Abstract
AIM: To study the effects of obstructive jaundice on liver regeneration after partial hepatectomy.
METHODS: Hepatocyte growth factor (HGF), its receptor, c-Met, vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1) mRNA expression in both liver tissue and isolated liver cells were investigated after biliary obstruction (BO) by quantitative reverse-transcription polymerase chain reaction (RT-PCR) using a LightCycler. Immunohistochemical staining for desmin and α-smooth muscle actin (α-SMA) was also studied. Regenerating liver weight and proliferating cell nuclear antigen (PCNA) labeling index, and growth factor expression were then evaluated after 70% hepatectomy with concomitant internal biliary drainage in BO rats or sham-operated rats.
RESULTS: Hepatic TGF-β1 mRNA levels increased significantly 14 days after BO, and further increased with duration of cholestasis. Meanwhile, HGF and VEGF tended to increase, but was not significant. In cell isolates, TGF-β1 mRNA was found mainly in the hepatic stellate cell (HSC) fraction. Immunohistochemical studies revealed an increased number of HSCs (desmin-positive cells) and activated HSCs (α-SMA-positive cells) in portal areas after BO. In a hepatectomy model, liver regeneration was delayed in BO rats, as compared to sham-operated rats. TGF-β1 mRNA was significantly up-regulated up to 48 h after hepatectomy, and the earlier HGF mRNA peak was lost in BO rats.
CONCLUSION: BO induces HSCs proliferation and activation, leading to up-regulation of TGF-β1 mRNA and suppression of HGF mRNA in livers. These altered expression patterns may be strongly involved in delayed liver regeneration after hepatectomy with obstructive jaundice.
Keywords: Biliary obstruction, Liver regeneration, Hepatocyte growth factor, Transforming growth factor-β, Hepatic stellate cells, Hepatectomy
INTRODUCTION
Recently, major hepatectomy has been performed for treatment of advanced hepatic and biliary carcinomas [1]. However, major hepatectomy associated with obstructive jaundice is often complicated by hepatic failure [2,3], suggesting that biliary obstruction (BO) may influence liver regeneration and cause hepatic failure after major hepatectomy. Although several previous studies regarding liver regeneration after hepatectomy with obstructive jaundice have been reported [4-6], it is not clear how BO affects liver regeneration. Aronson et al [4] reported that extrahepatic cholestasis inhibits liver regeneration after hepatectomy, whereas Mizuno et al [5], demonstrated that it has no effects on liver regeneration. Thus, the effect of BO on liver regeneration after hepatectomy is still open to discussion even in an experimental model. In clinical cases, whether preoperative biliary drainage before surgery is beneficial or not is also a matter of debate.
In the liver regeneration process, several growth factors are reported to play a crucial role in regulation of regeneration by providing either stimulatory or inhibitory signals for hepatocytes [7]. Epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and hepatocyte growth factor (HGF) stimulate DNA synthesis in hepatocytes in vivo and in culture, but HGF is known to be the most powerful mitogen of hepatocytes [8,9]. In liver, HGF is produced by nonparenchymal cells, mainly hepatic stellate cells (HSCs), and acts on hepatocytes in a paracrine manner via its receptor, c-Met [10-12]. Vascular endothelial growth factor (VEGF) is also reported to be the only angiogenic factor that stimulates proliferation of sinusoidal endothelial cells (SECs) [13-15]. On the other hand, transforming growth factor-β1 (TGF-β1) is a potent growth inhibitor of hepatocytes [16-19]. TGF-β1 mRNA levels are very low or undetectable in normal liver, but increase significantly after partial hepatectomy [20-22]. In cell isolates from regenerating normal liver, the TGF-β1 mRNA was relatively abundant in SECs, Kupffer cells, and HSCs [23]. Meanwhile, in a liver injury model, induced by carbon tetrachloride or D-galactosamine administration, TGF-β1 mRNA expression was up-regulated mainly in HSCs [23- 25].
HSCs are known to be located in the space of Disse, below the SECs lining, in close contact to and partially intercalated between hepatocytes, with their long processes extending along sinusoids [26]. HSCs express desmin, a cytokeletal intermediate filament characteristic of muscle cells [27], but once HSCs are activated, they transform into myofibroblast-like cells, and express α-smooth muscle actin (α-SMA). Furthermore, myofibroblast-like cells derived from HSCs produce large amounts of TGF-β1, which stimulates activated HSCs to produce more TGF-β1 in an autocrine manner [28,29]. On the other hand, activated HSCs lose HGF productivity, although HGF is primarily produced from non-activated HSCs [10].
