Abstract
AIM: To investigate the effect of firing noise on gastrointestinal transit and probe its mechanism by measuring the levels of plasma polypeptide hormones.
METHODS: A total of 64 SD rats were randomly divided into a control group and three stimulating groups. Firing noise of different intensity by sub-machine guns was used as inflicting factor. The effect of firing noise on liquid substance gastrointestinal transit and solid substance gastrointestinal transit was observed by measuring the ratio of carbon powder suspension transmitting and barium sticks transmitting respectively. Plasma levels of polypeptide hormones were measured by radio-immunoassay.
RESULTS: The noise accelerated gastrointestinal transit of solid food by more than 80 db;and accelerated gastrointestinal transit of liquid food significantly by more than 120 db. Meantime, plasma levels of plasma motilin (MTL)(157.47±16.08; 151.90±17.08), somatostatin (SS)(513.97±88.77; 458.25±104.30), substance P (SP)(115.52±20.70; 110.28±19.96) and vasoactive intestinal peptide (VIP) (214.21±63.17; 251.76±97.24) remarkably changed also.
CONCLUSION: Within a certain intensity range, the firing noise changes the levels of rat plasma gastrointestinal hormones, but the gastrointestinal transit is still normal. Beyond the range, the noise induces plasma hormone levels disturbance and gastrointestinal transit disorder.
Keywords: Explosive noise, Gastrointestinal transit, Gastrointestinal hormone
INTRODUCTION
Explosive noise may produce an adverse effect on gastrointestinal tract, and there is higher incidence of digestive system disease in war time. Current studies mainly focus on morphological changes of gastrointestinal tract[1], and there is little information available in literature about the effect of firing noise on the gastrointestinal transit. The aim of this study was to show the effects of explosive noise on gastrointestinal transit, and probe its mechanism by measuring the levels of plasma polypeptide hormones.
MATERIALS AND METHODS
Materials
Healthy SD rats were purchased from Animal Center of the Fourth Military Medical University (FMMU). The ND2 volume level meter was provided by the School of Aerospace Medicine of FMMU. Radioimmunoassay kits were supplied by Naval Radioimmunoassay Technology Center. An FJ-2003/8PS radioimmunity counter and a high-speed and low-temperature centrifuge were used in this experiment.
Experimental procedures
After an adaptive phase of 7 d, 64 SD male rats were randomly divided into 4 groups: Group A, consisting of 16 rats, which were not stimulated; Group B, which were stimulated with 40 dB noise; Group C, which were stimulated with 80 dB noise; and Group D, which were stimulated with 120 dB noise. Different groups received different intensity noise stimulations respectively. Then 8 rats of each group (including Group A) were intragastricly administrated with carbon powder suspension[2,3], anesthetized 20 min later, and then decapitated. Blood was sampled and the ratio of carbon powder suspension transmitting measured. The other 8 rats were intragastricly administrated with barium small sticks[4], anesthetized 10 h later, and decapitated. Blood was sampled in the same way and the ratio of barium sticks transmitting observed under X-ray.
Noise stimulation
After 12-h abrosia, rats were put into sound-proof room. Firing noise of submachine guns acted as an inducing factor which had been recorded and was played to rats through a loudspeaker at a distance of 20-30 cm for 12 h. Examined with ND2 volume level meter and frequency spectrum analyzer, the intensity of the firing noise was measured as 0 dB (Group A), 40 dB (Group B), 80 dB(Group C) and 120 dB(Group D) respectively and their frequency as 0.25-4.00 kHz.
Liquid substance gastrointestinal transit
Rats were intragastricly administrated with 1 mL carbon powder suspension, consisting of carbon powder (50 g/L), gum arabic (100 g/L) and water(850 g/L), then anesthetized and dissected 20 min later. Small intestine was taken out of abdomen and tiled on the bench. The distance of carbon powder transmission from pyloric sphincter to the end of small intestine was measured, and the ratio of carbon powder suspension transmitting calculated [5,6] (distance of carbon powder transmission/total length of small intestine ×100%).
Solid substance gastrointestinal transit
Rats were intragastricly administrated with 10 barium small sticks (length: 5 mm, diameter: 1 mm), then anesthetized and dissected 10 h later. The sticks were taken out of gastrointestinal tract. Barium small sticks which remained in each segment of the gastrointestinal tract were counted.
Measurement of plasma MTL, SS, VIP and SP
Rats were decapitated to sample the blood. Blood plasma was separated by centrifugation at 3500 r/min at 4 °C, then concentrations of MTL, SS, SP and VIP were tested by radio-immunoassay[7], according to instruction of RIA kits strictly.
