Abstract
AIM: To evaluate whether multiple determinations of intramucosal pH (pHi) in acute pancreatitis (AP) patients could provide additional information of the disease severity during early hospitalization.
METHODS: Twenty-one patients suffering from acute pancreatitis were monitored by gastric tonometry in the first 72 h after hospital admission.
RESULTS: In the survivor group (n = 15) the initially low pHi values returned to normal level (pHi ≥ 7.32) within 48 h (median pHi: d 1: 7.21; d 2: 7.32; d 3: 7.33). In contrast, pHi values in the non-survivor group n = 6) were persistently either below or in the low normal range (median pHi 7.12; 7.12; 7.07 respectively), but pHi differences between the two groups reached significance only after 24 h (P < 0.01). Mucosal acidosis detected at any time during the monitored period was associated with the emergence of single or multiple organ dysfunction (P < 0.01).
CONCLUSION: Prolonged gastric mucosal acidosis was associated with remote organ dysfunction and failure in Acute Pancreatitis, however, correlation with the fatal outcome became significant only 24 h after admission. Due to its non-invasive nature gastric tonometry may supplement the pro-inflammatory markers to achieve a multi-faceted monitoring of the disease.
Keywords: Acute pancreatitis, Human studies, Intramucosal pH, Mucosal barrier dysfunction, Septic complications, Bacterial translocation, Multiple organ dysfunction
INTRODUCTION
Necrotizing acute pancreatitis (AP) is currently recognized as a two-phase systemic disease, where the early phase (within the first two weeks) is characterized by sterile pancreatic necrosis and concomitant development of systemic inflammatory response syndrome (SIRS). If the patient survives the early MODS, the disease may progress to a second phase with the development of infected pancreatic necrosis and septic complications associated with multiple organ failure (MOF)[1].
Since an abundance of Gram negative bacteria[2] and other pathogens of gastrointestinal origin are commonly detected in pancreatic infections, the gut is considered to be the main source of pancreatitis related septic complications[3]. This is in accordance with experimental data suggesting that bacterial translocation through the damaged gut barrier is the most important mechanism for the contamination of pancreatic necrosis[4]. Recent evidence indicates that in case of host stress and low availability of intestinal luminal nutrition, non-pathogenic commensal intestinal bacteria can rapidly switch on virulence genes and mount a toxic offensive for their survival. Therefore, when-highly virulent traits of enteral bacteria emerge by virulence phenotype switching, gut-derived sepsis may be more likely to occur in severe AP[5].
Severe AP associated intestinal mucosal dysfunction may be the consequence of several deleterious local and systemic factors, such as disturbance of perfusion (ischemia-reperfusion phenomenon), oxidative stress during systemic inflammatory response syndrome, absence of mucosal feeding during parenteral nutrition, etc. This will eventually lead to metabolic failure in the gut, inducing intramucosal acidosis. Measurement of gastric intraluminal pCO2 by balloon tonometry and calculation of intramucosal pH (pHi) provides a quantitative indicator of the adequacy of intestinal milieu. Several studies have confirmed the value of pHi as a predictor of morbidity and mortality in the critically ill[6].
Gastric tonometry has been applied in human acute pancreatitis, however, the available clinical data are limited. The aim of this study was to analyze the relationship between intramucosal acidosis and AP associated complications (remote organ dysfunction and infection) with special attention to the outcome of the disease.
MATERIALS AND METHODS
Patients
The Ethics Committee of Semmelweis University Medical School has approved the study protocol, and written informed consent was obtained from each patient.
Gastric tonometry is generally considered to be useful in critically ill patients therefore the enrollment into this study was focused on patients hospitalized for suspected severe acute necrotizing pancreatitis . For control purposes patients with moderate and mild severity of pancreatitis were also included. The diagnosis of AP was based on laboratory findings and imaging studies (abdominal ultrasound and/or computer tomography) in association with the typical clinical picture. Exclusion criteria were as follows: pancreatitis associated with pancreatic cancer, history of recurrent AP within 3 mo, chronic, post-traumatic or post-operative pancreatitis, childhood, pregnancy, as well as administration of immune suppressive drugs (including steroids) in the previous one month. Twenty-one patients suffering from acute pancreatitis were enrolled; they were either admitted to the surgical intensive care unit (ICU) or to general surgical ward, all of them were monitored by gastric tonometry for the first three days. The end point of the study was the outcome of the disease. Based on this two groups were created in a retrospective fashion; patients with fatal outcome were enrolled in Group 1, whereas patients who survived were enrolled in Group 2.