At present, the influence of BO on HSCs phenotype, especially their TGF-β1 expression during cholestasis and their influence after hepatectomy, is not yet determined. In this study, HGF, c-Met, VEGF and TGF-β1 mRNA expression were investigated after BO in both liver tissue and isolated liver cells, by means of in situ collagenase perfusion and counterflow elutriation, to determine potential cellular sources of these growth/inhibitory factors. Immunohistochemical staining with desmin and α-SMA antibody was also studied to evaluate the number of HSCs and their activation status. To determine the effect of BO on liver regeneration, we also investigated changes in hepatic HGF, c-Met, VEGF and TGF-β1 mRNA expression after 70% hepatectomy with concomitant internal biliary drainage in BO rats, and compared them to those in sham-operated rats. Regenerating liver weight and PCNA labeling index were also studied to evaluate the relationship between growth factor expression and liver regeneration after hepatectomy.
MATERIALS AND METHODS
Animals
Male Wister rats (SLC, Inc. Shizuoka, Japan), weighing 200 to 300 g were used in this study. All animals were housed in a temperature- and humidity-controlled environment with a 12-h light dark cycle, and allowed to drink water and eat ad libitum. All surgical procedures were performed under light diethyl ether anesthesia. The operative procedure was carried out using clean, but not sterile technique. Experiments with the animals followed our institution’s criteria for the care and use of laboratory animals in research, which conform to National Institutes of Health guidelines.
Experiment 1
The rats were subjected to BO or sham surgery (sham-operated control). In the BO rats, the extrahepatic bile duct was isolated and a polyethylene tube with an outer diameter of 0.8 mm (Natsume, Tokyo, Japan) was inserted into the extrahepatic bile duct, according to the cut-down technique. The other end of the tube was then ligated to induce BO. In the sham-operated rats, the extrahepatic bile duct was isolated but was not occluded. The liver was carefully excised before surgery, and 14 and 21 d (n = 10 at each time point) after surgery. HGF, c-Met, VEGF and TGF-β1 mRNA levels in liver tissue were investigated by quantitative reverse-transcription polymerase chain reaction (RT-PCR), using a LightCycler (Roche Diagnostics, Mannheim, Germany). Furthermore, to determine potential cellular sources of these growth factors, liver cells (hepatocytes, SECs, Kupffer cells and HSCs) were isolated from liver tissue 14 d after BO, by means of in situ collagenase perfusion and counterflow elutriation. The levels of HGF, c-Met, VEGF and TGF-β1 mRNA in each cell fraction were then investigated by quantitative RT-PCR. Immunohistochemical staining with anti-desmin and anti-α-SMA antibody was also performed, to evaluate the number and activation status of HSCs.
Experiment 2
Fourteen days after BO, rats were subjected to 70% hepatectomy with concurrent internal biliary drainage. The tied end of the polyethylene tube was released and embedded in the duodenum. 70% of the liver was then resected according to the method of Higgins and Anderson [30]. In the sham-operated group, rats underwent internal biliary drainage with concurrent 70% hepatectomy 14 d after sham operation. For the assessment of the HGF, c-Met, VEGF and TGF-β1 expressions, the right inferior lobe of the liver was carefully excised before and 6, 12, 24, 48, 72, 120, 168, and 240 h (n = 10 at each time point) after hepatectomy. The levels of HGF, c-Met, VEGF and TGF-β1 mRNA in liver tissue were investigated by quantitative RT-PCR. In addition, remnant liver weight ratio and PCNA labeling index were also evaluated and compared between BO and sham-operated rats.
