Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause for elevated liver enzymes in the developed nations. Beyond prevention programs which are of particular interest because of the increasing number of overweight children, treatment should be focussed on the most important risk factors, obesity and insulin resistance. As a consequence of elucidating the pathomechanisms of NAFLD, the number of potential therapeutic options increased. However, many studies investigating the therapeutic effect show shortcomings in at least one of the following points: lack of a serial liver biopsy, short term of treatment and limited number of included patients. The second generation insulin sensitizer pioglitazone and rosiglitazone show the most promising improvements in NAFLD, but weight gain and potential hepatotoxicity calls for attention. In conclusion, a general recommendation for the application of specific drugs cannot be given. Besides controlled clinical trials, weight reduction and physical activity to improve insulin sensitivity in obese patients should be the priority objective.
Keywords: Nonalcoholic fatty liver disease, Treatment
INTRODUCTION
Insulin resistance and obesity represent the most important risk factors for development of NAFLD[1]. Fatty liver has a benign prognosis, whereas up to 20% of patients with NASH develop cirrhosis[2,3]. Risk factors for development of fibrosis are age, BMI>30, glutamic-oxaloacetic transaminase (AST)/glutamic-pyruvic transaminase (ALT)>1, and diabetes[4]. In addition, the risk for development of hepatocellular carcinoma (HCC) is comparable to that of patients with hepatitis C infection[5]. The pathophysiology of NAFLD is described as a “two hit model”. The first hit is supposed to be the increase of free fatty acids in hepatocytes which results in a decrease of β-oxidation. Downregulation of β-oxidation further aggravates accumulation of fatty acids. The second step includes all mechanisms contributing to the development of inflammation and fibrosis[6]. In detail, increase of fatty acids enhances the expression of cytochrome peroxidase 2E1 (CYP2E1). CYP2E1 stimulates generation of oxidative species and thereby enhances lipid peroxidation of the hepatocyte membrane[7,8]. Endotoxin and TNF-α have been demonstrated to play a harmful role in development of alcoholic steatohepatitis[9]. Injection of lipopolysaccharide (LPS) in leptin-deficient, obese ob/ob mice resulted in a significantly more severe liver injury probably caused by TNF-α compared to lean control animals[10]. Administration of anti-TNF-α-antibody ameliorated liver damage in this model of NAFLD[11]. Both endotoxin and oxidative stress upregulate expression of CD95 ligand and contribute to apoptotic cell death[12]. In fact, increased hepatocyte apoptosis correlating with disease severity was described in patients with NASH[13].
Here we discuss the role of a drug-free management in improvement of insulin resistance and NASH and give a critical summary of recent data on medical treatment. The potential concepts of treatment are summarized in Figure 1.
Figure 1.
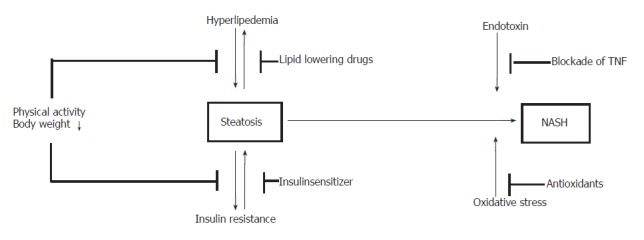
Potential treatment options of NAFLD
REDUCTION OF BODY WEIGHT
Although insulin resistance occurs in patients with normal BMI and anthropometric measurements, the majority of these patients are adipose with increased visceral fat. So, in overweight or obese patients, weight loss is usually recommended as the first line management[14]. The American Gastroenterological Association recommends a target of 10% of baseline weight as an initial goal of weight loss if BMI exceeds 25 kg/m2. Weight loss should proceed at a rate of 1-2 lb/wk. Rapid weight loss due to a very low energy diet (<500 kcal daily) or jejunoileal bypass has been associated with exacerbation of steatohepatitis in obese patients[15-17]. Weight loss should be achieved by restricting calorie intake and physical exercise. Both have been shown to improve insulin resistance[18]. Physical exercise is proven to be beneficial for coronary heart disease and peripheral vascular disease. Rollins demonstrated that moderate to high-intensity exercise (30 min 3-5 times/wk) reduces the risk of complications associated with obesity[19]. However, it is not clear whether patients with NAFLD would benefit from merely increasing physical activity. The goal of weight management is to induce a negative calorie balance. A calorie deficit of 500-1 000 