Target site: 622-642) | siRNA-expressing vector No.3 |
| Oligo3 F: 5´-tcgagaacgcttcaaccgctggttcttcaagagagaaccagcggttgaagcgttcttttt-3´ | |
| Oligo 3 R: 5´-ctagaaaaagaacgcttcaaccgctggttctctcttgaagaaccagcggttgaagcgttc-3´ | |
| Control: 5´-gaggaccgttactagatcata-3´ | Control siRNA- expressing vector |
| Oligo F: 5´-tcgagaggaccgttactagatcatattcaagagatatgatctagtaacggtcctcttttt-3´ | |
| Oligo R: 5´-ctagaaaaagaggaccgttactagatcatatctcttgaatatgatctagtaacggtcctc-3´ |
Cell culture and transfection
The human esophageal cancer cell line Eca-109 was obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Eca-109 cells were cultured in medium RPMI-1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS) and penicillin (100 kU/L) and streptomycin (100 mg/L) at 37°C in a humidified incubator with 5% CO2. For cell transfection, lipofectamine 2000 (Invitrogen) was used for transfecting the siRNA-expressing plasmids (or the control plasmids) following the manufacturer’s instructions. The transfected cells were cultured for 5 h and then transferred to fresh medium with 10% FBS.
Semiquantitative RT-PCR analysis for Bcl-XL gene expression
Cells were harvested 48 h after transfection. Total RNA was purified using the Total RNA Isolation System (Qiagen). Reverse transcription-PCR was performed with the isolated total RNA (100 ng) using the Omniscript RT kit and HotStarTaq PCR kit (Qiagen) according to the manufacturer’s instructions. The primers were bcl-xl: 5’-GGCCTGAAGCCGGTGCAC -3’, 5’-CACGGCGATACC GCTGGA -3’; β-actin: 5’-CTGGATGCGATTCCAAGCAC-3’, 5’-GAAGGACTTGGGATCGTCCGG-3’. When RT-PCR was finished, 1 μL from the reaction mixture was withdrawn and analyzed by agarose gel electrophoresis followed by ethidium bromide staining. The 500 bp bcl-xl bands were cut from the gel and extracted using a DNA gel extaction kit (Qiagen). DNA concentration was determined using the GeneQuant pro RNA/DNA Calculator (Biochrom Ltd). Bcl-XL gene expression was calculated by dividing the concentration of the RT-PCR product of the treated cells by the concentration of the RT-PCR product of the untreated cells (taken as 100%). Each point represents the average of triplicate tests.
Western blotting analysis
Seventy-two hours after the transfection, cells were washed twice in PBS and total protein was extracted in 150 mmol/L NaCl, 50 mmol/L Tris·HCl (pH 7.5), 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 5 mmol/L EDTA, 10 mg/mL leupeptin, 1% aprotinin and 2 mmol/L PMSF. Ten micrograms of protein sample was loaded onto a 10% SDS-PAGE and electroblotted onto a PVDF nylon membrane (Millipore, Bedford). Membranes were blocked in 0.05% Tween 20 (v/v) PBS containing 5% skim milk,and then incubated with rabbit polyclonal Bcl-XL antibodies and rabbit polyclonal β-Actin antibodies (Santa Cruz Biotechnology). Membranes were then incubated with a HRP-linked goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology). Finally, the membrane was reacted with DAB reagent and washed with PBS once protein bands had appeared.
Cell growth assay
Untreated cells or the transfected cells were harvested and reseeded at 1 × 104 cells/well in a 12-well, flat-bottomed plate. Cells were cultivated with RPMI 1640 medium in the CO2 incubator at 37°C. The total cell number was determined every two days with a hematocytometer and under an inverted microscope (Olympus). Cell viability was determined by trypan blue staining. Each value represents the average of triplicate wells.
