Abstract
AIM: To investigate the effect of Qinggan Huoxuefang (QGHXF) on improvement of liver function and pathology in rats, and to analyze the mechanism.
METHODS: Wistar rats were divided into three groups at random: normal control group (12),micro-amount carbon tetrachloride group (CCl4)(12) and model group A (60). The model group A was ingested with the mixture (500 mL/L alcohol, 8 mL/kg per day; corn oil, 2 mL/kg per day; pyrazole, 24 mg/kg per day) once a day and intraperitoneal injections of 0.25 mL/kg of a 250 mL/L solution of CCl4 in olive oil twice a week for 12 wk. The CCl4 group received intraperitoneal injections only. At the end of 8 wk the model group A (60) was divided into 5 subgroups: model group, Xiaochaihu Chongji (XCH) group, QGHXF high dose group, moderate dose group and low dose group, and were given the drugs respectively. At the end of 12 wk, all the rats were killed and blood samples collected, as well as liver tissue. Blood samples were used for evaluation of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (γ-GT). Liver specimens were obtained for routine HE, apoptosis gene array and flow cytometry analysis.
RESULTS: A liver fibrosis animal model was successfully established. Fibrosis was obviously reduced in QGHXF high dose group, and no fibrosis formed in CCl4 group. Compared with model group the QGHXF group and XCH group could obviously decrease the level of ALT, AST, ALP, and GGT (P < 0.05). QGHXF high dose group was better than XCH group in ALT (615 ± 190 vs 867 ± 115), and AST(1972 ± 366 vs 2777 ± 608). Moreover, QGHXF could reduce liver inflammation, fibrosis-induced hepatic stellate cell (HSC) apoptosis and regulate apoptosis gene expression. The HSC apoptosis rates of QGHXF groups were 22.4 ± 3.13, 13.79 ± 2.26 and 10.07 ± 1.14, higher than model group, 6.58±1.04 (P < 0.05). Compared to model group, 39 genes were up-regulated, 11 solely expressed and 17 down-regulated in high dose group.
CONCLUSION: QGHXF can improve liver fibrosis and induce HSC apoptosis.
Keywords: Qinggan Huoxuefang, Alcoholic liver fibrosis, Apoptosis, Gene array
INTRODUCTION
Alcohol abuse and dependence have been a public problem all over the world. In the United States, 45 billion dollars were spent on alcoholic intemperance and its related problems[1,2]. In recent years morbidity rate of alcoholic liver diseases (ALD) has risen rapidly. It has become the second liver disease after viral hepatitis in China[3,4]. Liver disease in the alcoholics is due not only to malnutrition but also to ethanol’s hepatotoxicity linked to its metabolism by means of the alcohol dehydrogenase and cytochrome P450 2E1 (CYP2E1) pathways and the resulting production of toxic acetaldehyde[5-8]. Alcoholic liver disease is a major source of alcohol-related morbidity and mortality. Heavy drinkers and alcoholics may progress from fatty liver to alcoholic hepatitis to cirrhosis, and it is estimated that 10 to 15 percent of alcoholics will develop cirrhosis[9]. Alcoholic liver fibrosis (AF) is one of the alcoholic liver diseases. It is the excessive accumulation of extracellular matrix (ECM) proteins including collagen that occurs in chronic liver diseases. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension and often requires liver transplantation[10]. However, recent evidence indicates that even advanced fibrosis is reversible[11-13]. Thus it has become the focus of researches. Fibrogenesis in human ethanol injury is due to the activity of stellate cells, Kupffer cells, and to a lesser extent, to endothelial cells[14]. Above all of the factors, hepatic stellate cell (HSC) is the key to fibrosis. Activated HSCs are the source of collagen deposition in the liver[15]. This study aimed to explore the mechanism of QGHXF (a traditional Chinese herb) on AF through examination of liver function, and histopathology, detection of HSC apoptosis and gene array, in an effort to search for the experimental basis for traditional Chinese medicine in AF therapy.