calories/d for those who are overweight or obese appears to be rational, although there are no specific studies on this subject. Dietary recommendation should include reduction of dietary carbohydrates, because lipid profile of overweight patients improves[20,21]. However, weight loss is rarely achieved or maintained over a long period. Most studies to date have been short term with a small number of patients included. Children with NASH may benefit from weight loss, because serum liver enzymes normalized and sonographic abnormalities disappeared[22]. Another study investigated 48 patients with elevated liver enzymes and clinical, histological, and sonographic characteristics of fatty liver disease[23]. Eighty-one percent of these patients were obese, 73% were glucose-intolerant or diabetic, and 85% had dyslipidemia. Dietary intervention as well as lipid-lowering medication and oral antidiabetics as needed were included into the treatment protocol. These dietary interventions resulted not only in a moderate weight loss, but also in a reduction of serum liver enzymes in 96% of patients. However, it remains unclear, if improved liver enzymes were accompanied by an improvement of liver histology, because serial liver biopsies were not performed. In another study, 15 obese patients followed a restricted diet (25 calories per kilogram ideal body weight) and exercise regimen over a 3 mo period[24]. Liver enzymes decreased in all patients and steatosis determined by biopsy was reduced compared with patients from control group.
A recently published study analyzed the effect of short term weight loss on liver histology[25]. Twenty-three obese patients (BMI>25kg/m2) with biopsy-proven NASH received standardized nutritional counseling to reduce insulin resistance and body weight. Each subject received individualized nutrition counseling in order to achieve dietary goals. The dietary adjustments included increased intake of fiber and a decreased intake of calories. The daily calory intake consists of 40%-45% carbohydrates with an emphasis on complex carbohydrates with fiber, 35%-40% fat with emphasis on mono- and polyunsaturated fats, and 15%-20% protein. In addition, all participants were encouraged to increase their physical activity to achieve a heart rate of 70% of the calculated target heart rate. Food frequency questionnaires were performed to assess dietary intake and the Paffenberger Activity Questionnaire was used to evaluate the level of physical activity. Sixteen patients successfully completed 12 mo of intense dietary intervention. Mean weight decreased from 98.3 kg to 95.4 kg. There was also a reduction of mean waist circumference, visceral fat, fasting glucose, insulin resistance, triglycerides, serum levels of liver enzymes and histological score, but the differences were not statistically significant. Fifteen patients underwent repeat liver biopsy. Nine of these 15 patients had a histological response, 6 patients had a stable score and none had worsened.
Pharmacological treatment of obesity may be applied to patients with a BMI>27 kg/m2 and obesitiy-associated comorbidities. Sibutramine is a serotonin reuptake antagonist which should not be used in patients with coronary heart disease and moderate or severe hypertension[26]. The intake of orlistat results in fat malabsorption. Sabuncu and coworkers analyzed the effects of orlistat and sibutramine in obese patients with clinically presumed NASH. Both orlistat and sibutramine improved liver enzymes and decreased the sonographic hallmarks of fatty liver. However, liver biopsies were not performed and the level of alkaline phosphatase increased[27].
Those patients with a BMI>35 kg/m2 and obesity-associated comorbidities may be considered for more aggressive weight management, including bariatric surgery. Because liver failure occurs after jejuno-ileal bypass, the later has been replaced by the proximal gastric bypass operation. Two studies demonstrated improvement of liver histology after weight reduction and stabilization of weight for long term[28,29]. However, occasional cases of worsening liver function can also occur during period of rapid weight loss following this procedure. The results of studies investigating the safety of such surgery in patients with severe NASH have to be awaited. Patients considered for this procedure should be monitored carefully and the pros and cons should be discussed with the patient in detail.