Analysis of apoptosis
Cell apoptosis was assessed under an inverted fluorescence microscope (Olympus).The green fluorescent DNA intercalant dye YO-PRO-1 was purchased from Invitrogen Company. Dual staining with YO-PRO and PI made it possible to detect early apoptotic cells that have undergone initial changes in permeability to small molecules. At 72 h post cell transfection, YO-PRO-1 dyes were added to the culture medium at 0.1 μmol/L for 30 min. Cell apoptosis was quantified by determining the percentage of cells that were YO-PRO-1-positive in every 200 cells. Each value represents the average of triplicate wells.
Statistical analysis
Statistical analysis was performed by the Student’s t test or χ2 test. P < 0.05 was considered statistically significant. Data are expressed as mean ± SD. All statistical calculations were performed using SPSS10.0 statistical software package.
RESULTS
siRNA-expressing vector inhibiting Bcl-XL mRNA expression
We examined three siRNA-expressing vectors which target human Bcl-XL as shown in Figure 1A. Of the three siRNA-expressing vectors, siRNA-expressing vector No.1 potently suppressed the synthesis of Bcl-XL mRNA in human esophageal cancer cells. RT-PCR product quantification showed that siRNA expressing vector No.1 suppressed Bcl-XL mRNA production to 32.5% of that in the control as shown in Figure 1B. On the basis of these results, we selected siRNA-expressing vector No.1 as the most highly functional siRNA-expressing vector in further studies.
Figure 1.
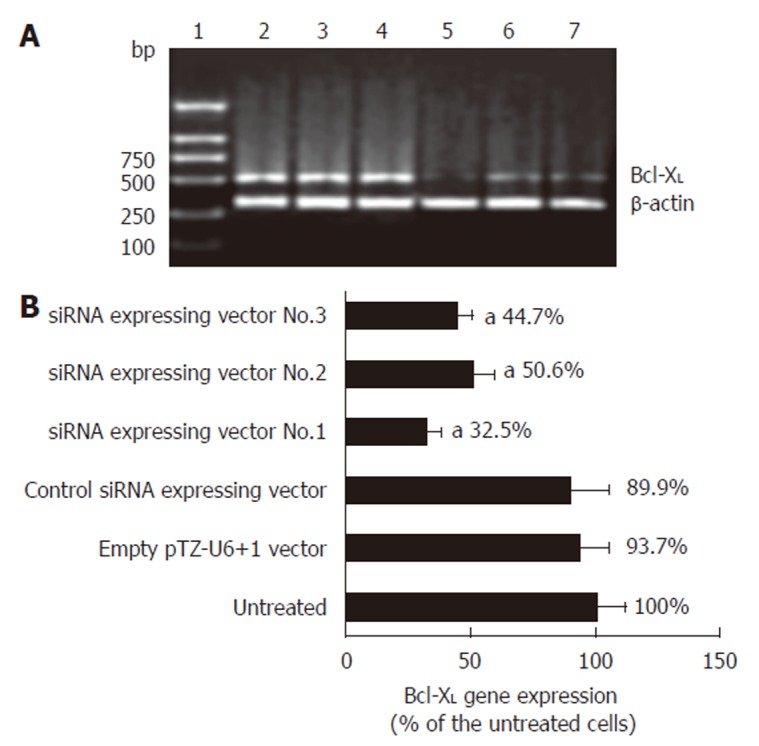
RT-PCR analysis of the effect of siRNA-expressing vector on Bcl-XL gene expression. A: Argarose gel electrophoresis of the RT-PCR products. L1: DNA marker; L2: RT-PCR product of the untreated cells; L3: RT-PCR product of the empty pTZ-U6+1 vector treated cells; L4: RT-PCR product of the control siRNA-expressing vector treated cells; L5: RT-PCR product of the siRNA-expressing vector No.1 treated cells; L6: RT-PCR product of the siRNA-expressing vector No.2 treated cells; L7: RT-PCR product of the siRNA-expressing vector No.3 treated cells. B: Quantification of the RT-PCR products (mean ± SD, n = 3, aP < 0.05 vs untreated esophageal cancer cells).