Statistical analysis
Analysis of variance was performed to investigate the effects of firing noise on gastrointestinal transit and plasma polypeptide hormone level in rats of four groups. All data were presented as mean ± SD. P < 0.05 was taken as significant.
RESULTS
Liquid substance gastrointestinal transit and plasma peptides levels
Ratios of carbon powder suspension transmitting in groups B and C were 78.1% ± 7.6% and 77.1%± 6.1% respectively, and were not significantly different from that of group A (control group, 77.8% ± 8.8%). Ratio of carbon powder suspension transmitting in group D was 86.7% ± 3.3%, significantly higher than that of group A(P < 0.05). The liquid substance gastrointestinal transit of group D (120 dB noise stimulated group) was accelerated significantly (Figure 1).
Figure 1.
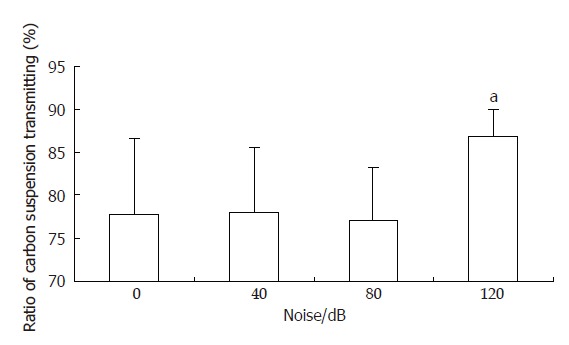
Effect of explosive noise on liquid substance gastrointestinal transit (n = 8, vs 0 dB), aP < 0.05.
At the same time, all of the plasma MTL, SS, SP and VIP concentrations in groups B and C were increased compared with that of group A. In group D, plasma MTL, SS and SP concentrations were increased, but plasma VIP concentration decreased to some extent (Table 1). It could be seen that plasma peptides in groups B and C were increased gradually, but plasma peptides in group D were changed irregularly.
Table 1.
Plasma polypeptide hormones after noise stimulation (mean ± SD, n=8, μg/L)
| Noise/dB | MTL | SP | SS | VIP |
|
0 |
128.0 ± 5.1 |
52.5 ± 20.1 |
184.6 ± 49.6 |
254.3 ± 129.1 |
|
40 |
130.6 ± 15.6 |
74.4 ± 17.4 |
382.8 ± 79.1b |
471.1 ± 145.4a |
|
80 |
133.2 ± 30.5 |
86.6 ± 15.2b |
386.6 ± 59. 6b |
460.6 ± 173.8a |
| 120 | 157.5 ± 16.1b | 115.5 ± 20.7b | 514.0 ± 88.8b | 214.2 ± 63.2 |
aP < 0.05. bP < 0.01 vs 0 dB(control).
Solid substance gastrointestinal transit and plasma peptides levels
Percentage of barium sticks remained in the gastrointestinal tract in group A was 40.6% ± 25.4%, and that of group B was 36.9% ± 22.1%. There was no significant difference between groups A and B. Percentage of barium sticks remained in groups C and D was 16.2% ± 10.8% and 22.5% ± 16.1% respectively, significantly lower than that of group A(control group)(P < 0.05)(Figure 2). There was smaller percentage of barium sticks remained in the gastrointestinal tract, indicating that barium sticks gastrointestinal transmit was faster. So the solid substance gastrointestinal transit of groups C and D (80,120 dB noise stimulated group) was accelerated.
Figure 2.
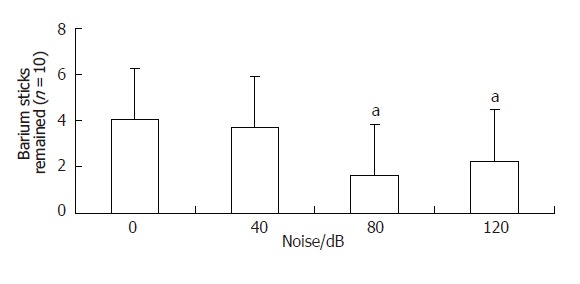
Effect of explosive noise on gastrointestinal transit aP < 0.05 vs 0 dB.
All of the plasma peptide concentrations in group B was increased compared with that of group A. In groups C and D, plasma MTL, SS and SP concentrations were increased, but plasma VIP concentration decreased to some extent(Table 2). It could be seen that plasma peptides in group B were increased stepwise, but plasma peptides in groups C and D were irregularly disturbed.
Table 2.