ANTIOXIDANTS
Oxidative stress is proposed to act as the “second hit” in the pathogenesis from steatosis to NASH and fibrosis. Therefore, using antioxidant substances seems to be rational in the treatment of steatohepatitis. Several in vitro and animal in vivo studies revealed that application of vitamin E decreased levels of profibrogenic TGF beta, improved liver histology and inhibited hepatic stellate cell activation[30-32]. Two open-label pilot trials examined the effect of vitamin E in patients with NAFLD[33,34]. Eleven pediatric patients with presumed NASH were prescribed 400-1 200 IU of oral vitamin E. Diagnosis was based on the presence of chronically elevated levels of AST and ALT, and fatty liver on ultrasound. Other causes for hepatitis were excluded. Two patients had biopsy-proven NASH. Treatment resulted in normalization of liver function test. However, serial biopsies were not performed, liver remained increased echogenic during treatment and improvement of enzymatic values was not sustained after discontinuation of vitamin E[33]. In another study[34], 10 patients with the clinical diagnosis of NAFLD and 12 patients with biopsy proven NASH were treated with vitamine E (300 mg/d) for 1 year. Treatment resulted in a significant improvement of liver enzymes. In the nine patients with steatohepatitis who had a posttreatment liver biopsy, the degree of steatosis, inflammation, or fibrosis also improved or remained unchanged. The plasma levels of TGF-β decreased significantly with vitamin E. However, these promising results were not confirmed in a subsequent randomized, double-blind, placebo-controlled trial[35]. In this study, vitamin E (1 000 IU/d) in combination with vitamin C (1 000 mg/d) to potentially enhance the regeneration of oxidized vitamin E was given to 23 patients with NASH, while 22 patients were randomized to placebo. The duration of treatment was 6 mo. In addition, a low fat, low calorie diet in combination with increased physical activity was recommended. The degree of adherence to these recommendations remained unclear. The results showed a significant improvement of ALT levels in the placebo group but not in the treatment group. The fibrosis stage of 11 (48%) patients of the vitamin group and 9 (41%) of the placebo group improved by at least one stage. The authors concluded from this within group comparison that vitamin C and vitamin E are effective in improving liver fibrosis, although only two more patients in the vitamin group showed a regression of fibrosis. Adams and Angulo[36] criticized that the effect of placebo treatment was ignored, because no comparison between groups was performed. A between group analysis revealed that 6 mo of therapy with the combination of vitamin E and C is not better than placebo for patients with NASH.
URSODEOXYCHOLIC ACID
This hydrophilic bile acid is approved for the treatment of primary biliary cirrhosis. Ursodeoxycholic acid (UDCA) has been shown to reduce the portion of hydrophobic bile acids which contribute to oxidative stress. This is of particular importance, because fatty hepatocytes reveal an increased sensitivity to hydrophobic bile acids[37]. A pilot study published in 1996 analyzing the effect of UDCA on serum liver enzymes and histology in patients with NAFLD showed promising results[38]. The hepatic steatosis decreased on repeat liver biopsy in 12 of 19 patients and there was also a statistically significant improvement in liver enzymes, but there were no changes in the histological grade of inflammation or fibrosis. In a subsequent controlled trial 166 patients were randomized with liver-biopsy proven NASH to receive 13 and 15 mg/kg of UDCA daily[39]. One hundred and twenty-six patients completed 2 years of therapy and serial liver biopsies were available in 107 patients. Analysis of serum liver chemistry, changes in the degree of steatosis, necroinflammation or fibrosis revealed no significant difference between the verum and placebo-treated groups. However, the results from this study showed a high rate of spontaneous improvement in hepatic steatosis in the placebo arm probably explaining in part why the data were negative. In addition, the dose of 13 and 15mg/kg pre day was possibly insufficient to improve NAFLD, so effect of higher doses needs to be evaluated in further studies.