MATERIALS AND METHODS
Materials
Jianzhuang Alcohol (520) and corn oil were purchased from Lianhua Supermarket. Formaldehyde, 400 g/L, olive oil, carbon tetrachloride, hematoxylin and eosin were supplied by Shanghai Chemicals Company. The test kit of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (γ-GT), and alkaline phosphatase (ALP) were purchased from Shanghai Rongsheng Biotech Co. Ltd. QGHXF (bupleurum root 9 g, scutellaria root 9 g, red sage root 15 g, carapax trionycis 9 g, Radix puerariae 15 g), concentrated to 2.6 kg/L were processed by Department of Pharmacy, Longhua Hospital, Shanghai University of Traditional Chinese Medicine. XCH was from Shanghai Shikang Technology Co. Ltd (ZZ-3484 No.081006). In situ cell death detection kit (Cat. No. 1684817)and DAB substrate (Cat. No.1718096) were purchased from Roche Diagnostics Ltd.
Animal preparation[16-20]
Eighty-four specific pathogen free (SPF) male Wistar rats weighing 150 ± 20g were purchased from Shanghai Experimental Animal Co. Ltd. All the rats were randomly assigned into three groups: normal group(12),micro-amount CCl4 group(12)and model group A (60). The model group A was ingested the mixture (500 mL/L alcohol, 8 mL/kg per day; corn oil, 2 mL/kg per day; pyrazole, 24 mg/kg per day) once a day and intraperitoneal injections of 0.25 mL/kg of a 25 % solution of CCl4 in olive oil twice a week for 12 wk. The CCl4 group received intraperitoneal injections only. Normal group was ingested saline (10 mL/kg per day). At the end of 8 wk the model group A (60) was divided into 5 subgroups: model group, XCH group, QGHXF high dose group, moderate dose group and low dose group, and drugs were given respectively. Model group was given saline (5 mL/kg per day); QGHXF high dose group was given QGHXF 2.6 g/kg, 5 mL/kg; moderate dose group was given 1.3 g/kg, 5 mL/kg; low dose group was given 0.65 g/kg, 5 mL/kg; XCH group was given XCH (3 g/kg per day,); and model group and CCl4 group were given saline. At the end of 12 wk all the rats were anaesthetized and killed. Blood sample and liver tissue specimens were collected. A portion of liver was fixed for histopathology. Another portion was for flow cytometry assay and the remaining tissue stored at -80˚C until assayed.
Serum ALT, AST, ALP and GGT determination
ALT, AST, ALP and GGT were evaluated in samples of serum obtained at the end of the experiment. The activity was evaluated by using a commercial clinical test kit (Shanghai Rongsheng Biotech Co. Ltd.) according to instructions of the kit.
Histopathology and estimation criterion
Liver tissue was instantly fixed in 40 g/L phosphate bu-ffered formaldehyde, processed by routine histology pro-cedures, embedded in paraffin, cut into 5 μm section and mounted on the slide. The samples were stained with hematoxylin and eosin (HE) for histopathological examination[21]. Histopathological criteria refer to the report[22].
Flow cytometry assay[23,24]
Six liver specimens were obtained randomly from each group. The liver tissue was rapidly removed, weighed, and placed into 10 mL of ice-cold PBS containing 2 g/L bovine serum albumin (BSA) (Sigma), 0.01 mol/L EDTA, and 10 g/L deoxyribonuclease I (Sigma), and then the tissue was broken in a glass homogenizer and passed through a 40 μm nylon cell stainer (Becton Dickinson). The suspension was centrifuged at 500 g for 10 min at room temperature. The pellet was resuspended in 500 μL of PBS with BSA and transferred into a fresh tube. The cells obtained by the above method were fixed with ice-cold 700 mL/L ethanol in PBS at 4˚C for 8 h, then incubated with RNase (20 mg/L) for 30 min at 37˚C and labeled with propidium iodide (50 mg/L). DNA contents were measured by an FACSCalibur cytometer (Becton Dickinson). Multicycle software (CellQuest software, Becton Dickinson) was used to produce histograms of DNA content frequency. Sub-diploid DNA peaks were quantified from the DNA content data.
The tests were completed in Flow Cytometry Labo-ratory of Shanghai Institute for Biological Sciences, Chin-ese Academy of Sciences.
Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay
Tissue sections were dewaxed and rehydrated according to standard protocols. They were incubated for 15-30 min at 21-37 °C with proteinase K working solution (10-20 mg/L in 10 mmol/L Tris/HCl, 7.4-8). Slides were rinsed twice with PBS. Fifty microliter TUNEL reaction mixture was added to the sample tissue and incubated for 60 min at 37 °C in a humidified atmosphere in the dark. Slides were rinsed 3 times with PBS. Fifty microliter Converter-POD was added to the sample. Slides were incubated in a humidified chamber for 30 min at 37 °C, rinsed 3 times with PBS, then DAB substrate was added and counterstained with hematoxylin, mounted under glass coverslip and analyzed under light microscope.
Apoptosis gene array[25-29]
Three liver specimens were taken from each group of QGHXF high dose group, model group and normal group, then homogenized and mixed for apoptosis gene array, including RNA isolation, RNA yield and quality assessment, probe synthesis, hybridization, chemiluminescent detection, image and data acquisition and analysis. The data were analyzed and calculated by Gearray Analyzer software.
Statistical analysis
Data were expressed as mean ± SD and analyzed by the ANOVA and post-hoc test. P < 0.05 was regarded as statistically significant.
RESULTS
Rat condition
When the rats were given alcohol they became excited and ran around the cage. After that they could not walk, and at last fell into a profound sleep. As the time prolonged spoor time changed from 1~2 h to about 5 h. The weight of rats fell obviously and rats maintained cachexia. Conditions of the rats of QGHXF groups and XCH group were better than those of model group and CCl4 group.
Liver function
Serum levels of ALT, AST, ALP, and γ-GT in model group were higher than those in normal group. All the indexes were improved in each medicine control group (P < 0.05 or 0.01), especially in QGHXF high dose group each index was better than that in XCH group. Details of the data are shown in Table 1.
Table 1.
Impact of QGHXF on ALT, AST, ALP, and -GT in AF rats (mean±SD)
| Group | n | ALT (nkat/L) | AST (nkat/L) | ALP (nkat/L) | GGT (nkat/L) |
| Norma | 12 | 464 ± 90 b | 1156 ± 2 50b | 1065 ± 315b | 360 ± 98b |
| CCl4 | 12 | 674 ± 172 | 1678 ± 293a | 1695 ± 406a | 748 ± 242a |
| Model | 10 | 926 ± 154 | 3344 ± 330 | 2806 ± 639 | 1376 ± 215 |
| Low dose | 11 | 747 ± 113 | 2552 ± 388b | 1748 ± 462a | 982 ± 236a |
| Moderate dose | 10 | 718 ± 156 | 2215 ± 650b | 1632 ± 502a | 869 ± 303a |
| High dose | 10 | 615 ± 190bc | 1972 ± 366bc | 1570 ± 497a | 770 ± 240b |
| XCH | 10 | 867 | 2777 ± 608a | 2413 ± 609a | 768 ± 292b |
P < 0.05,
P < 0.01 vs model group;
P < 0.05 vs XCH.
Effects of QGHXF on liver pathology
The normal group showed normal lobular architecture with central veins and radiating hepatic cords (Figure 1A). The establishment of the model group was successful with marked fatty degeneration, slight confluence, inflammatory infiltrates of monocytes, portal inflammation and necrosis, obvious collagen deposition, and decreased density of hepatocytes (Figure 1B). The CCl4 group only developed micro- and moderate steatosis. Inflammation and fibrosis could not be found (Figure 1C). The treatment group (QGHXF high dose group) could markedly improve those pathological parameters. Inflammation and collagen deposition decreased obviously, and steatosis could nearly not be found (Figure 1D).
Figure 1.