All patients were treated according to the usual AP protocols adjusted to their current condition. Initial management was conservative. During the acute phase, the therapy consisted of adequate fluid replacement through a central venous catheter with hemodynamic monitoring, and assistance of respiratory or renal function if needed. A modified nasogastric tube was inserted to keep the stomach empty and measure intraluminal pCO2. Analgesics were given as required to all patients, and they received proton pump inhibitor (PPI) (2 × 40 mg omeprazole intravenously) to prevent stress ulcers. Prophylactic antibiotics (ofloxacin/metronidazole or imipenem) were administered as soon as the presence of necrosis was evident, or CRP value was over 150 mg/L. Surgical intervention was performed when infected necrosis was diagnosed (2 cases), or if the patient’s condition deteriorated despite intensive medical therapy (1 case). In one case laparoscopic cholecystectomy was performed after the patient’s recovery from AP.
All patients were monitored by gastric tonometry at least twice daily during the first three days of hospitalization. Attending clinicians did not use the pHi values to guide the patients’ management.
Gastric Tonometry
Gastric tonometry was performed using a semi-automated method[7] according to the modification of the original technique described by Fiddian-Green et al[8]. A tonometry tube (TonometricsTM 16F Catheter, Datex-Ohmeda Division, Instrumentarium Corp., Helsinki, Finland) was inserted into the lumen of the stomach via the nasogastric route. This catheter is a nasogastric tube with an additional smaller diameter conduit equipped with a CO2 permeable silicone balloon attached to the tip. The balloon was inflated with room air and its line was connected to a bedside CO2 monitor (TonocapTM Monitor, Datex-Ohmeda). Gastric intraluminal pCO2 pressure was measured after an equilibration period, and actual pHi was automatically calculated using additional data (arterial pH, arterial pCO2) obtained from the patient’s
arterial blood samples. Since it is generally presumed that systemic metabolic acidosis confounds the interpretation of gastric pHi, all the pHi values were excluded from analysis where systemic metabolic acidosis was present. We have applied the simplified gastric pHi measurement protocol proposed by Bonham et al, where the daily lowest pHi value was used to characterize the mucosal condition[9]. Routine PPI administration has been employed to improve the accuracy of mucosal pCO2 measurements[9], since gastric acid secretion blockade prevents the spurious elevation of luminal pCO2 (resulting from the artifactual carbon-dioxide accumulation due to the reaction of bicarbonate with acid derived H+-s). Mucosal acidosis was diagnosed if pHi ≤ 7.32 (the generally accepted cut off value[11]).
Laboratory investigations
Serum amylase and lipase activities were measured by the standard spectrophotometry method (reagents from Boehringer Diagnostics, Mannheim, Germany), where the upper limit of normal values was 220 U/L and 190 U/L respectively. Acute pancreatitis was diagnosed if serum amylase level exceeded 600 U/l. CRP was determined routinely by immunoturbidimetric assay (Orion Diagnostica, Espoo, Finland); the upper limit of normal value was 10 mg/L.
Statistical analysis
All data were presented as: median (range). Statistical analysis was performed using the Mann-Whitney U-test. Results were considered significant if P values were < 0.05.
RESULTS
Twenty-one patients with acute pancreatitis (6 women and 15 men) were involved in the study with a median age of 60 (29-77) years. The overall mortality rate was 6 of 21. There were no significant differences between the non-survivor (Group 1) and survivor (Group 2) groups in median age, gender and duration of symptoms before hospital admission. Ranson’s and modified Glasgow scores showed no significant differences between the two groups, in contrast, APACHE II scores were significantly higher in the non-survivor group from the second day after admission. Ethanol was the leading etiological factor in the first group, whereas biliary origin in the second (Table 1).
Table 1.