Effect of siRNA-expressing vector on Bcl-XL protein expression
We evaluated the effect of siRNA-expressing vector No.1 on target protein Bcl-XL by Western blotting analysis. Figure 2 shows that siRNA-expressing vector No.1 markedly down-regulated the expression of Bcl-XL in human esophageal cancer cells as compared with untreated cells. However, treatment with control siRNA-expressing vector and empty pTZ-U6+1 vector did not change the expression of Bcl-XL in human esophageal cancer cells as compared with untreated cells.
Figure 2.

Western blotting analysis and effect of siRNA-expressing vectors. L1: Untreated esophageal cancer cells; L2: Esophageal cancer cells were transfected with empty pTZ-U6+1 vector; L3: Esophageal cancer cells were transfected with control siRNA expressing vector; L4: Esophageal cancer cells were transfected with siRNA-expressing vector No.1.
Effect of siRNA-expressing vector on cell proliferation
We have analyzed siRNA-expressing vector No.1 on the cell proliferation of Eca-109 esophageal cancer cells. Figure 3 shows that siRNA-expressing vector No.1 target Bcl-XL significantly inhibited the cell growth in esophageal cancer cells as compared with the untreated cells (P < 0.05). Cells treated with control siRNA expressing vector and empty pTZ-U6+1 vector showed only slight cell growth inhibition and had no difference as compared with the untreated cells.
Figure 3.
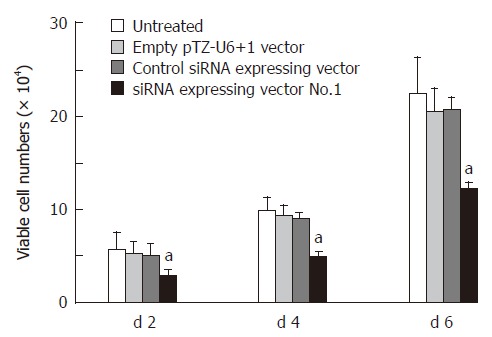
Effect of siRNA-expressing vector on cell growth post transfection (mean ± SD, n = 3, aP < 0.05 vs untreated esophageal cancer cells).
Bcl-XL siRNA-expressing vector potentiating apoptosis of esophageal cancer cells
YO-PRO-1 is a membrane-impermeable DNA-binding dye and is generally excluded from variable cells, whereas early-stage apoptotic cells are YO-PRO-1-positive. Typical photographs of YO-PRO-1 staining are shown in Figure 4A. The nucleus of early-stage apoptotic cell was stained green under the inverted fluorescence microscope. Figure 4B shows that the apoptotic cells significantly increased in siRNA-expressing vector No.1 treated cells compared to untreated cells (P < 0.05). However, treatment with control siRNA expressing vector and empty pTZ-U6+1 vector did not significantly affect the apoptosis of esophageal cancer cells.
Figure 4.
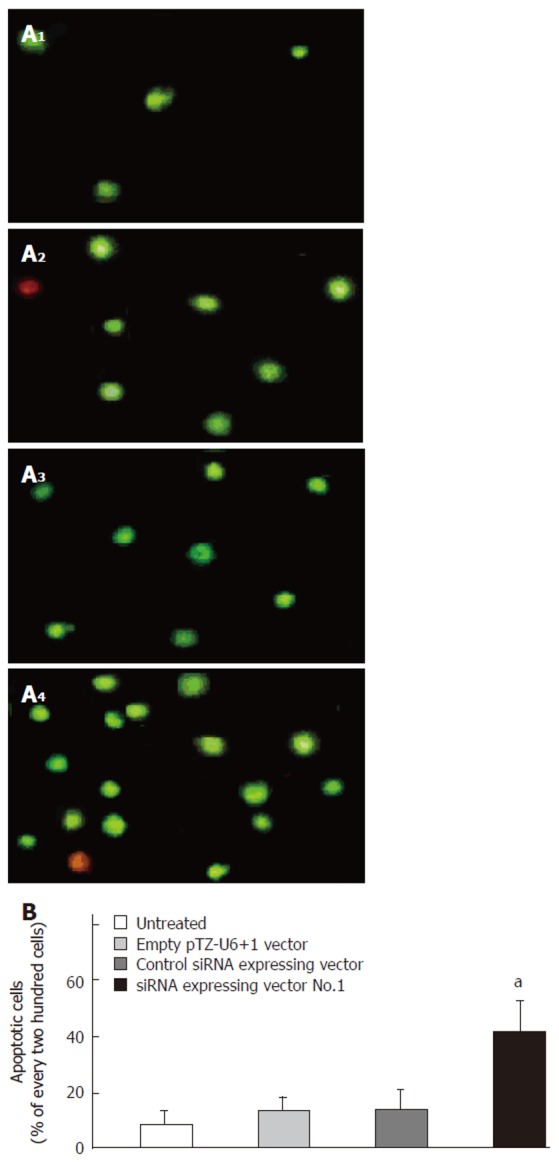