INSULINSENSITIZER
The association of insulin resistance and hyperinsulinemia with NAFLD suggests a possibility of therapeutical intervention. The first evidence came from leptin-deficient, obese ob/ob mice. Metformin, a biguanide that reduces hyperinsulinemia and improves hepatic insulin resistance, reduced hepatomegaly and hepatic steatosis in ob/ob mice, whereas caloric restriction did not result in a substantial improvement[40]. The authors postulated that metformin improve hepatic insulin resistance by decreasing hepatic expression of TNF-α, a cytokine that promotes insulin resistance. A pilot study evaluated the effect of metformin in 20 patients with histological proven NASH[41]. When compared with the six patients not complying with treatment, intake of metformin for 4 mo significantly reduced levels of transaminases. They normalized in 50% of treated individuals. Also, insulin sensitivity improved significantly and liver volume decreased by 20%. Metformin was well tolerated and there was no case of lactic acidosis. However, the authors did not provide a serial liver biopsy to evaluate the effect of metformin on liver histology. These promising results were only in part supported by another open label trial [42]. Fifteen patients with biopsy proven NAFLD completed 12 mo of treatment with metformin (20 mg/kg). During the initial 3 mo, liver enzymes improved significantly. There was also an improvement in insulin sensitivity detectable. However, after 3 mo, insulin sensitivity did not further improve and levels of AST and ALT gradually increased back to pretreatment levels. Among the 10 patients with posttreatment biopsy, three showed improvement in steatosis, two showed improvement in inflammation score and one in fibrosis.
Another trial evaluating the effect of metformin was performed by a Turkish group from Ankara[43]. Uygun and coworkers randomized 36 patients with NASH into two groups. The first group was given lipid and calorie-restricted diet alone, while the second group was treated with metformin in addition to the diet for a period of 6 mo. The comparison between both groups showed no significant differences in inflammatory activity or fibrosis, although more patients in the treatment group showed an improved liver histology. The improvements of liver enzymes, insulin, insulin resistance index and c-peptide levels in the metformin group were significantly greater than those detected in the group with dietary treatment alone. The most recently published controlled trial by Bugianesi and colleagues demonstrated a better effect of metformin on improvement of liver enzymes compared to a prescriptive diet or the administration of vitamin E[44]. Unfortunately, the histological data were limited to support an association between improvement of liver chemistry and histological findings.
Another class of agents presumably improving insulin sensitivity is the thiazolinediones. These compounds are ligands for the peroxisome proliferator-activated receptor gamma (PPAR gamma), which is expressed at high levels in adipocytes. Troglitazone, a first generation thioglitazone, and metformin were shown to inhibit the expression of sterol regulatory element binding protein-1 (SREBP-1), a key regulator of lipogenic enzymes[40,45]. Troglitazone was investigated in a pilot study including 6 patients with biopsy-proven NASH[46]. Patients received 200 mg of troglitazone twice daily, which was well tolerated. Levels of ALT normalized in 4 of 6 patients on therapy and they persisted in normal range 3 mo after discontinuation of troglitazone. However, the Food and Drug Administration removed troglitazone from the market in March 2 000 because of serious hepatotoxicity[47,48]. The second-generation thioglitazones rosiglitazone and pioglitazone appear to be safer, although their use is currently contraindicated in the presence of active liver disease or of ALT more than 2.5 times normal. An open label trial including 26 biopsy-proven NASH patients analyzed the effect of rosiglitazone, 4 mg twice daily for 48 wk[49]. All patients were overweight, and 23% had a BMI>35 kg/m2. Twenty-six patients had posttreatment biopsy. The mean necroinflammatory score significantly improved with treatment and biopsies of 10 patients did not fulfill published criteria for NASH anymore after treatment . Twenty-five patients completed 48 wk of treatment and showed a significant improvement in liver enzymes and insulin resistance. However, 3 patients had to be withdrawn because of adverse events. One of these individuals discontinued because of increased ALT levels. In addition, weight gain occurred in more than two-thirds of participants and liver enzyme levels increased to near pretreatment level 6 mo after discontinuation of study medication. Another pilot study demonstrated similar results in 18 patients with biopsy proven NASH, who were treated with pioglitazone[50]. By 48 wk, levels of ALT normalized in 72% of patients. Hepatic fat content and size decreased which was determined by magnetic resonance imaging. There was also a significant improvement in liver histology regarding features of steatosis, inflammation and fibrosis. Histological improvement occurred in two-thirds of patients. The main side effect in this study was also weight gain and increase in total body adiposity.
