Abstract
AIM: To examine the histological and immunohistochemical findings of biopsy specimens taken from the major duodenal papilla of autoimmune pancreatitis (AIP) patients.
METHODS: The major duodenal papilla in the resected pancreas of 3 patients with AIP and of 5 control patients [pancreatic carcinoma (n = 3) and chronic alcoholic pancreatitis (n = 2)] was immunostained using anti-CD4-T cell, CD8-T cell and IgG4 antibodies. Forceps biopsy specimens taken from the major duodenal papilla of 2 patients with AIP and 5 control patients with suspected papillitis were prospectively taken during duodenoscopy and immunohistochemically examined.
RESULTS: Moderate or severe lymphoplasmacytic infiltration including many CD4-positive or CD8-positive T lymphocytes and IgG4-positive plasma cells (≥10/HPF), was observed in the major duodenal papilla of all 3 patients with AIP. The same findings were also detected in the biopsy specimens taken from the major duodenal papilla of 2 patients with AIP, but in controls, there were only a few (≤3/HPF) IgG4-positive plasma cells infiltrating the major duodenal papilla.
CONCLUSIONS: An abundant infiltration of IgG4-positive plasma cells is specifically detected in the major duodenal papilla of patients with AIP. Although this is a preliminary study, IgG4-immunostaining of biopsy specimens taken from the major duodenal papilla may support the diagnosis of AIP.
Keywords: Autoimmune pancreatitis, IgG4, Major duodenal papilla
INTRODUCTION
Autoimmune pancreatitis (AIP) is a unique form of pancreatitis in which autoimmune mechanisms are involved in the pathogenesis. It is characterized by irregular narrowing of the main pancreatic duct, enlargement of the pancreas, increased levels of serum γ globulin or IgG, presence of autoantibodies, and responsiveness to steroid therapy[1,2]. Histological findings of the pancreas of AIP are also characteristic, and include dense lymphoplasmacytic infiltration with fibrosis[3]. Recently, it was reported that serum concentrations of IgG4 are significantly and specifically raised in patients with AIP[4]. However, the sensitivity of raised serum IgG4 concentrations is 67%[5]- 68%[6] in some reports.
AIP occurs predominantly in elderly males and frequently presents as obstructive jaundice[7]. This presentation is also typical of pancreatic carcinoma. In typical cases of AIP that show diffuse change of the pancreas, the diagnosis can be easily made based on the combination of computed tomography (CT) and endoscopic retrograde cholangiopancreatography (ERCP) findings. However, in segmental mass-forming cases, the differentiation between AIP and pancreatic carcinoma remains difficult. The “double-duct sign”, representing stricture in both the pancreatic and bile ducts, is often found in patients with pancreatic head carcinoma. This finding, however, is also frequently observed in AIP[7], because the inflammatory process compresses both the main pancreatic duct and the distal common bile duct[3]. As most patients with AIP respond to oral steroid therapy[2], an accurate diagnosis of AIP can avoid unnecessary laparotomy or pancreatic resection. On the other hand, histopathological approach to the pancreas is sometimes difficult.
We previously reported that IgG4-positive plasma cells abundantly infiltrated various organs, as well as the pancreas of a patient with AIP, and that an abundant infiltration of IgG4-positve plasma cells was not observed in the organs of patients with pancreatic carcinoma or chronic alcoholic pancreatitis[3,8]. In order to find a useful new method to diagnose AIP on the basis of histological and immunohistochemical studies of the resected pancreas, we prospectively examined the histological and immunohistochemical findings of biopsy specimens obtained from the major duodenal papilla of AIP patients.
MATERIALS AND METHODS
The major duodenal papilla in the resected pancreas of 3 patients with AIP who underwent pancreatoduodenectomy due to suspected pancreatic carcinoma was histologically examined. The specimens were fixed in 10% formaldehyde. Serial sections were cut from paraffin-embedded tissue blocks, and immunostained using anti-CD4-T (Novocastra, Newcastle upon Tyne, UK), CD8-T (DakoCytomation, Glostrup, Denmark) cell subsets, as well as IgG4 (The Binding Site, Birmingham, UK) antibodies with avidin-biotin-peroxidase complex (ABC). The number of immunohistochemically identified cells per high power field (HPF) in each section was counted.