Clinical characteristics of the patients investigated in this study
| Group 1 non-survivors (n = 6) | Group 2 survivors (n = 15) | P value | |
| Age (yr)1 | 69 (41-77) | 52 (29-69) | 0.22 |
| Sex ratio (M/F) | 5/1 | 10/5 | |
| Duration of symptoms before admission (h)1 | 49.5 (12-104) | 17 (4-120) | 0.09 |
| Etiology | |||
| Ethanol/gallstone/other | 3/2/1 | 5/8/2 | |
| Disease severity | |||
| Predicted severity by Ranson’s score1 | 5.5 (1-8) | 3 (2-7) | 0.18 |
| Predicted severity by Glasgow criteria1 | 2.5 (2-6) | 2 (0-6) | 0.45 |
| APACHE II at the time of admission1 | 12 (4-15) | 6 (3-16) | 0.128 |
| APACHE II on the third hospital day1 | 14 (10-29) | 7 (0-10) | 0.005 |
| Outcome | |||
| Necrosis/No necrosis/No data2 | 5/0/1 | 5/9/1 | |
| Remote organ dysfunction1 | 3 (2-5) | 0 (0-2) | 0.0016 |
Values are represented as median (range). APACHE: Acute Physiology and Chronic Health Evaluation.
No CT scan or autopsy proven data of necrosis was available.
In the non-survivor group 3 of the 6 patients died within the first 3 d (two patients due to cardiorespiratory failure, whereas the third one due to early MOF). Three other patients deceased later (on d 9, 17, 18 respectively), all of them developed sepsis and late MOF (Table 1.) Bacteria responsible for fatal septic complications were Klebsiella, Staphylococci, Enterococci, and unidentified Gram-positive cocci.
In the survivor group local complications (peripancreatic fluid collection, necrosis) evolved in 5 of 15 patients (Table 1), and remote organ dysfunction (circulatory, pulmonary) was observed in 6 cases. Blood culture positivity was observed in 8 of 15 patients. Klebsiella, Enterobacter, Pseudomonas aeruginosa, Staphylococci and alpha hemolytic Streptococci were identified in the samples.
At the time points when the tonometry measurements were performed, serum CRP levels were significantly higher in Group 1. Other inflammatory markers, such as white blood cell counts, and the manifestations of the systemic metabolic disturbances as reflected by the daily lowest arterial pH and actual base excess values did not differ significantly between the two groups (Table 2).
Table 2.
Selected markers of inflammation, values of gastric tonometry measurements, blood test results in the patient groups
| Group 1 non-survivors (n = 6) | Group 2 survivors (n = 15) | P value | |
| Markers of inflammation | |||
| C-reactive protein (mg/L)1 | 408 (181-427) | 234 (95-317) | 0.048 |
| White blood cell count (G/L)1 | 19.1 (14.5-22.6) | 15.8 (10.2-23.2) | 0.14 |
| Positive bacterial hemoculture | 3 | 8 | |
| Gastric Tonometry | |||
| Lowest pHi within the first 72 h1 | 7.11 (6.97-7.32) | 7.31 (6.94-7.46) | 0.0001 |
| Lowest pHi during the third day1 | 7.07 (6.97-7.25) | 7.33 (7.27-7.46) | 0.0004 |
| Arterial blood gas results | |||
| Arterial pH12 | 7.35 (7.18-7.46) | 7.38 (7.34-7.44) | 0.18 |
| Actual base excess (mmol/L)12 | -9.4 (-12.5 - -4.5) | -7 (-13.3 - -1) | 0.11 |
Values are median (range). pHi: Calculated gastric intramucosal pH.
Daily minimum values within the first 72 h.
Using our pHi measurement protocol there were neither failures of tonometer placement, nor tonometer related complications.
In general, the daily lowest pHi values were permanently below 7.32 in the non-survivor group (Group 1), whereas pHi was normal, or only temporarily low in the survivor group (Group 2). The pHi value differences between the two groups did not reach statistical significance on the first hospital day (Figure 1); in contrast, on the second and third days pHi values in non-survivors became significantly lower (pH 7.12 and 7.07 respectively), than those of the survivors (Figure 1).
Figure 1.
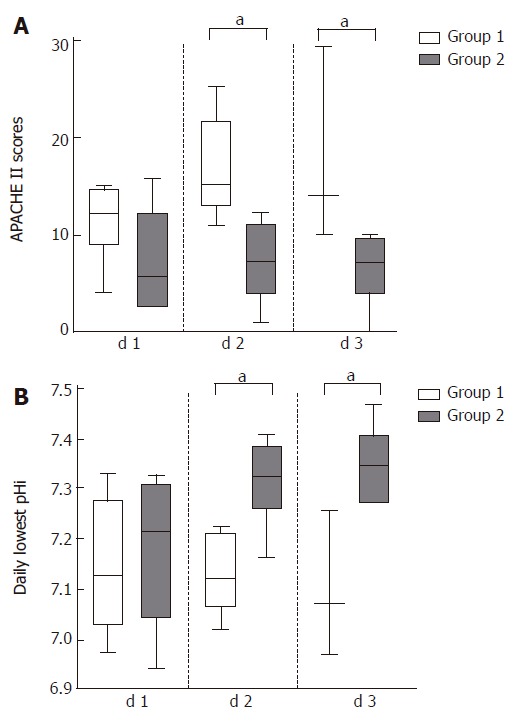
The daily lowest pHi and APACHEIIvalues in acute pancreatitis in the survivor and non-survivor group in the first three days.