In a recently published pilot study by Sanyal and coworkers 20 nondiabetic patients with biopsy-confirmed NASH were randomized to take the combination of pioglitazone (30 mg daily) and vitamin E or vitamin E (400 IU daily) alone for a period of 6 mo[51]. ALT levels normalized in all patients within 6 mo. Compared to baseline, treatment with vitamin E alone resulted in a significant decrease in steatosis, whereas the combination therapy produced a significant improvement in steatosis, cytologic ballooning, Mallory´s hyaline and pericellular fibrosis. Although vitamin E did not have any significant effects on metabolic endpoints, combination therapy improved insulin sensitivity, lowered fasting free fatty acid (FFA) levels and decreased metabolites of FFA oxidation. However, like in the previous trial by Neuschwander-Tetri and coworkers[49] , one of 10 patients receiving pioglitazone plus vitamin E had a significant increase in ALT level and was withdrawn from the study.
LIPID LOWERING DRUGS
Because NAFLD frequently occurs with a disordered lipid homeostasis, lipid-lowering drugs are considered as possible treatment for NAFLD. Hypertriglyceridemia and reduced HDL-cholesterol level are typical dyslipidemias associated with NAFLD. Gemfibrozil reduces very low-density lipoprotein triglyceride production. In a small controlled study of 46 patients with NASH, levels of AST were significantly decreased in 74% of the gemfibrozil group compared with 30% in the control group after 4 wk of treatment[52]. There was no correlation with pretreatment serum triglyceride levels. Posttreatment liver biopsies were not performed and the duration of biochemical response was not evaluated.
In NASH patients with hyperlipidemia statins are another potential treatment option. However, existing data are predominantly uncontrolled with a small number of patients. One study analyzed 28 hyperlipidemic patients with biopsy-proven NASH. Patients were given atorvastatin 20 mg daily for 24 wk. Both significant reduction of LDL-cholesterol and liver enzymes were detectable after treatment[53]. Statin-induced hepatoxicity did not occur and the risk seems to be not increased in patients with presumed NAFLD[54]. However, controlled trials with a bigger number of patients are required to demonstrate the benefit and elucidate potential risks of administrating statins.
BLOCKADE OF TNF-α
Adipose tissue produces several cytokines and biologically active proteins, denoted as adipokines, regulating hepatic and peripheral glucose and lipid metabolism. These adipokines include leptin, resistin, adiponectin and TNF-α. Expression of resistin is not increased in patients with insulin resistance, although resistin inhibits insulin action in animal models[55]. NASH patients show increased serum leptin levels, suggesting the attempt to overcome hepatic leptin resistance to stimulate hepatic lipid turnover[56]. In several studies investigating the pathomechanisms of fatty liver disease increased TNF-α levels have also been demonstrated[57,58]. TNF-α contributes to insulin resistance and thereby increases hepatic steatosis and plays a potentially proinflammatory role[59]. This was supported by studies in leptin deficient ob/ob mice. Treatment of anti-TNF-α antibody improved liver histology, reduced hepatic total fatty acid content, and decreased ALT levels[11]. However, studies of ob/ob mice lacking type I and II TNF receptors have suggested that TNF-α is not involved in the liver disease[60]. Further evidence of the involvement of TNF-α came from studies of pentoxifylline which acts as an inhibitor of TNF-α[61,62]. In these two studies 20 patients and 18 patients, respectively, with biopsy confirmed NASH were enrolled. Pentoxifylline was given for 6 or 12 mo. Both studies demonstrated a significant improvement of AST and ALT levels after application of pentoxifylline in patients with NASH, although histological evidence of its benefit remains unknown.