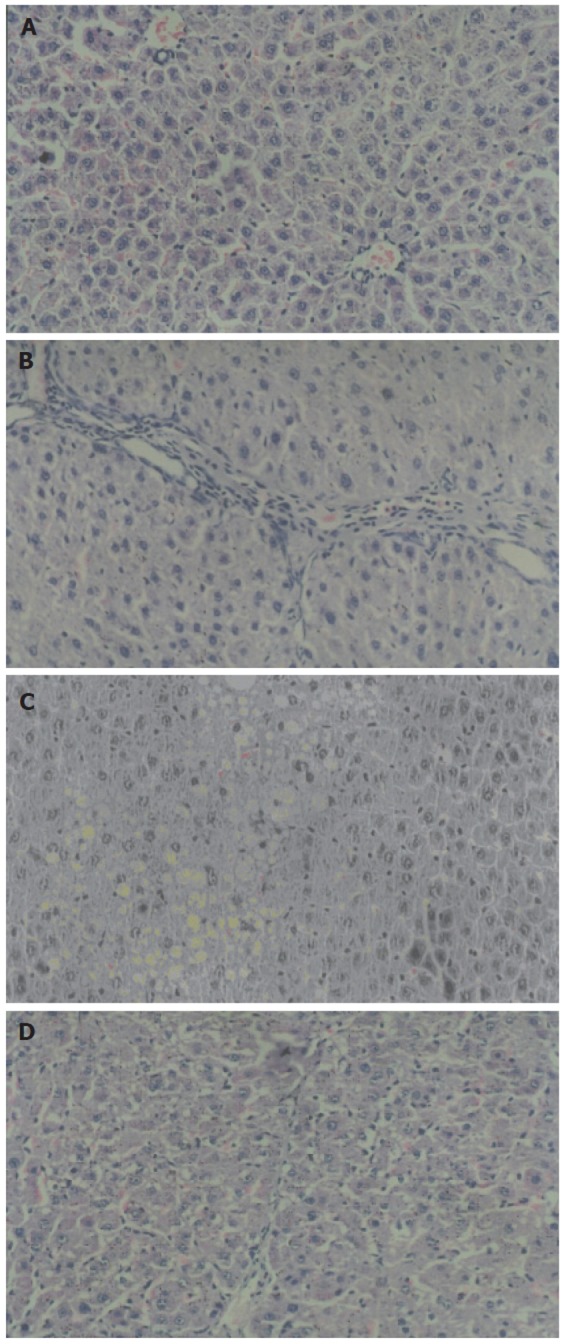
Analysis of liver pathology of each group (HE×200). A: normal group; B: model group; C: CCl4 group; D: high dose group.
Apoptosis of HSC
Apoptosis was measured in the liver tissue with FC-AS (Figure 2). All QGHXF groups had increased apoptosis rate of HSC, and decreased proliferation compared with the model group. The differences were significant (Table 2).
Figure 2.
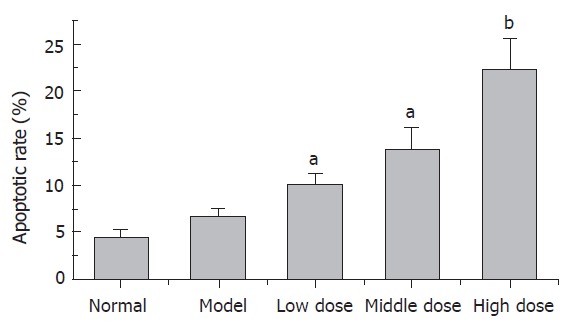
QGHXF induced apoptotic rate of HSC (n = 6, mean ± SD). There were significant differences between model group and QGHXF groups. aP < 0.05 vs model group. bP < 0.01 vs model group.
Table 2.
Effect of QGHXF on proliferation and cell cycle of HSC (n=6; mean±SD)
| Group | Apoptotic rate (%) | Cell cycle | |||
| G0/G1 | S | G2/M | PI value (%) | ||
| Normal | 4.47 ± 0.8 | 79.30 ± 1.03 | 1.69 ± 0.73 | 19.02 ± 1.67 | 20.71 ± 1.03 |
| Low dose | 10.07 ± 1.14a | 37.47 ± 0.68b | 1.46 ± 0.46a | 61.07 ± 0.33b | 62.53 ± 0.68b |
| Moderate dose | 13.79 ± 2.26a | 68.31 ± 0.92a | 2.11 ± 0.46a | 29.58 ± 1.38 | 31.69 ± 0.92a |
| High dose | 22.4 ± 3.13b | 71.25 ± 1.05b | 2.70 ± 0.46 | 26.06 ± 1.47b | 28.75 ± 1.05b |
| Model | 6.58 ± 1.04 | 64.55 ± 1.53 | 3.69 ± 0.66 | 31.76 ± 0.87 | 35.45 ± 1.53 |
Proliferative index = 100 %×(S+G2/M)/( G0/G1+S+G2/M).