If the relationship between the daily lowest pHi values and the APACHE II scores of the corresponding day were analyzed, the non-survivor group was characterized by a day-by-day decrease of pHi, which was associated with the expected rise in the APACHE II scores. Conversely, the originally subnormal pHi values of the survivor group have returned to the normal range with a corresponding decrease in the APACHE II scores (Figure 1).
We have found that assessment of the daily lowest pHi value was able to predict the development of non-infectious complications of acute pancreatitis. Prolonged mucosal acidosis -reflected by constantly subnormal pHi values- was associated with the development of MODS and fatal outcome. In contrast, in this study low pHi values were unsuitable to predict infections (Figure 2).
Figure 2.
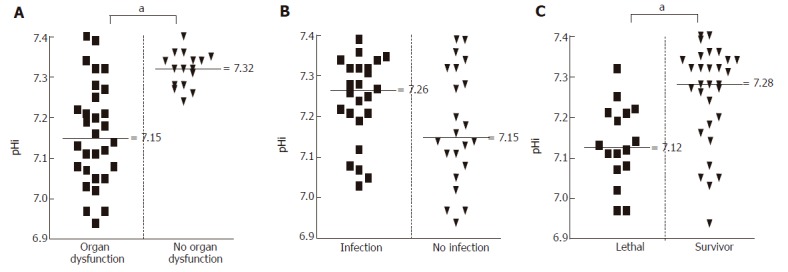
Evaluation of the predictor capacity of pHi values, if single measurements are considered.
We have also analyzed the dynamics of the daily lowest pHi values from the viewpoint of the development of multiple organ dysfunction/failure (Figure 3). The pHi data in the survivor group were subdivided to represent the emergence or absence of MODS. By definition, MODS in this group was responding to treatment, therefore it was transient in nature. The pHi data curve in the subgroup with the absence of remote organ dysfunction characteristically started slightly below the normal value and returned to normal level by d 3. In contrast, in the subgroup of patients with remote organ dysfunction the curve started low, but similarly, a return to normal values was noted.
Figure 3.
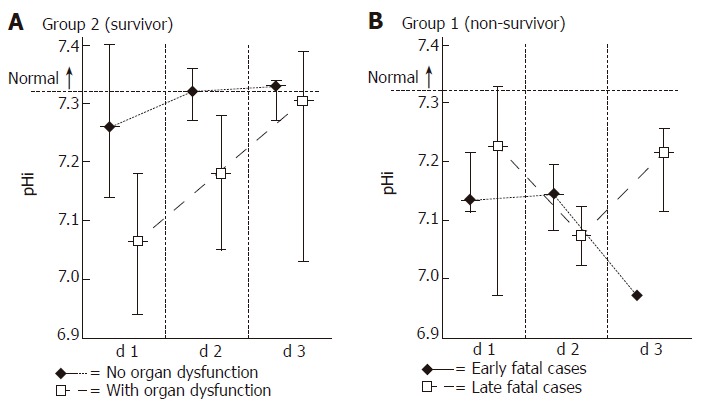
Dynamics of the daily lowest pHi values in relation to the different outcomes of acute pancreatitis. In the survivor group (Group 2) pHi data were sorted to reflect the emergence or absence of MODS. In the absence of MODS pHi values stayed close to the physiological range, returning from the initial depression to normal by d 3. In patients with MODS initial pHi values were considerably lower, but a return to normal values was detectable. In the non-survivor group (Group 1) each patient developed MOF, therefore values were sorted to reflect the early or late appearance of this complication. All pHi values continued to stay at subnormal levels from the day of admission, and never returned to normal. (Values represent median and range).
Since in the non-survivor group (Group 1) each patient developed MODS with deterioration into MOF (not responding to treatment), the values were sorted to reflect the early or late appearance of this complication. Although such review of the data had limited statistical power (due to the low number of cases), it could be concluded that all pHi values -irrespective of the early or late emergence of MODS/MOF- stayed at subnormal levels from d 1 and never returned to normal.