Plasma polypeptide hormones after noise stimulation (mean±SD, n=8, μg/L)
| Noise/dB | MTL | SP | SS | VIP |
| 0 | 128.0 ± 5.1 | 52.3 ± 23.0 | 308.9 ± 222.8 | 275.5 ± 125.5 |
| 40 | 130.2 ± 8.9a | 82.6 ± 15.7b | 329.3 ± 95.6 | 359.2 ± 227.8 |
| 80 | 134.9 ± 12.6b | 110.3 ± 20.0b | 458.3 ± 104.3a | 251.8 ± 97.2 |
| 120 | 151.9 ± 17.1b | 115.2 ± 20.4b | 503.7 ± 65.3b | 257.8 ± 142.2 |
P < 0.05.
P < 0.01 vs 0 dB (control).
DISCUSSION
There is a higher incidence of digestive system disease in war time than in peace time. Explosive noise is one of important factors that induce human body stress in war time, and it may produce an adverse effect on gastrointestinal tract[8-10]. Liu found that explosive noise could injure gastric mucosa. Our results indicated that explosive noise could accelerate gastrointestinal transit, and that the effect was related to the intensity of the noise. There was no apparent change in gastrointestinal transit(P > 0.05), after stimulated with lower intensity noise; however, not only solid substance but also liquid substance gastrointestinal transits were accelerated significantly(P < 0.05), after stimulated with high level noise.
Gut hormone is a main factor in regulatory mechanism of gastrointestinal motility, and plays an important regulatory role in gastrointestinal function in stress state[11-13]. Gut hormone is generally divided into two categories, erethitic hormone and inhibitive hormone. They are contradictory regulating factors in the blood. When balance between them is lost, the disturbance of gastrointestinal motility occurs[14]. We observed plasma concentrations of two kinds of erethitic braingut peptides and two kinds of inhibitive braingut peptides, after stimulation of rats with different intensity noise, so as to probe the mechanism of explosive noise impacting gastrointestinal transit.
We found that concentrations of all four plasma peptides were increased, when rats were stimulated by low level noise, and the balance between stimulatory and inhibitory gut hormones was normal. When rats were stimulated with high level noise, three of peptides (including MTL, SS and SP) were increased in comparison with normal controls, but plasma VIP was decreased.
It is well known that MTL and SP are erethitic gut hormones. They could promote GI transit[15,16]. Our results showed that plasma MTL and SP concentration increased after stimulation with firing noise. After lower intensity sound stimulations, their plasma levels increased to some extent and after high intensity sound stimulations, their plasma levels increased obviously. In all, we found that plasma erethitic gut hormones concentration was increased and was positively correlated with intensity of noise.
SS exerts depressive effect on GI motility[17]. Previous findings showed that plasma SS level was higher in stress state[18,19]. Similarly, we found that plasma SS concentration increased along with the augmentation of the sound intensity. VIP is another kind of inhibitive gut hormone[20]. In our study, after lower intensity sound stimulation, plasma VIP concentration increased also; but it declined after stimulation by high intensity noise.
Rats, after stimulation by low intensity noise, could keep the GI transit normal, and the balance between the erethitic and inhibitive gut hormones was kept under moderate stress still, which helped to keep GI transmission function normal. At same time, we found that disturbance of GI transmission function occurred after stimulation with high intensity noise, and that the balance between the erethitic and inhibitive gut hormones was lost because of severe stress, which induced GI transmission function disorder. Therefore we presumed that changes of plasma gut hormone level were related to GI transmission function disorder induced by explosive noise and were one of the important underlying reasons.
In summary, our results indicate that explosive noise could induce stress in rats and exert some negative effect upon gastrointestinal transit. After explosive noise stimulation, secretion of many kinds of gut hormone is chaotic, which plays an important role in occurrence of gastrointestinal transmission disorder.