P < 0.05,
P < 0.01 vs model group.
TUNEL assay
TUNEL assay demonstrated standard apoptotic HSC in space of Disse and collagenous fibers in high dose group (Figure 3A), and in model group apoptotic hepatocytes could be seen (Figure 3B). There were no obvious apo-ptotic cells in normal group (Figure 3C).
Figure 3.
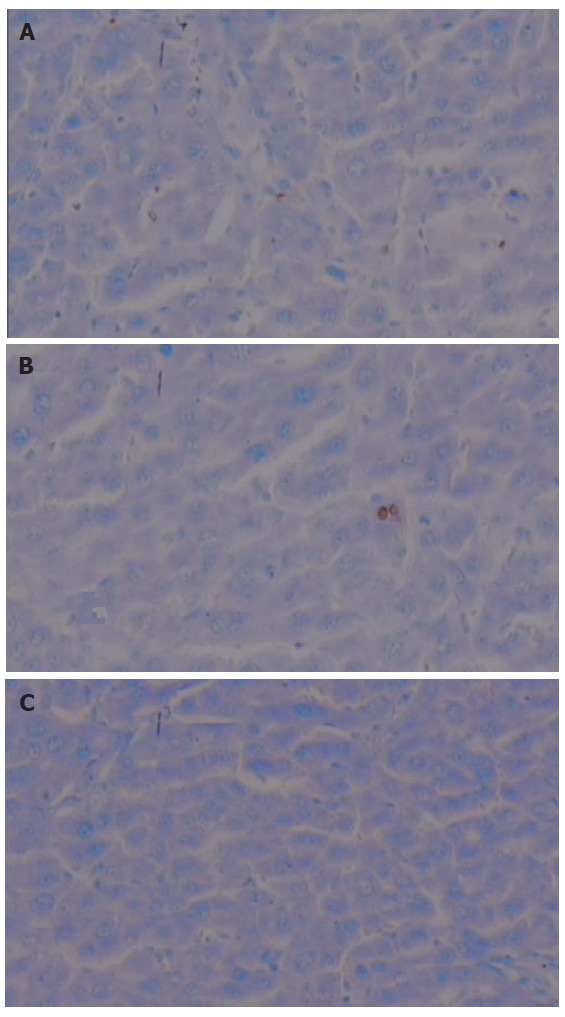
TUNEL assay of each group. A: High dose; B: Model; C: Normal; Counterstained with hematoxylin ( original magnification, ×400).
Gene array analysis
There were 112 genes in the apoptosis chip, and difference was defined when the same gene ratio was more than 2 or less than 0.5 between different groups. Compared to model group 39 genes were up-regulated (ratio >2) in high dose group, 11 solely expressed and 17 down-regulated (ratio < 0.5). Compared with normal group 16 genes were up-regulated (ratio > 2) in model group, 6 solely expressed and 35 down-regulated(ratio < 0.5). Compared with normal group 38 genes were up-regulated (ratio > 2) in high dose group , 9 solely expressed and 21 down-regulated (ratio < 0.5). These different expression genes were mainly of TNF receptor family, Bcl-2 family, caspase family, and p53 and ATM pathway. Moreover, in model/normal group chip, only a few genes were consistent with those of high/model group chip, and they were Bad, Bak, Bik, NAIP1, 4-1BBL, TRIP (up-regulated) and TRAF2 (down-regulated). However, in high/model group compared with high/normal group, the up-regulated genes were nearly the same. The unique expressive genes in high/model group were Bar, Bcl-x, Bid, Casper, Caspase-7, chk1, DAPkinase, TNFb Mcl-1, OPG, FASL, and CD30L. In high/normal group they were Apaf-1, ATM, Bax, Bim, Bruce RAD53/chk2, TNFR1, TNFS, F11, and P53. Among down-regulated genes one fourth were similar. They were Caspase-1, NOP30-like, RIP, TNFRF11A, and TRAF2. In comparison of model/normal group with high/normal group we found the up-regulated gene expression trend resembled the former result. The same genes were Bcl-2, hrk, IAP2, IAP3 Caspase-11, Caspase-14, and CD30. Down-regulated genes only expressed in high/normal group were CRAF1, CD27, TNFRF11A, RIP, NOP30-like, and Caspase-1 (Figure 4).