Adiponectin is exclusively secreted from adipose tissue in inverse proportion to BMI[63,64]. Although the three dimensional structure of adiponectin closely resembles that of TNF-α[65], these two proteins have completely opposite effects. Adiponectin and TNF-α suppress each other´s production and antagonize their biological effects[66]. Adiponectin acts to reduce body fat[67], improve hepatic and peripheral insulin sensitivity[68] and decrease fatty acid levels[69]. Adiponectin has also antiinflammatory effects which could prevent liver disease. Xu and coworkers replenished recombinant adiponectin in mice fed with a high fat ethanol containing diet and in obese ob/ob mice with NASH. In both mice, administration of adiponectin ameliorated hepatomegaly, steatosis, and elevated ALT levels[70]. Both hepatic TNF-α expression and serum levels of TNF-α significantly decreased, which is further evidence for a harmful role of TNF-α.
This concept was further supported by a study of over 100 patients with NAFLD[71]. Multivariate analysis revealed that decreased serum adiponectin levels and increased TNF-α and soluble TNFR2 levels correlated with the presence of NASH independent of the presence of insulin resistance. NASH patients showed lower adiponectin levels than patients with simple steatosis. Levels of adiponectin correlated with the degree of hepatic necroinflammation. These data provide evidence for the involvement of TNF-α and adiponectin in human NAFLD. Therefore, studies evaluating the effect of adiponectin in treatment of NASH are required.
LIVER TRANSPLANTATION
NASH is considered the most common cause of cryptogenic cirrhosis[72]. Patients with pure steatosis have a benign prognosis, whereas the risk for developing cirrhosis and hepatocellular carcinoma in NASH patients is increased[73,74]. Complications of cirrhosis or hepatocellular carcinoma may require liver transplantation. In one study by Laurin and colleagues six of eight patients who underwent transplantation for NASH developed recurrent NASH. In three of these six patients, perivenular fibrosis recurred[75]. The patients with recurrences revealed post-transplant hyperlipidemia and increases in body weight. In two subsequent studies the recurrence rate of steatosis was between 60 to 100% of transplant recipients[76,77]. In one third of these patients progression to steatohepatitis occurred.
CONCLUSIONS
Because of increasing incidence of obesity and insulin resistance NAFLD has become increasingly the focus of basic and clinical research. Whereas fatty liver shows a benign prognosis, patients with NASH should be treated. Progress in understanding the pathomechanisms which contribute to aggravation of fatty liver pathology offers potential treatment options. However, a standard therapy has not been established. The most promising results came from trials with second generation insulin sensitizer in obese patients with insulin resistance. Blockade of TNF-α by adiponectin showed impressive improvement of NASH in an animal model. Clinical trials investigating therapeutic effect of inhibiting TNF-α in NAFLD have to be awaited. So, beyond clinical studies, the first step in treatment should be improvement of insulin sensitivity by weight loss and physical activity.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Bai SH
References
- 1.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 2.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594–598. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 3.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two "hits". Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- 9.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 10.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 12.Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, Krammer PH, Galle PR. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- 13.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 14.American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–1704. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- 15.Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224–229. doi: 10.1016/0168-8278(91)90942-5. [DOI] [PubMed] [Google Scholar]
- 16.Luyckx FH, Scheen AJ, Desaive C, Dewe W, Gielen JE, Lefebvre PJ. Effects of gastroplasty on body weight and related biological abnormalities in morbid obesity. Diabetes Metab. 1998;24:355–361. [PubMed] [Google Scholar]
- 17.Drenick EJ, Simmons F, Murphy JF. Effect on hepatic morphology of treatment of obesity by fasting, reducing diets and small-bowel bypass. N Engl J Med. 1970;282:829–834. doi: 10.1056/NEJM197004092821502. [DOI] [PubMed] [Google Scholar]
- 18.Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr. 2004;80:308–316. doi: 10.1093/ajcn/80.2.308. [DOI] [PubMed] [Google Scholar]
- 19.Rollins G. Moderate exercise reduces the risk of heart disease and death in men with type 2 diabetes. Rep Med Guidel Outcomes Res. 2003;14:10, 12. [PubMed] [Google Scholar]
- 20.Archer WR, Lamarche B, Dériaz O, Landry N, Corneau L, Després JP, Bergeron J, Couture P, Bergeron N. Variations in body composition and plasma lipids in response to a high-carbohydrate diet. Obes Res. 2003;11:978–986. doi: 10.1038/oby.2003.135. [DOI] [PubMed] [Google Scholar]