Effect of siRNA-expressing vector targeting Bcl-XL on apoptosis of esophageal cancer cells. A: Photographs of YO-PRO-1 staining under the inverted fluorescence microscope at 72 h after transfection. 1: Untreated esophageal cancer cells; 2: Esophageal cancer cells were transfected with empty pTZ-U6+1 vector; 3: Esophageal cancer cells were transfected with control siRNA-expressing vector; 4: Esophageal cancer cells were transfected with siRNA-expressing vector No.1. B: Percentage of apoptotic cells in every 200 cells (mean ± SD, n = 3, aP < 0.05 vs untreated cells).
DISCUSSION
Members of the Bcl-2 family of proteins play important roles in regulating cell survival and apoptosis. The Bcl-2 family includes pro-apoptotic members and antiapoptotic proteins such as Bcl-2 and Bcl-XL that inhibit apoptosis by blocking the release of cytochrome C. Bcl-XL is over-expressed in numerous types of cancer including myelomas, lymphomas, hepatomas, gastric carcinomas and ovarian cancers[6,7,9,18,19]. This over-expression of Bcl-XL is associated with decreased apoptosis in tumors, resistance to chemotherapeutic drugs and a poor clinical outcome. In esophageal cancer, some investigators observed that Bcl-XL expression correlated well with depth of tumor invasion, lymphatic invasion, and lymph node metastasis in superficial squamous cell carcinoma of the esophagus. Patients with high Bcl-XL expression showed significantly shorter survival than those with low Bcl-XL expression[20,21]. However, the precise role of Bcl-XL in the development of esophageal cancer remains to be elucidated. Thus, the performance to specifically reduce Bcl-XL level by genetic means in established esophageal cancer cell lines should be helpful for a better understanding of its role in maintaining the malignant phenotype. Several approaches have been developed to inhibit the function of Bcl-XL including antisense, peptide nucleic acid, small organic compounds[22-24]. Most of these antagonists of Bcl-XL were reported to elicit spontaneous apoptosis of cancer cells in vivo as well as in vitro and to enhance the sensitivity to chemotherapy in cancer cells. Bcl-XL has been successfully down-regulated by RNAi in some previous studies, but the down-regulation of Bcl-XL expression by siRNA-expressing vector or other approaches in esophageal squamous cell carcinoma has not been performed until now.