We recently treated 2 patients with AIP, a 73-year-old male and a 60-year-old female. Both patients had obstructive jaundice due to stenosis of the lower bile duct. Segmental narrowing of the main pancreatic duct was observed in the pancreatic head on ERCP, and focal enlargement of the pancreatic head was detected on CT. Serum IgG4 concentrations were elevated to 325 mg/dL in the male and 825 mg/dL in the female. The major duodenal papilla was normal on duodenoscopy during ERCP. Forceps biopsy specimens from the major duodenal papilla were taken during duodenoscopy. Histological and immunohistochemical studies were done on these biopsy specimens. After the biopsy was taken, steroid therapy was given to both patients, who showed marked responsiveness both morphologically and serologically. In 1 patient, rebiopsy from the major duodenal papilla was done after steroid therapy.
Histological and immunohistochemical studies were also done on control specimens. Controls consisted of the major duodenal papilla specimens of the pancreases resected by pancreatoduodenectomy for pancreatic head carcinoma (n = 3) and chronic alcoholic pancreatitis (n = 2), as well as endoscopically biopsied specimens taken from the major duodenal papilla of patients with suspected papillitis (n = 5).
RESULTS
Moderate or severe lymphoplasmacytic infiltration was observed in the major duodenal papilla of all 3 patients with AIP. Immunohistochemically an abundant infiltration of CD4- or CD8-positive T lymphocytes and IgG4-positive plasma cells (≥10/HPF) was observed in these 3 major duodenal papillae. Moderate or severe lymphoplasmacytic infiltration which included many CD4- or CD8-positive T lymphocytes and IgG4-positive plasma cells (≥10/HPF) was also observed in the biopsy specimens taken from the major duodenal papilla of 2 AIP patients (Figures 1 and 2). Although infiltration of CD4- or CD8-positive T lymphocytes was detected to some extent in the major duodenal papilla of controls, there were few IgG4-positive plasma cells infiltrating the major duodenal papilla of controls (≤3/HPF). The abundant infiltration of CD4- or CD8-positive T lymphocytes and IgG4-positive plasma cells disappeared in the biopsy specimen taken from the major duodenal papilla of 1 patient after steroid therapy.
Figure 1.
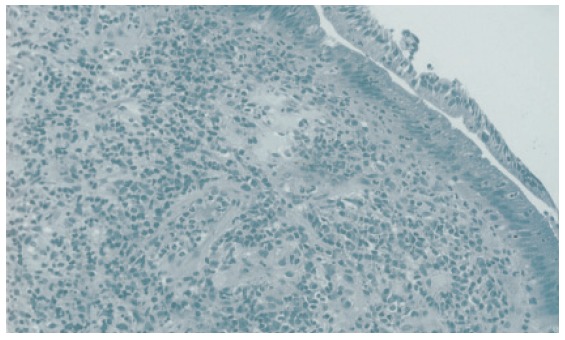
Histology of biopsy specimen taken from the major duodenal papilla of a patient with autoimmune pancreatitis showing severe lymphoplasmacytic infiltration.
Figure 2.
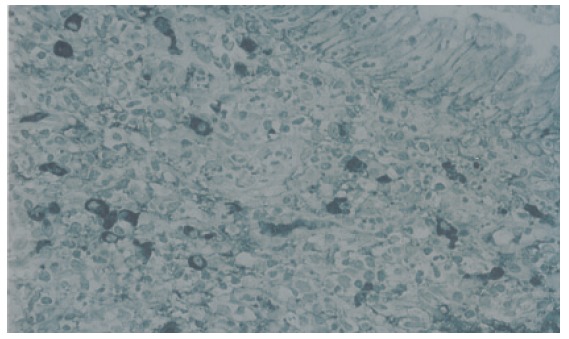
IgG4-immunostaining of biopsy specimen taken from the major duodenal papilla of a patient with autoimmune pancreatitis showing an abundant infiltration of IgG4-positive plasma cells (≥10/HPF).