Figure 4.
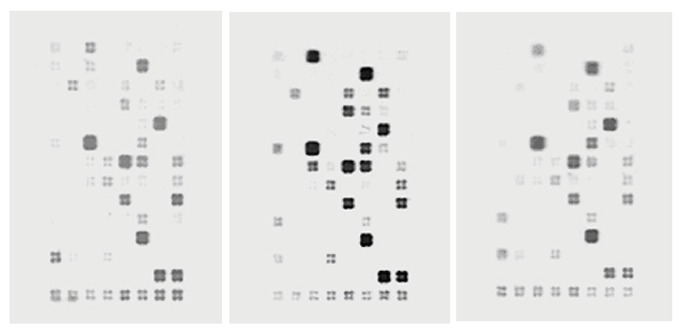
Gene expression profile of each group.
DISCUSSION
In contrast with the traditional view that cirrhosis is an irreversible disease, recent evidence indicates that even advanced fibrosis is reversible[11] . It has been demonstrated that apoptosis is the major mechanism by which activated HSCs are removed during recovery from fibrosis[30-32]. As a prophase of AC the reversibility of AF has been repor-ted[33]. The toxicity of ethanol, acetaldehyde, reactive ox-ygen and other metabolic products are the main causes of AF. These factors lead to hepatocyte inflammation, necrosis and apoptosis. AF was historically thought to be a passive and irreversible process due to the collapse of the hepatic parenchyma and its substitution with a colla-gen-rich tissue. Currently, it is considered a model of the wound-healing response to chronic liver injury. HSCs comprise 15 % of the total number of resident liver cells, but they play a very important role in the course of AF[34]. They have been identified as the main collagen-producing cells in the liver. Following chronic injury, HSCs activate or transdifferentiate into myofibroblast-like cell, produce large amounts of extracellular matrix (ECM) and prevent ECM degradation[34,35]. So it is beneficial to induce HSC apoptosis in order to reduce its proliferation and ECM accumulation.
The aim of this study was to explore the mechanism of QGHXF on AF through induction of HSC apoptosis. According to TCM theory, “alcohol is pungent and hot”. “Jingyue Quanshu” has already pointed out: if a man drinks too much he will be “Shuigu”. Through our long-term clinical practice and epidemiological research we find dampness, heat and gore are the major pathogenesis of ALD, which is in consistent with TCM theory. Therefore, we used QGHXF to clear dampness and heat of the liver and eliminate the gore of the blood. There are five components in QGHXF: bupleurum root, scutellaria root, red sage root, Carapax trionycis, and Radix puerariae. Bupleurum root (bitter and pungent in flavor, slightly cold in property, acting on liver and gallbladder channel) can expel pathogenic factors from the exterior to reduce fever, sooth the depressed liver and invigorate the spleen-yang. Scutellaria root (bitter in flavor, cold in property, acting on the lung, gallbladder, stomach and large intestine channels) can clear away heat and dampness, purge fire, remove toxins from the body, stop bleeding, and prevent miscarriage. Red sage root (bitter in flavor, slightly cold in property, acting on the heart, pericardium and liver channel) can invigorate blood circulation, remove blood stasis, cool the blood, treat carbuncles and tranquilize the disturbed mind by nourishing the blood. Radix puerariae (sweet and pungent in flavor, cool in property, acting on the spleen and stomach channels) can dispel pathogenic factors from the superficial muscles to allay fever, promote the production of fluid to quench thirst, promote the eruption of measles and invigorate the spleen-yang to stop diarrhea. carapax trionycis (salty in flavor, slightly cold in property, acting on the liver and kidney channels) can nourish yin and suppress hyperactive yang, reducing fever and resolving hard lumps. So the QGHXF can clear dampness and heat of the liver and eliminate the gore of the blood.
