Abstract
AIM: To investigate the histological and ultra-structural characteristics of liver graft during different of warm ischemia time (WIT) in rats and to predict the maximum limitation of liver graft to warm ischemia.
METHODS: The rats were randomized into 7 groups undergoing warm ischemia injury for 0, 10, 15, 20, 30, 45 and 60 min, respectively. All specimens having undergone warm ischemia injury were investigated dynamically by light and electron microscopy, and histochemistry staining. After orthotopic liver transplantation (OLT),the recovery of morphology of liver grafts after 6, 24 and 48 h was observed.
RESULTS: The donor liver from non-heart-beating donors (NHBD) underwent ischemia injury both in the warm ischemia period and in the reperfusion period. Morphological changes were positively related to warm ischemia injury in a time-dependent manner during the reperfusion period. The results demonstrated that different degrees of histocyte degeneration were observed when WIT was within 30 min, and became more severe with the prolongation of WIT, no obvious hepatocyte necrosis was noted in any specimen. In the group undergoing warm ischemia injury for 45 min, small focal necrosis occurred in the central area of hepatic lobule first. In the group undergoing warm ischemia injury for 60 min, patchy or diffused necrosis was observed and the area was gradually extended, while hepatic sinusoid endothelial cells were obviously swollen. Hepatic sinusoid was obstructed and microcirculation was in disorder.
CONCLUSION: The rat liver graft undergoing warm ischemia injury is in the reversible stage when the WIT is within 30 min. The 45 min WIT may be a critical point of rat liver graft to endure warm ischemia injury. When the WIT is over 60 min, the damage is irreversible.
Keywords: Liver transplantation, Warm ischemia injury, Morphological observation
INTRODUCTION
In the past 40 years, liver transplantation has achieved a great success and has become the most effective method to treat the end-stage hepatic diseases. Liver transplantation is developing rapidly as a result of perfect perioperative care and widespread applications of potent immunosuppressants. However, there is an obvious problem of donor organ shortage, especially in countries where the “brain-death” cases have not been legitimated. At present, a high percentage of liver grafts comes from non-heart-beating donor (NHBD) in these countries. Under these circumstances, liver grafts unavoidably encounter a period of warm ischemia injury and undergo further injuries in preservation and reperfusion process[1-3]. Poor quality of liver grafts is considered an important risk factor greatly reducing the liver transplantation effectiveness[4-6]. Clinical practice suggests that the warm ischemia time (WIT) should not be longer than 5 min[7], and 10 min of WIT may be the upper limit. According to this, many donor livers are useless, thus aggravating the problem of donor liver shortage. This study was to observe the changing patterns of histological structure and ultrastructure of liver graft undergoing warm ischemia injury.
MATERIALS AND METHODS
Animals and grouping
Two hundred and ten healthy male adult Sprague-Dawley (SD) rats weighing 250-300g (Experimental Animal Center at Sun Yat-Sen University) were used in the study. The mean weight of recipient rats was slightly heavier than that of donor rats. According to WIT, 210 SD rats were randomly divided into seven groups. The duration of WIT was 0, 10, 15, 20, 30, 45 and 60 min respectively. Forty-two SD rats (6 each group) were used for the observation of warm ischemia injury. The other 168 were divided into 7 subgroups. Orthotopic liver transplantation (OLT) was performed in each group according to the predetermined WIT, 12 as donors and 12 as recipients. Histological, histochemical and ultrastructural changes were observed 6, 24 and 48 h respectively after reperfusion. Specimens taken from the right hepatic lobe of rats were divided into 4 types, one for routine olefin sections after fixation in formalin solution, one for glycogen staining after fixation in Gendre solution, one for enzyme histochemical staining after quick freezing in liquid nitrogen and one for ultrathin sections.