DISCUSSION
Chronic pancreatitis and several other pancreaticobiliary diseases may be associated with inflammation of the major duodenal papilla, histologically showing infiltration of neutrophils or lymphocytes, or fibrosis[9]. In 2002, Unno et al[10]reported that a swollen major duodenal papilla was detected in 41% of 17 patients with AIP, and many infiltrating T lymphocytes were present in the biopsy specimens taken from the swollen papillary tissue. In 2004, Sahin et al [11]reported that dense T-lymphocytic infiltration was present in the resected major duodenal papilla of 2 patients with AIP. In the present study, dense infiltration of CD4- or CD8-positive lymphocytes was also detected in the resected major duodenal papilla of 3 patients with AIP. Furthermore, an abundant infiltration of IgG4-positive plasma cells was detected in the major duodenal papilla of these patients and was not observed in the major duodenal papilla of controls.
Our previous immunohistochemical studies[3,8] of resected pancreases taken from AIP patients showed that the infiltrating inflammatory cells consisted of CD4- or CD8-positive T lymphocytes and IgG4-positive plasma cells, and that an abundant infiltration IgG4-positive plasma cells in the pancreas was not detected in other diseases. In addition, an abundant infiltration of IgG4-positive plasma cells was detected in various organs of patients with AIP, including peripancreatic retroperitoneal tissue, biliary tract, salivary glands, lymph nodes, and others. We therefore proposed the existence of a novel clinicopathological entity, an IgG4-related systemic disease characterized by extensive IgG4-positive plasma cell infiltration of organs together with CD4- or CD8-positive T lymphocytes[8]. Based on this concept, the dense infiltration of IgG4-positive plasma cells along with CD4- or CD8-positive lymphocytes that are detected in the major duodenal papilla of patients with AIP seems to be induced by the same mechanism as is operative in the pancreas.
These findings led us to do a prospective immunohistochemical study using an anti-IgG4 antibody to study the biopsy specimens taken from the major duodenal papilla of AIP patients. An abundant IgG4-positive plasma cell infiltration was detected in the biopsy specimens taken from the non-swollen major duodenal papilla of 2 AIP patients, and was not detected in the biopsy specimens taken from controls. Of note, the abundant infiltration of IgG4-positive plasma cells disappeared after steroid therapy. Although the number of examined cases is small, IgG4-immunostaining of biopsy specimens taken from the major duodenal papilla may be useful to support the diagnosis of AIP.
In conclusion, an abundant infiltration of IgG4-positive plasma cells was detected in the major duodenal papilla of patients with AIP. Although this is a preliminary study, IgG4-immunostaining of biopsy specimens taken from the major duodenal papilla may be useful to support the diagnosis of AIP.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Zhang Y
References
- 1.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Morphological changes after steroid therapy in autoimmune pancreatitis. Scand J Gastroenterol. 2004;39:1154–1158. doi: 10.1080/00365520410008033. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Okamoto A, Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas. 2005;31:28–31. doi: 10.1097/01.mpa.0000167000.11889.3a. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki K. Autoimmune pancreatitis is increasing in Japan. Gastroenterology. 2003;125:1557–1558. doi: 10.1016/j.gastro.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Chang HS, Han J, Kim JS, Min YI. The clinical significance of papillitis of the major duodenal papilla. Gastrointest Endosc. 2002;55:877–882. doi: 10.1067/mge.2002.124559. [DOI] [PubMed] [Google Scholar]
- 10.Unno H, Saegusa H, Fukushima M, Hamano H. Usefulness of endoscopic observation of the main duodenal papilla in the diagnosis of sclerosing pancreatitis. Gastrointest Endosc. 2002;56:880–884. doi: 10.1067/mge.2002.129605. [DOI] [PubMed] [Google Scholar]
- 11.Sahin P, Pozsár J, Simon K, Illyés G, László F, Topa L. Autoimmune pancreatitis associated with immune-mediated inflammation of the papilla of Vater: report on two cases. Pancreas. 2004;29:162–166. doi: 10.1097/00006676-200408000-00012. [DOI] [PubMed] [Google Scholar]