In this experiment the levels of ALT, AST, GGT, and ALP were obviously decreased in XCH group and each of QGHXF groups(P < 0.05) and QGHXF high dose group had the best effect. Moreover, QGHXF high dose group had better effect than XCH group on ALT and AST(P < 0.05). In addition, histopathology proved the effect of the drug. In model group we could see marked fatty degeneration, slight confluence, inflammatory infiltrates of monocytes, portal inflammation and necrosis, obvious collagen deposition, fibrosis and hepatocyte loosening. The treatment group (QGHXF groups) could markedly improve those pathological changes. Inflam-mation and collagen deposition decreased obviously, and steatosis could nearly not be seen. To clarify that the fibrosis was caused by alcohol but not CCl4 , we established the CCl4 group in which only micro- and moderate steatosis were found, while inflammation and collagen deposition were not found. It suggested that alcohol was the real reason for fibrosis. To analyse mechanism of the effect we used FCAS and TUNEL assay. The results showed QGHXF groups could not only increase apoptosis rate of HSC but also inhibit it from proliferation. It partly proved our hypothesis that QGHXF could induce HSC apoptosis to reverse liver fibrosis. However, the HSC proliferation rate of low dose group was higher than model group, the reason for which needs to be further studied.
What excited us was, from the FCM analysis, the double regulating function of Chinese medicine. Considering the multi- target effect of Chinese medicine we selected a gene chip for further investigation. According to gene chip result, the gene expression of p53 and ATM pathway, Caspase family, Bcl-2 family, TNF receptor family, death effector domain family all changed in each group. It meant either alcohol or drug could affect the apoptosis gene expression of liver. As a mixed cell tissue of liver, the types and apoptosis pathway of apoptotic cells were so different that both up-regulated and down-regulated genes in model/normal groups were quite different from that of high/model groups. As we all know, alcohol can induce hepatocytic apoptosis so many apoptotic genes expressed in model/normal group, but these genes were not expressed in high/model group. Thus we deduced the types of apoptotic cells were not the same, and the result of TUNEL also supported this point.
By combination of the TUNEL results with that of FCM analysis (apoptotic rate of HSC was the lowest in model group, while it was the highest in high dose group), we could conclude that QGHXF can induce HSC apoptosis, prevent hepatocytic apoptosis and necrosis. As regards which pathway plays the key role in HSC apoptosis more profound studies are needed. QGHXF has been proven effective on AF from this experiment.
ACKNOWLEDGEMENTS
We thank Professor Liu and Mr Zhang from School of Phar-macy, East China University of Science and Technology for their help, as well as teachers of Shanghai Institutes for Biological Sciences, CAS for FCM analysis.
Footnotes
Supported by Shanghai Rising-Star program, No. 03QMH1410
S- Editor Pan BR L- Editor Zhu LH E- Editor Wu M
References
- 1.Ji G, Wang YQ, Cao CL. [Clinical study on treatment of alcoholic liver disease by qinggan huoxue recipe] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:13–16. [PubMed] [Google Scholar]
- 2.Qiu DK, MA X. Therapy of alcoholic liver disease. Linchuang. Neike Zazhi. 2004;21:77–79. [Google Scholar]
- 3.Wang LS, PEI X. Alcoholic liver disease. Linchuang Huicui. 2001;16:1141–1143. [Google Scholar]
- 4.Ling QH, Qing Du X. Pay more attention to ALD research. Zhonghua. Xiaohua Zazhi. 2001;21:517–518. [Google Scholar]
- 5.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220–231. [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Leo MA, Lieber CS. Lycopene attenuates alcoholic apoptosis in HepG2 cells expressing CYP2E1. Biochem Biophys Res Commun. 2003;308:614–618. doi: 10.1016/s0006-291x(03)01435-9. [DOI] [PubMed] [Google Scholar]
- 8.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 9.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209–219. [PMC free article] [PubMed] [Google Scholar]