DISCUSSION
In this study we have shown a strong correlation between persistent low gastric mucosal pHi and therapy resistant MOF associated with severe AP. This finding is in accordance with the original results of Bonham et al[9]. They suggested that if the lowest pHi -measured at any time during the hospitalization- was below the 7.25 cut-off value, such severe mucosal acidosis was predictive of mortality in AP.
In a study by Juvonen et al[12] pHi values generally did not discriminate mild AP from the severe form, but the pHi values measured at 48 h were significantly lower in the severe patient group. Our data showed that the course of gastric pHi alterations was indeed of considerable interest, since not only in mild cases, but also in patients with responsive severe disease, the originally low values returned to the normal range. This was in sharp contrast with the persistently subnormal pHi values characterizing the therapy resistant MOF cases. With respect to the early predictive capacity of gastric tonometry, however, pHi values at the time of admission did not prognosticate the outcome.
Recent advances in the comprehension of intestinal mucosal pathophysiology in AP deserve further comments. Previous studies used a somewhat simplistic framework of low-flow state and regional perfusion failure as being responsible for the development of mucosal acidosis and the related complications.
Bonham’s group proposed that a mucosal ischemia-reperfusion injury was the culprit of gut barrier failure in AP. Soong et al[13] emphasized the role of hyperinflammation in the pathomechanism of mucosal injury, since peak endotoxin concentrations were detected before pHi fell to its lowest level. Mucosal acidosis correlated with the consumption of endotoxin core IgM antibodies as a reflection of antecedent circulating endotoxin exposure. Hynninen et al[14] have found that intramucosal acidosis in severe AP was concomitant with, or rapidly followed by increases in circulating cytokines (IL-6, IL-8, and IL-10), but they could identify no correlation between endotoxemia and low pHi.
It can be suspected that further -yet unexplored- elements may influence the mucosal integrity in AP including nitric oxide dependent vasoregulatory imbalance[15], mucosal oxidative stress, up-regulation of cellular adhesion molecules, and activation of adherent polymorphonuclear leukocytes with consecutive, destructive oxygen free radical production, as well as alteration of intestinal bacterial virulence[16,17]. Consequently, acidic gastric pHi should not be considered as a simple sign of the splanchnic bed hypoperfusion, but rather as a more complex pathophysiological representation of the involvement of the gut in SIRS, evolving together with the stress related alteration in the intestinal milieu.
Although our concept of mucosal barrier failure and bacterial invasion has been enriched by new details recently, the information concerning the general well-being of the mucosa provided by gastric tonometry still seems to be of value in cases of severe AP. Cumulating evidence suggests, however, that on the other side of the mucosal barrier (i.e. in the blood and tissues) a relative immune-deficient state evolves independently from mucosal acidosis in the late course of the disease. The exaggerated SIRS is followed by compensatory anti-inflammatory response syndrome (CARS) leading to immune deactivation[18], in which the activation induced cell death (AICD) of polymorphonuclear leukocytes[19] may be an important constituent.
The additive effects of these key elements may well represent the decisive step towards sepsis, and indeed, gastric tonometry will reflect the mucosal part only. (Despite the apparent mucosal dysfunction detected in our severe AP cases, a statistically significant association between low pHi values and infectious complications could not be established in this study.)
In conclusion, intramucosal pH values showed characteristic, time dependent alterations distinguishing mild acute pancreatitis from the severe form, but detection of mucosal acidosis at admission did not improve outcome prediction. The complex sequence of pathophysiological events responsible for the development of intramucosal acidosis and septic complications is not fully elucidated in acute pancreatitis. However, future investigations should continue to benefit from gastric tonometry as a non-invasive adjunct in monitoring the intestinal mucosal function during the course of the disease.