Quantitative RT-PCR analysis of HGF, c-Met, VEGF, and TGF-β1 mRNA expression
Total RNA was extracted from liver tissues or freshly isolated liver cells by the acid guanidium-thiocyanate/phenol /chloroform method, and 1 mg of extracted total RNA was subjected to a reverse transcription reaction, using Ready To GoTM T-primed 1st strand cDNA synthesis kit (Amersham Pharmacia Biotech, Buckinghamshire, England). The cDNA from 33 ng of total RNA was used as a template. HGF, c-Met, VEGF, and TGF-β1 mRNA levels were quantified by means of a LightCycler (Roche Diagnostics, Mannheim, Germany), using the double-strand-specific dye SYBE Green I. Details of the primers used in this study are summarized in Table 1. The PCR condition was as follows: initial denaturation at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing for 10 s, and extension at 72 °C for 20 s. The expression level of each angiogenic factor was adjusted using the level of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA, and expressed as ratio to GAPDH mRNA.
Table 1.
Primer Sequences for RT-PCR
| Gene | Primer Sequence | T (°C) | |
| HGF | Sense | 5’-TTATGGGGAATGAGAAATGC | 60 |
| Antisense | 5’-TCGAACAAAAATACCAGGAC | ||
| c-Met | Sense | 5’-CAGACGCCTTGTATGAAGT | 60 |
| Antisense | 5’-CATAAGTAGCGTTCACATGG | ||
| TGF-β1 | Sense | 5’-ATGACATGAACCGACCCTTC | 60 |
| Antisense | 5’-TGTGTTGGTTGTAGAGGGCA | ||
| VEGF | Sense | 5’-AATTGAGACCCTGGTGGACA | 56 |
| Antisense | 5’-TAGTGACGTTGCTCTCCGAC | ||
| GAPDH | Sense | 5’-TGAACGGGAAGCTCACTGG | 60 |
| Antisense | 5’-TCCACCACCCTGTTGCTGTA | ||
| β-actin | Sense | 5’-CCTGTATGCCTCTGGTCGTA | 60 |
| Antisense | 5’-CCATCTCTTGCTCGAAGTCT | ||
T : annealing temperature .
Isolated liver cells from liver tissue
Liver cells 14 d after BO were isolated, to determine potential cellular sources of TGF-β1 and HGF mRNA. Rat hepatocytes were isolated according to the methods of Gumucio et al [31]. The SECs, Kupffer cells and HSCs were also isolated by means of in situ collagenase perfusion and counterflow elutriation, as described by Knook et al [32], with minor modification. A JE-5.0 elutriator rotor (Beckman Instruments, Palo Alto, CA) was used in a J6-MI Beckman centrifuge. The separation process was started by adding the nonparenchymal cell suspension to a sample-mixing chamber. The HSCs were eluted at flow rates of 16 to 18 mL/min, and at a speed of 3 200 r/min. The SECs were then eluted at flow rates of 23 to 26 mL/min, and Kupffer cells at flow rates of 36 to 39 mL/min, at a speed of 2 500 r/min. The purity of HSCs was > 90%, as assessed by their positive staining for desmin [33]. The purity of SECs and Kupffer cells was > 90% and > 92%, respectively, as assessed by typical cobblestone morphology and positive staining for ED-1 [34], respectively. To evaluate expression of HGF and TGF-β1 mRNA in each cell fraction, freshly isolated cells were used for total RNA extraction.
Immunohistochemical staining
Resected liver tissue specimens from rats were fixed in 4% paraformaldehyde (Wako Chemical Co. Osaka, Japan) in phosphate-buffered saline, washed with phosphate-buffered saline, dehydrated with 30%, 70%, 95%, and 100% ethanol and xylene, and then embedded in paraffin. Four-micrometer sections were cut and mounted onto superfrosted slide glass (Matsunami Glass Ind., Ltd., Osaka, Japan). Sections were incubated with methanol-1% hydrogen peroxide to destroy endogenous peroxidase, and blocked with nonspecific staining blocking reagent (Dako, Glostrup, Denmark). After overnight incubation at 4 °C with mouse monoclonal anti-desmin antibody (diluted 1∶100; Dako), or mouse monoclonal anti-α-SMA antibody (diluted 1∶100; DAKO) sections were processed according to the standard immunoperoxidase method, using a streptavidin biotin peroxidase complex kit (Dako LSAB + Kit/HRP; Dako). The peroxidase reaction was then developed with diaminobenzidine (Dako).