Establishment of animal model
Warm ischemia injury was induced by clamping the basilar part of the heart and blocking the thoracic aorta of the donor animals after the donor rat received 0.2 mL heparin sodium solution (1250 U heparin sodium) via dorsum of penis vein to establish the non-heart-beating donor model. Then the liver graft was dissected. The liver was then perfused in situ through the abdominal aorta with 20 mL chilled lactic acid Ringer’s solution (50 U/mL heparin sodium) and stored in a bath of cold lactate Ringer’s solution before transplantation. Immediately prior to the portal vein clamping, orthotopic liver transplantation was performed as previously described[8] with minor modifications[9]. The cold ischemia time (CIT) was 50 ± 3.5 min and the anhepatic period was 20 ± 2.5 min.
Observation indexes and methods
Specimens were fixed in formalin solution, routine 4-6 μm paraffin sections were stained with HE for light microscopy.
Specimens were disposed by the typical ultra-histology methods, and the sections were observed under transmission electron microscope and scanning electron microscope.
Hepatic glycogen was stained by PAS reaction after the fresh specimens were fixed in Gendre’s solution for 6 h. Tetrazolium blue, PPDA, magnesium activation were respectively adopted to observe the activities of SDH, CO and ATPase on 5-6 μm thick cryo-sections.
RESULTS
Observation under light microscope
Histological structure changed slightly when WIT was shorter than 30 min. Cytoplasm loosening, cell edema, focal vacuole degeneration were noted when WIT was over 30 min, especially in the lobule center area. Leukocyte infiltration was noted in the portal area and acidophilus was obvious in some hepatocytes. The above pathologic changes aggravated when WIT was prolonged to 60 min. Cell degeneration was diffuse or extended to a focal area, even lipid degeneration could be seen. The degree of degeneration was dependent on the duration of WIT, but necrosis could hardly be observed under light microscope. After 6 and 24 h reperfusion, injury to liver graft became severer and hepatocytes presented with obvious edema and some ballooning degeneration in the group undergoing warm ischemia injury for 30 min (Figures 1A and B). Focal like necrosis could be noted in the lobule center area in the group undergoing warm ischemia injury for 45 min (Figure 1C), the change aggravated when WIT was prolonged. Forty-eight hours after reperfusion, hepatic injury resumed gradually in the group undergoing warm ischemia injury for < 45 min. Hepatocytes presented with plaque or diffused necrosis and the pathologic change was irreversible in the group undergoing warm ischemia injury for 50 min (Figure 1D).
Figure 1.
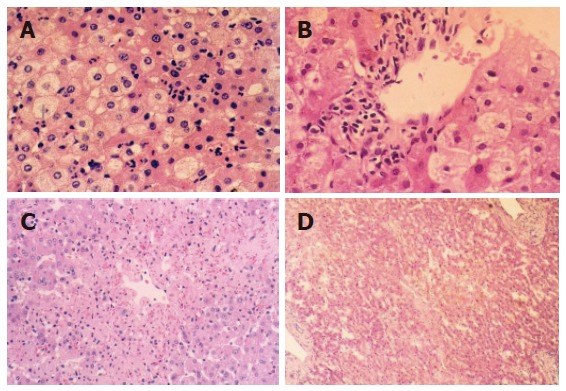
Cytoplasm loosening, cell edema, focal vacuole degeneration after reperfusion (10 × 10) in the group undergoing warm ischemia injury for < 30 min (A); obvious cell edema, ballooning-like degeneration after reperfusion (40 × 10) in the group undergoing warm ischemia injury for 30 min (B); focal necrosis around central lobule area after reperfusion (10 × 10) in the group undergoing warm ischemia injury for 45 min (C); plaque-like area necrosis after reperfusion (40 × 10) in the group undergoing warm ischemia injury for 60 min (D).