Footnotes
Supported by the Hungarian National Scientific Research Fund (OTKA), No. T 016630, and the Hungarian Ministry of Health (ETT), No. 276/2001
Co-first-author: Geza Telek
S- Editor Wang J L- Editor Chiarioni G E- Editor Bi L
References
- 1.Schmid SW, Uhl W, Friess H, Malfertheiner P, Büchler MW. The role of infection in acute pancreatitis. Gut. 1999;45:311–316. doi: 10.1136/gut.45.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luiten EJ, Hop WC, Endtz HP, Bruining HA. Prognostic importance of gram-negative intestinal colonization preceding pancreatic infection in severe acute pancreatitis. Results of a controlled clinical trial of selective decontamination. Intensive Care Med. 1998;24:438–445. doi: 10.1007/s001340050593. [DOI] [PubMed] [Google Scholar]
- 3.Meakins JL, Marshall JC. The gut as the motor of multiple organ system failure. In: Marston A, Bulkley GB, Fiddian-Green RG, Haglund UH, editors. Splanchnic Ischemia and Multiple Organ Failure. St Louis: CV Mosby; 1989. pp. 339–348. [Google Scholar]
- 4.Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127–1132. doi: 10.1023/a:1010786701289. [DOI] [PubMed] [Google Scholar]
- 5.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31:598–607. doi: 10.1097/01.CCM.0000045576.55937.67. [DOI] [PubMed] [Google Scholar]
- 6.Brown SD, Gutierrez G. Does gastric tonometry work? Yes. Crit Care Clin. 1996;12:569–585. doi: 10.1016/s0749-0704(05)70263-3. [DOI] [PubMed] [Google Scholar]
- 7.Salzman AL, Strong KE, Wang H, Wollert PS, Vandermeer TJ, Fink MP. Intraluminal "balloonless" air tonometry: a new method for determination of gastrointestinal mucosal carbon dioxide tension. Crit Care Med. 1994;22:126–134. doi: 10.1097/00003246-199401000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Fiddian-Green RG, Pittenger G, Whitehouse WM. Back-diffusion of CO2 and its influence on the intramural pH in gastric mucosa. J Surg Res. 1982;33:39–48. doi: 10.1016/0022-4804(82)90007-5. [DOI] [PubMed] [Google Scholar]
- 9.Bonham MJ, Abu-Zidan FM, Simovic MO, Windsor JA. Gastric intramucosal pH predicts death in severe acute pancreatitis. Br J Surg. 1997;84:1670–1674. [PubMed] [Google Scholar]
- 10.Parviainen I, Vaisänen O, Ruokonen E, Takala J. Effect of nasogastric suction and ranitidine on the calculated gastric intramucosal pH. Intensive Care Med. 1996;22:319–323. doi: 10.1007/BF01700453. [DOI] [PubMed] [Google Scholar]
- 11.Doglio GR, Pusajo JF, Egurrola MA, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Fernandez S, Palizas F, Gutierrez G. Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med. 1991;19:1037–1040. doi: 10.1097/00003246-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Juvonen PO, Alhava EM, Takala JA. Gastric tonometry in assessing splanchnic tissue perfusion in acute pancreatitis. Scand J Gastroenterol. 2000;35:318–321. doi: 10.1080/003655200750024218. [DOI] [PubMed] [Google Scholar]
- 13.Soong CV, Lewis HG, Halliday MI, Rowlands BJ. Intramucosal acidosis and the inflammatory response in acute pancreatitis. Am J Gastroenterol. 1999;94:2423–2429. doi: 10.1111/j.1572-0241.1999.01368.x. [DOI] [PubMed] [Google Scholar]
- 14.Hynninen M, Valtonen M, Markkanen H, Vaara M, Kuusela P, Jousela I, Piilonen A, Takkunen O. Intramucosal pH and endotoxin and cytokine release in severe acute pancreatitis. Shock. 2000;13:79–82. doi: 10.1097/00024382-200013010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Ba ZF, Chaudry IH. Nitric oxide. To block or enhance its production during sepsis? Arch Surg. 1994;129:1137–1142; discussion 1142-1143. doi: 10.1001/archsurg.1994.01420350035003. [DOI] [PubMed] [Google Scholar]
- 16.Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, Chang E, Ohman D, Suh S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232:480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljungdahl M, Lundholm M, Katouli M, Rasmussen I, Engstrand L, Haglund U. Bacterial translocation in experimental shock is dependent on the strains in the intestinal flora. Scand J Gastroenterol. 2000;35:389–397. doi: 10.1080/003655200750023958. [DOI] [PubMed] [Google Scholar]
- 18.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood. 1999;93:425–439. [PubMed] [Google Scholar]
- 19.Telek G, Kovacs GC, Pasquier C, Jakab F, Hamar J, Rozé C. Polymorphonuclear leukocytes enter into apoptosis early in the course of taurocholate-induced acute pancreatitis in rats. The role of oxidative stress. Gastroenterology. 2000;118 Suppl 2:5319. [Google Scholar]