In this study, we constructed three siRNA expressing vectors targetting human Bcl-XL. Down-regulation of Bcl-XL gene expression was observed in the esophageal carcinoma cell line Eca109 transfected with these three siRNA-expressing vectors. Of the three siRNA-expressing vectors, siRNA-expressing vector No.1 potently suppressed the synthesis of Bcl-XL mRNA in semiquantitative RT-PCR assay. RT-PCR product quantification showed that siRNA-expressing vector No.1 suppressed Bcl-XL mRNA production to 32.5% of that in the untreated esophageal cancer cells. Western blotting analysis showed that siRNA-expressing vector No.1 decreased the synthesis of Bcl-XL protein in esophageal cancer cells. To further investigate the role of Bcl-XL in the pathogenesis of esophageal cancer, cell growth and apoptosis were analyzed to determine the functional consequence of the siRNA-expressing vectors mediated decrease of Bcl-XL in established esophageal cancer cells. Our data showed that knockdown of Bcl-XL by siRNA-expressing vector suppressed cell growth and potentiated apopotosis in established esophageal cancer cells in a stable manner. These results were consistent with previous reports, Zhu et al[25] found that knockdown of Bcl-XL protein expression by small interfering RNA inhibited the proliferation of 5-FU-resistant human colon cancer cells. Lei X et al[26] demonstrated that siRNA targeting Bcl-XL genes specifically suppressed Bcl-XL expression and increased spontaneous apoptosis in the human gastric cancer cell line MGC-803. All these investigations suggest that Bcl-XL may serve as a potential target in cancer therapy. In summary, our study indicates that down-regulation of Bcl-XL by siRNA-expressing vectors can suppress cell growth and induce apoptosis in human esophageal cancer cells and siRNA technique may provide a novel therapeutic approach in the treatment of human esophageal cancer.
ACKNOWLEDGMENTS
We thank Dr. John J. Rossi (Division of Molecular Biology, Beckman Research Institute of the City of Hope, Duarte, California 91010, United States) for providing the pTZ-U6+1 vector.
Footnotes
Supported by Science and Technology Fund of Sichuan Province, No. 2003A067
S- Editor Wang GP L- Editor Ma JY E- Editor Liu WF
References
- 1.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 2.Krasna MJ, Mao YS, Sonett JR, Tamura G, Jones R, Suntharalingam M, Meltzer SJ. P53 gene protein overexpression predicts results of trimodality therapy in esophageal cancer patients. Ann Thorac Surg. 1999;68:2021–2024; discussion 2024-2025. doi: 10.1016/s0003-4975(99)01146-7. [DOI] [PubMed] [Google Scholar]
- 3.Rokudai S, Fujita N, Kitahara O, Nakamura Y, Tsuruo T. Involvement of FKHR-dependent TRADD expression in chemotherapeutic drug-induced apoptosis. Mol Cell Biol. 2002;22:8695–8708. doi: 10.1128/MCB.22.24.8695-8708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cen X, Zhu P, Yu J, Shi Y, Ma M. Effective chemotherapy induce apoptosis in vivo in patients with leukemia. Chin Med J (Engl) 2003;116:74–77. [PubMed] [Google Scholar]
- 5.Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 6.Xerri L, Parc P, Brousset P, Schlaifer D, Hassoun J, Reed JC, Krajewski S, Birnbaum D. Predominant expression of the long isoform of Bcl-x (Bcl-xL) in human lymphomas. Br J Haematol. 1996;92:900–906. doi: 10.1046/j.1365-2141.1996.423958.x. [DOI] [PubMed] [Google Scholar]
- 7.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, et al. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- 8.Okaro AC, Deery AR, Hutchins RR, Davidson BR. The expression of antiapoptotic proteins Bcl-2, Bcl-X(L), and Mcl-1 in benign, dysplastic, and malignant biliary epithelium. J Clin Pathol. 2001;54:927–932. doi: 10.1136/jcp.54.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