- 10.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 12.Fan K, Huang HT, Zhang DZ. [Study on a recombinant keratinocyte growth factor variant in treating experimental rat liver fibrosis] Zhonghua Gan Zang Bing Za Zhi. 2005;13:229–230. [PubMed] [Google Scholar]
- 13.Gu S, Wang PL. [Recent developments in the investigation of anti-liver fibrosis compositions of herbs] Zhonghua Gan Zang Bing Za Zhi. 2005;13:479–480. [PubMed] [Google Scholar]
- 14.Nanji AA, French SW. Animal models of alcoholic liver disease--focus on the intragastric feeding model. Alcohol Res Health. 2003;27:325–330. [PMC free article] [PubMed] [Google Scholar]
- 15.Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, Brenner DA, Rippe RA. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 16.Casey CA, McVicker BL, Donohue TM Jr, McFarland MA, Wiegert RL, Nanji AA. Liver asialoglycoprotein receptor levels correlate with severity of alcoholic liver damage in rats. J Appl Physiol. 2004;96:76–80. doi: 10.1152/japplphysiol.00375.2003. [DOI] [PubMed] [Google Scholar]
- 17.Senthilkumar R, Nalini N. Glycine prevents hepatic fibrosis by preventing the accumulation of collagen in rats with alcoholic liver injury. Pol J Pharmacol. 2004;56:121–128. [PubMed] [Google Scholar]
- 18.Li YL, FU BY, Wang BY, Cui W, Ling H. Comparison of experimental chemical and alcoholic liver fibrosis animal model. Zhongguo Yikedaxue Bao. 2005;34:25–27. [Google Scholar]
- 19.Zhao HC, Fang L, Li JT, Ma AL, Wang TL. Prevention and treatment of acute alcoholical hepatic liver injury with anetholtrithione. Zhongguo. Xinyao Zazhi. 2005;14:853–856. [Google Scholar]
- 20.Zhang Y, Chen SH, Ding W, YU ZH, LI YM. Iron involved in the mechanisms of alcoholic liver disease. Zhejiang Yixue. 2004;26:190–192. [Google Scholar]
- 21.Ronis MJ, Hakkak R, Korourian S, Albano E, Yoon S, Ingelman-Sundberg M, Lindros KO, Badger TM. Alcoholic liver disease in rats fed ethanol as part of oral or intragastric low-carbohydrate liquid diets. Exp Biol Med (Maywood) 2004;229:351–360. doi: 10.1177/153537020422900410. [DOI] [PubMed] [Google Scholar]
- 22.Wang TL. Diagnosis standard and classification of liver pathology. Zhonghua Ganzangbing Zazhi. 2001;9:312–313. [Google Scholar]
- 23.Yuan YZ, Bao Y, Xia L, Zhang YP, Zhang XJ. Study of Kang-laite-induced apoptosis on human pancreatic cancer cells by CDNA microarray. Zhonghua Xiaohua Zazhi. 2004;24:445–455. [Google Scholar]
- 24.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 25.Liu XJ, Yang L, Luo FM, Wu HB, Qiang Q. Association of differentially expressed genes with activation of mouse hepatic stellate cells by high-density cDNA microarray. World J Gastroenterol. 2004;10:1600–1607. doi: 10.3748/wjg.v10.i11.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seth D, Leo MA, McGuinness PH, Lieber CS, Brennan Y, Williams R, Wang XM, McCaughan GW, Gorrell MD, Haber PS. Gene expression profiling of alcoholic liver disease in the baboon (Papio hamadryas) and human liver. Am J Pathol. 2003;163:2303–2317. doi: 10.1016/S0002-9440(10)63587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang H, Xie WF, Zhang ZB, Zhang X, Zhang XR, Chen YX, Yang XJ. Identification of hepatic fibrosis related genes with gene chip. Ganzang. 2003;8:5–8. [Google Scholar]
- 28.Wong CK, Ip WK, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor-induced adhesion molecule expression on eosinophils by p38 mitogen-activated protein kinase and nuclear factor-[kappa] B. Am J Respir Cell Mol Biol. 2003;29:133–147. doi: 10.1165/rcmb.2002-0289OC. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004;34:849–861. doi: 10.1016/j.bone.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–557. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 33.Li ZH, Ye YA, Wang YZ, Liu YH, Li YH, An Y, SU L. The Correlative Study on The Alcoholic Liver Fibrosis and Alcoholic Consumption. Zhongguo Yi Kan. 2005;40:35–36. [Google Scholar]
- 34.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 35.Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]