Observation under electron microscope
The structure of mitochondria and endoresticule was normal when WIT was shorter than 15 min (Figure 2A). Mitochondria became swollen, density of basal plasma was reduced, endoresticule was enlarged and glycogen particles were reduced in the group undergoing warm ischemia injury for 30 min. Mitochondria crista became fuzzy and pale in the group undergoing warm ischemia injury for 45 min. While fuzzy or ruptured mitochondria crista, vacuole degeneration and broken endoresticule were noted in the group undergoing warm ischemia injury for 60 min. Six hours after reperfusion, damage to liver graft became severer and 24 h after reperfusion, mitochondria became swollen and basal density reduction was aggravated, but glycogen particles increased in the group undergoing warm ischemia injury for < 30 min (Figure 2B). Mitochondria became swollen, vacuole was degenerated, and rough endoreticule was broken, apoptosis of hepatic and endothelial cells was increased with chromosome margination, karyopyknosis and karyorrhexis in the group undergoing warm ischemia injury for > 30 min (Figures 2C and D). Endothelial gaps were enlarged, the sieve plate was mingled, some endothelial cells broke off, and therefore, some sinusoids were blocked with cytoplasmic bleb accumulation in the groups undergoing warm ischemia injury for 45 and 60 min (Figure 3A). Forty-eight hours after reperfusion, swollen mitochondria resumed gradually, glycogen particles increased obviously in the group undergoing warm ischemia injury for < 45 min. Mitochondria were extended with crista turbulence, vacuole, membrane rupture, and cell apoptosis and necrosis (with karyopyknosis, karyorrhexis and karyolysis) could be noted in the group undergoing warm ischemia injury for 60 min. The above changes were irreversible (Figure 3B). Most endothelial cells underwent necrosis and shedding, many hepatic sinusoids were full of cytoplasmic blebs, reticular fibrosis and hemocytes (Figure 3C). Under scanning electron microscope, endothelial cells presented with bleb or ballooning like degeneration, sinusoids were blocked, thus the microcirculation underwent irreversible disturbance (Figure 3D).
Figure 2.
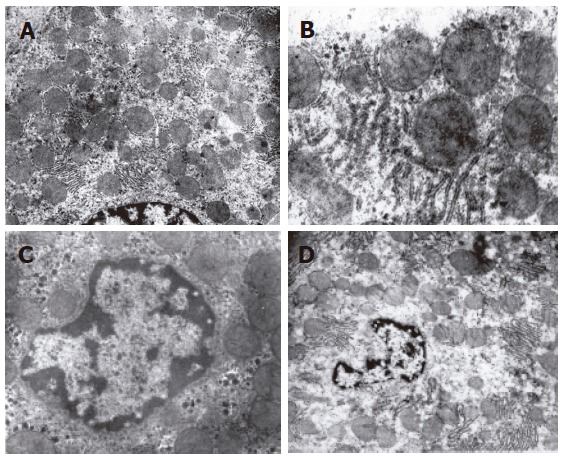
Swollen mitochondria, ruptured crista, rough endoplasmic reticulum degranulation (× 15 000) in the group undergoing warm ischemia injury for 30 min (A); phanero-swollen mitochondria and nuclear chromosome margination, and karyopyknosis after reperfusion (× 10 000) in the group undergoing warm ischemia injury for 45 min (B and C); significant swollen mitochondria, crista extinction, nuclear membrane rupture, karyolysis and karyorrhexis after reperfusion (× 5000) in the group undergoing warm ischemia injury for 60 min (D).
Figure 3.