Footnotes
Co-correspondence: Yu-Xin Huang
S- Editors Pan BR and Wang J L- Editor Zhu LH E- Editor Bai SH
References
- 1.Liu GS, Huang YX, Li SW, Pan BR, Wang X, Sun DY, Wang QL. Experimental study on mechanism and protection of stress ulcer produced by explosive noise. World J Gastroenterol. 1998;4:519–523. doi: 10.3748/wjg.v4.i6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittelstadt SW, Hemenway CL, Spruell RD. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J Pharmacol Toxicol Methods. 2005;52:154–158. doi: 10.1016/j.vascn.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Lee HT, Chung SJ, Shim CK. Small intestinal transit does not adequately represent postoperative paralytic ileus in rats. Arch Pharm Res. 2002;25:978–983. doi: 10.1007/BF02977023. [DOI] [PubMed] [Google Scholar]
- 4.Zhan SQ, Luo JY, Gong J, Yang WD, Song CY, Wang SJ. The physiological and pathophysiological study of gastrointestinal transit time. Xi'an Yike Daxue Xuebao. 1998;19:590–592. [Google Scholar]
- 5.Appelbaum BD, Holtzman SG. Restraint stress has no effect on morphine-induced inhibition of gastrointestinal transit in the rat. Physiol Behav. 1985;34:995–997. doi: 10.1016/0031-9384(85)90026-5. [DOI] [PubMed] [Google Scholar]
- 6.Dong DL, Wang QH, Chen W, Fan JJ, Mu JW, Ke J, Yang BF. Contrasting effects of tetraethylammonium and 4-aminopyridine on the gastrointestinal function of mice. Eur J Pharmacol. 2005;509:179–185. doi: 10.1016/j.ejphar.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Demling L, Strunz U. Gastro-intestinal hormones. What remains. Arq Gastroenterol. 1978;15:130–135. [PubMed] [Google Scholar]
- 8.Al Moutaery AR. Effect of centrophenoxine on water-immersion restraint stress- and chemically-induced gastric ulcers in rats. Res Commun Mol Pathol Pharmacol. 2003;113-114:39–56. [PubMed] [Google Scholar]
- 9.Yates DA, Santos J, Söderholm JD, Perdue MH. Adaptation of stress-induced mucosal pathophysiology in rat colon involves opioid pathways. Am J Physiol Gastrointest Liver Physiol. 2001;281:G124–G128. doi: 10.1152/ajpgi.2001.281.1.G124. [DOI] [PubMed] [Google Scholar]
- 10.Beglinger C, Degen L. Role of thyrotrophin releasing hormone and corticotrophin releasing factor in stress related alterations of gastrointestinal motor function. Gut. 2002;51 Suppl 1:i45–i49. doi: 10.1136/gut.51.suppl_1.i45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunnett NW. The stressed gut: contributions of intestinal stress peptides to inflammation and motility. Proc Natl Acad Sci U S A. 2005;102:7409–7410. doi: 10.1073/pnas.0503092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muelas MS, Ramírez P, Parrilla P, Ruiz JM, Pérez JM, Candel MF, Aguilar J, Carrasco L. Vagal system involvement in changes in small bowel motility during restraint stress: an experimental study in the dog. Br J Surg. 1993;80:479–483. doi: 10.1002/bjs.1800800424. [DOI] [PubMed] [Google Scholar]
- 13.Fukudo S, Suzuki J. Colonic motility, autonomic function, and gastrointestinal hormones under psychological stress on irritable bowel syndrome. Tohoku J Exp Med. 1987;151:373–385. doi: 10.1620/tjem.151.373. [DOI] [PubMed] [Google Scholar]
- 14.Plourde V. Stress-induced changes in the gastrointestinal motor system. Can J Gastroenterol. 1999;13 Suppl A:26A–31A. doi: 10.1155/1999/320626. [DOI] [PubMed] [Google Scholar]
- 15.Depoortere I, Van Assche G, Thijs T, Geboes K, Peeters TL. Differential changes in ACh-, motilin-, substance P-, and K(+)-induced contractility in rabbit colitis. Am J Physiol. 1999;277:G61–G68. doi: 10.1152/ajpgi.1999.277.1.G61. [DOI] [PubMed] [Google Scholar]
- 16.Liu CQ, Sun T, Li ZX, Liu ZF, Fu SF, Shen JL. [Plasma polypeptide hormone levels in rats with gastric ulcer after exposure to intense noise] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21:48–50. [PubMed] [Google Scholar]
- 17.Eisenbraun J, Ehrlein HJ. Effects of somatostatin on luminal transit and absorption of nutrients in the proximal gut of minipigs. Dig Dis Sci. 1996;41:894–901. doi: 10.1007/BF02091528. [DOI] [PubMed] [Google Scholar]
- 18.Gui X, Pan G, Ke M. [Potential role of gut peptides in stress-induced colonic motor disorder] Zhonghua Yi Xue Za Zhi. 1997;77:31–34. [PubMed] [Google Scholar]
- 19.Wang JJ, Huang YX, Guo QD, Qin M, Gao W, Wang QL. Protective and therapeutic effects of electroacupuncture on gastric motor disorders and acute gastric mucosal lesions under psychological stress in rats. Disi Junyi Daxue Xuebao. 2001;22:806–810. [Google Scholar]
- 20.Li LS, Qu RY, Guo H, Wang W, Meng Y. Changes of SP and VIP in blood and ileum of cool stress rats. Shoudu Yike Daxue Xuebao. 2002;23:113–114. [Google Scholar]