- 21.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142:253–258. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 22.Vajro P, Fontanella A, Perna C, Orso G, Tedesco M, De Vincenzo A. Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr. 1994;125:239–241. doi: 10.1016/s0022-3476(94)70202-0. [DOI] [PubMed] [Google Scholar]
- 23.Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73–79. doi: 10.1093/qjmed/92.2.73. [DOI] [PubMed] [Google Scholar]
- 24.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 26.Ryan DH. Use of sibutramine to treat obesity. Prim Care. 2003;30:405–26, viii. doi: 10.1016/s0095-4543(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 27.Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12:189–192. [PubMed] [Google Scholar]
- 28.Luyckx FH, Scheen AJ, Desaive C, Thiry A, Lefébvre PJ. Parallel reversibility of biological markers of the metabolic syndrome and liver steatosis after gastroplasty-induced weight loss in severe obesity. J Clin Endocrinol Metab. 1999;84:4293. doi: 10.1210/jcem.84.11.6171-4. [DOI] [PubMed] [Google Scholar]
- 29.Luyckx FH, Desaive C, Thiry A, Dewé W, Scheen AJ, Gielen JE, Lefèbvre PJ. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222–226. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 30.Parola M, Muraca R, Dianzani I, Barrera G, Leonarduzzi G, Bendinelli P, Piccoletti R, Poli G. Vitamin E dietary supplementation inhibits transforming growth factor beta 1 gene expression in the rat liver. FEBS Lett. 1992;308:267–270. doi: 10.1016/0014-5793(92)81290-3. [DOI] [PubMed] [Google Scholar]
- 31.Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–1021. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- 32.Houglum K, Venkataramani A, Lyche K, Chojkier M. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069–1073. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- 33.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 34.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 35.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 36.Adams LA, Angulo P. Treatment of nonalcoholic steatohepatitis: antioxidants or insulin sensitizers. Clin Gastroenterol Hepatol. 2004;2:1059–1060. doi: 10.1016/s1542-3565(04)00462-8. [DOI] [PubMed] [Google Scholar]
- 37.Angulo P. Use of ursodeoxycholic acid in patients with liver disease. Curr Gastroenterol Rep. 2002;4:37–44. doi: 10.1007/s11894-002-0036-9. [DOI] [PubMed] [Google Scholar]
- 38.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 39.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 40.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 41.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 42.Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 43.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 44.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 45.Kakuma T, Lee Y, Higa M, Wang Zw, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci U S A. 2000;97:8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 47.Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95:272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 48.Menon KVN P, Lindor KD. Severe cholestatic hepatitis from troglitazone in a patient with nonalcoholic steatohepatitis and diabetes mellitus. Am J Gastroenterol. 2001;96:1631–1634. doi: 10.1111/j.1572-0241.2001.03809.x. [DOI] [PubMed] [Google Scholar]
- 49.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 50.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 51.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 52.Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. doi: 10.1016/s0168-8278(99)80243-8. [DOI] [PubMed] [Google Scholar]
- 53.Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, Karagiannopoulou G, Tzioufa V, Dimitrios K. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23:131–134. [PubMed] [Google Scholar]
- 54.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285:561–564. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 56.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity. Hepatology. 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 57.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 58.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 60.Memon RA, Grunfeld C, Feingold KR. TNF-alpha is not the cause of fatty liver disease in obese diabetic mice. Nat Med. 2001;7:2–3. doi: 10.1038/83316. [DOI] [PubMed] [Google Scholar]
- 61.Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–1952. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- 62.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 63.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 64.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 66.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 67.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 69.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin. Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 72.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 73.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 74.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 75.Kim WR, Poterucha JJ, Porayko MK, Dickson ER, Steers JL, Wiesner RH. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation. 1996;62:1802–1805. doi: 10.1097/00007890-199612270-00021. [DOI] [PubMed] [Google Scholar]
- 76.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 77.Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]