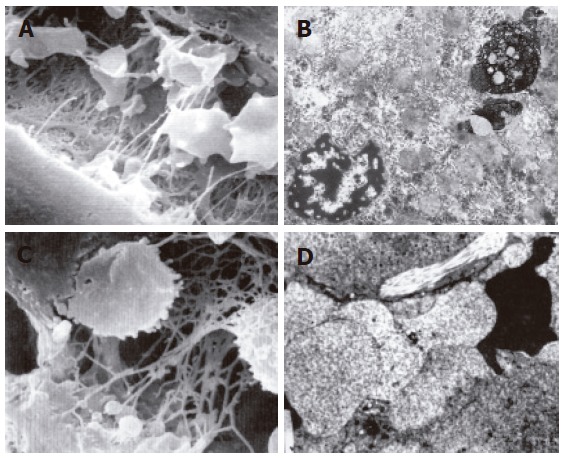
Cytoplasmic blebs and irregular endothelial sieve plate in some sinusoids after reperfusion (scanning electron microscope × 6000) in the group undergoing warm ischemia injury for 45 min (A); apoptosis and necrosis of hepatocytes after reperfusion (× 8000) in the group undergoing warm ischemia injury for 60 min (B); cytoplamic blebs, reticular cellulose and hemocytes after reperfusion (scanning electron microscope × 6000) in the group undergoing warm ischemia injury for 60 min (C); bleb or ballooning like swollen endothelial cells, blocked sinusoids and irreversible microcirculation disturbance after reperfusion (× 5000) in the group undergoing warm ischemia injury for 60 min (D).
Histochemical observation
Our previous study has demonstrated that hepatic glycogen begins to reduce when WIT is prolonged to 30 min and the activities of SDH, CO and ATPase begin to decrease when WIT is longer than 45 min[10]. The above changes were positively related to WIT in a time dependent manner during reperfusion in this study. PAS reaction and enzyme activities showed a recovering potency in the groups undergoing warm ischemia injury for 15 and 30 min. The liver graft underwent an irreversible injury and no evident recovery potency was found after implantation in the groups undergoing warm ischemia injury for 45 and 60 min.
DISCUSSION
How to evaluate the quality of liver grafts and how to ascertain the safety time limit for warm ischemia of liver grafts remain to be solved since warm ischemia injury affects the outcome of liver transplantation. Western transplantation community does not consider much of warm ischemia injury because their liver grafts are mainly taken from “brain-death” donors. Liver transplantation has become an effective management in the treatment of end-stage liver diseases, while the shortage of donor liver is a critical limiting factor for liver transplantation, thus people have begun to reconsider the marginal organ source like NHBD since 1990s[11-13]. Some laboratory studies support a controversial 60 min WIT limitation[14-16], but different experimental animals, warm ischemia models and experiment conditions may cause arguments about the WIT limitation. The argument about the WIT limitation has led to a worldwide investigation on warm ischemia-reperfusion injury[17,18].
In the present study, we observed the changes of histological structure and ultrastructure of liver grafts during different WIT. Cellular edema and vacuole degeneration could be noted in warm ischemia period. The pathologic changes were aggravated with the prolongation of WIT, but necrosis was absent. Hepatocyte vacuole degeneration was due to swollen mitochondria and outstretched endoresticule. Glycogen-absorbed vacuole also could be seen. Under electron microscope, pathologic changes were reversible, only part of cells underwent irreversible changes such as necrosis (with karyopyknosis, karyorrhexis and karyolysis) and apoptosis. However, liver grafts from NHBD underwent injuries both in the warm ischemia period and in the reperfusion stage. The degree of injury in the reperfusion stage was positively related to the duration of WIT. Histochemical observations showed that hepatic glycogen began to reduce when WIT was prolonged to 30 min. The activities of SDH, CO and ATPase began to decrease when WIT was longer than 45 min. The above changes were positively related to WIT in a time-dependent manner during the reperfusion period. PAS reaction and enzyme activities showed a recovering potency in the groups undergoing warm ischemia injury for 15 and 30 min. The liver graft underwent irreversible injury and no evident recovery potency was found after implantation in the groups undergoing warm ischemia injury for 45 and 60 min.
In conclusion, the pathologic changes of liver grafts undergoing only warm ischemia injury are reversible when WIT is shorter than 60 min, but the damage to liver graft would aggravate at the reperfusion stage, suggesting that rat liver grafts undergoing warm ischemia injury are in the reversible stage when WIT is within 30 min. The 45 min of WIT may be a critical point of rat liver grafts to tolerate warm ischemia injury and when WIT is prolonged to 60 min, the damage is irreversible.