- 10.Simonian PL, Grillot DA, Nuñez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- 11.Liu R, Page C, Beidler DR, Wicha MS, Núñez G. Overexpression of Bcl-x(L) promotes chemotherapy resistance of mammary tumors in a syngeneic mouse model. Am J Pathol. 1999;155:1861–1867. doi: 10.1016/S0002-9440(10)65505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebedeva I, Rando R, Ojwang J, Cossum P, Stein CA. Bcl-xL in prostate cancer cells: effects of overexpression and down-regulation on chemosensitivity. Cancer Res. 2000;60:6052–6060. [PubMed] [Google Scholar]
- 13.Li X, Marani M, Mannucci R, Kinsey B, Andriani F, Nicoletti I, Denner L, Marcelli M. Overexpression of BCL-X(L) underlies the molecular basis for resistance to staurosporine-induced apoptosis in PC-3 cells. Cancer Res. 2001;61:1699–1706. [PubMed] [Google Scholar]
- 14.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–838. [PubMed] [Google Scholar]
- 15.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 16.Czauderna F, Fechtner M, Aygün H, Arnold W, Klippel A, Giese K, Kaufmann J. Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA. Nucleic Acids Res. 2003;31:670–682. doi: 10.1093/nar/gkg141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K, Lin SY, Brunicardi FC, Seu P. Use of RNA interference to target cyclin E-overexpressing hepatocellular carcinoma. Cancer Res. 2003;63:3593–3597. [PubMed] [Google Scholar]
- 18.Kondo S, Shinomura Y, Kanayama S, Higashimoto Y, Miyagawa JI, Minami T, Kiyohara T, Zushi S, Kitamura S, Isozaki K, et al. Over-expression of bcl-xL gene in human gastric adenomas and carcinomas. Int J Cancer. 1996;68:727–730. doi: 10.1002/(SICI)1097-0215(19961211)68:6<727::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Marone M, Scambia G, Mozzetti S, Ferrandina G, Iacovella S, De Pasqua A, Benedetti-Panici P, Mancuso S. bcl-2, bax, bcl-XL, and bcl-XS expression in normal and neoplastic ovarian tissues. Clin Cancer Res. 1998;4:517–524. [PubMed] [Google Scholar]
- 20.Takayama T, Nagao M, Sawada H, Yamada Y, Emoto K, Fujimoto H, Ueno M, Hirao S, Nakajima Y. Bcl-X expression in esophageal squamous cell carcinoma: association with tumor progression and prognosis. J Surg Oncol. 2001;78:116–123. doi: 10.1002/jso.1130. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto M, Natsugoe S, Nakashima S, Okumura H, Sakita H, Baba M, Takao S, Aikou T. Clinical significance and prognostic value of apoptosis related proteins in superficial esophageal squamous cell carcinoma. Ann Surg Oncol. 2001;8:598–604. doi: 10.1007/s10434-001-0598-z. [DOI] [PubMed] [Google Scholar]
- 22.Vilenchik M, Raffo AJ, Benimetskaya L, Shames D, Stein CA. Antisense RNA down-regulation of bcl-xL Expression in prostate cancer cells leads to diminished rates of cellular proliferation and resistance to cytotoxic chemotherapeutic agents. Cancer Res. 2002;62:2175–2183. [PubMed] [Google Scholar]
- 23.Hayward RL, Macpherson JS, Cummings J, Monia BP, Smyth JF, Jodrell DI. Enhanced oxaliplatin-induced apoptosis following antisense Bcl-xl down-regulation is p53 and Bax dependent: Genetic evidence for specificity of the antisense effect. Mol Cancer Ther. 2004;3:169–178. [PubMed] [Google Scholar]
- 24.Kazi A, Smith DM, Zhong Q, Dou QP. Inhibition of bcl-x(l) phosphorylation by tea polyphenols or epigallocatechin-3-gallate is associated with prostate cancer cell apoptosis. Mol Pharmacol. 2002;62:765–771. doi: 10.1124/mol.62.4.765. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Guo W, Zhang L, Davis JJ, Teraishi F, Wu S, Cao X, Daniel J, Smythe WR, Fang B. Bcl-XL small interfering RNA suppresses the proliferation of 5-fluorouracil-resistant human colon cancer cells. Mol Cancer Ther. 2005;4:451–456. doi: 10.1158/1535-7163.MCT-04-0162. [DOI] [PubMed] [Google Scholar]
- 26.Lei XY, Zhong M, Feng LF, Yan CY, Zhu BY, Tang SS, Liao DF. Silencing of Bcl-XL expression in human MGC-803 gastric cancer cells by siRNA. Acta Biochim Biophys Sin (Shanghai) 2005;37:555–560. doi: 10.1111/j.1745-7270.2005.00077.x. [DOI] [PubMed] [Google Scholar]