Footnotes
Supported by the Key Clinical Project of Minister of Public Health, No. 97040230
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
References
- 1.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 2.Hines IN, Harada H, Wolf R, Grisham MB. Superoxide and post-ischemic liver injury: potential therapeutic target for liver transplantation. Curr Med Chem. 2003;10:2661–2667. doi: 10.2174/0929867033456396. [DOI] [PubMed] [Google Scholar]
- 3.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 4.White SA, Prasad KR. Liver transplantation from non-heart beating donors. BMJ. 2006;332:376–377. doi: 10.1136/bmj.332.7538.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuñez JR, Del Rio F, Lopez E, Moreno MA, Soria A, Parra D. Non-heart-beating donors: an excellent choice to increase the donor pool. Transplant Proc. 2005;37:3651–3654. doi: 10.1016/j.transproceed.2005.09.105. [DOI] [PubMed] [Google Scholar]
- 6.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 7.Huang JF. Theory and practice of liver transplantation. Guangzhou: Technology and Science Publishing-house of Guangdong Province; 1998. p. 132. [Google Scholar]
- 8.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64–69. [PubMed] [Google Scholar]
- 9.Ma Y, He XS, Chen GH. Surgical technique of the model of orthotopic liver transplantation and prevention of operational complication in rats. Zhonghua Xianwei Waike Zazhi. 2003;26:45–47. [Google Scholar]
- 10.He XS, Ma Y, Wu LW, Wu JL, Hu RD, Chen GH, Huang JF. Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J Gastroenterol. 2005;11:2662–2665. doi: 10.3748/wjg.v11.i17.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alessandro AM, Hoffmann RM, Knechtle SJ, Eckhoff DE, Love RB, Kalayoglu M, Sollinger HW, Belzer FO. Controlled non-heart-beating donors: a potential source of extrarenal organs. Transplant Proc. 1995;27:707–709. [PubMed] [Google Scholar]
- 12.Takada Y, Taniguchi H, Fukunaga K, Yuzawa K, Otsuka M, Todoroki T, Iijima T, Fukao K. Hepatic allograft procurement from non-heart-beating donors: limits of warm ischemia in porcine liver transplantation. Transplantation. 1997;63:369–373. doi: 10.1097/00007890-199702150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Schön MR, Hunt CJ, Pegg DE, Wight DG. The possibility of resuscitating livers after warm ischemic injury. Transplantation. 1993;56:24–31. doi: 10.1097/00007890-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gurkan A, Kacar SH, Varilsuha C, Karaca C, Kose S, Karaoglan M, Akman F, Basak K. Non-heart-beating donors: is it worthwhile? Ann Transplant. 2005;10:20–22. [PubMed] [Google Scholar]
- 15.Arias-Diaz J, Alvarez J, del Barrio MR, Balibrea JL. Non-heart-beating donation: current state of the art. Transplant Proc. 2004;36:1891–1893. doi: 10.1016/j.transproceed.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 16.Hirota M, Ishino K, Fukumasu I, Yoshida K, Mohri S, Shimizu J, Kajiya F, Sano S. Prediction of functional recovery of 60-minute warm ischemic hearts from asphyxiated canine non-heart-beating donors. J Heart Lung Transplant. 2006;25:339–344. doi: 10.1016/j.healun.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Van Deynse D, Dumont V, Lecomte C, Squifflet JP, Mourad M, Verschuren F, Dufrane D, Hantson P, Malaise J. Non-heart-beating donor, renewal of an old procedure: a Belgian experience. Transplant Proc. 2005;37:2821–2822. doi: 10.1016/j.transproceed.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 18.del Río Gallegos F, Nunez Pena JR, Soria García A, Moreno Roy MA, Varela A, Calatayud J. Non heart beating donors. Succesfully expanding the donor's pool. Ann Transplant. 2004;9:19–20. [PubMed] [Google Scholar]