PCNA labeling index
Immunohistochemical staining for PCNA was performed on formalin-fixed and paraffin-embedded liver tissue with anti-PCNA antibody as previously described [35,36]. A three-step immunoperoxidase method using strept-avidin biotin complex (Dako, Copenhagen, Denmark) was performed, according to the procedure described by Hall et al [36]. PC-10 monoclonal antibody (Dako, Copenhagen, Denmark) was used at a dilution of 1∶100, with overnight incubation at 4 °C. Evaluation of PC-10 immunostaining was performed based on the percentage of positive nuclei of 500 hepatocytes at high power (400 ×), and was expressed as a PCNA labeling index.
Statistical analysis
The results were expressed as mean ± SD. The Mann Whitney test was used for statistical analysis of unpaired data, and differences were considered significant at P < 0.05.
RESULTS
HGF, c-Met, VEGF and TGF-β1 mRNA expression after biliary obstruction
The expression of TGF-β1 mRNA (Figure 1D) was at low levels before BO, but increased significantly at 14 d (P < 0.05 vs sham) and further increased at 21 d (P < 0.03 vs sham) after BO. Meanwhile, the expression of HGF (Figure 1A) and VEGF (Figure 1C) mRNA tended to increase at 14 and 21 d after BO, but no significant differences were found, as compared to the sham-operated control. The expression of c-Met mRNA (Figure1B) was lower at 14 and 21 d after BO, but was not significantly different from the sham-operated control.
Figure 1.
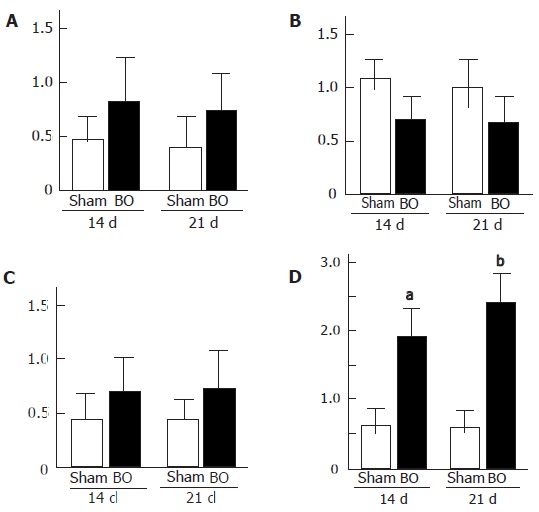
A: Hepatocyte growth factor (HGF); B: c-Met; C: vascular endothelial growth factor (VEGF); D: transforming growth factor-β1 (TGF-β1) mRNA expression in rat liver at 14 and 21 d after biliary obstruction (BO) and sham-operation (sham). (aP < 0.05 vs sham-operated control, bP < 0.03 vs sham-operated control). Results are expressed as mean ± SD of n = 10 for each period in each group.
HGF and TGF-β1 mRNA expression in isolated specific cell populations
To determine cellular sources of TGF-β1 mRNA during extrahepatic cholestasis, liver cells were isolated at 14 d after BO, because the expression of TGF-β1 mRNA was strongly induced by that time. In cell isolates, the mRNA for TGF-β1 was found mainly in the HSC fraction (Figure 2). On the other hand, the HGF mRNA expression was found in the nonparenchymal cell fraction, especially in the SEC fraction.
Figure 2.
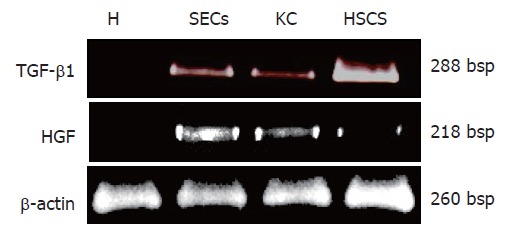
Hepatocyte growth factor (HGF) and transforming growth factor-β1 (TGF-β1) mRNA expression in isolated rat liver cells (H: hepatocytes; SECs: sinusoidal endothelial sells; KC: Kupffer cells; HSCs: Hepatic stellate cells) 14 d after biliary obstruction (BO).
Immunohistochemical staining for desmin and α-smooth muscle actin
In the sham-operated control, several desmin-positive cells, presumed to be HSCs, were seen around the portal area (Figure 3A). But 14 d after BO (Figure 3B), the number of positive cells increased, and further increased at 21 d (Figure 3C). On the other hand, α-SMA-positive cells were hardly seen in the sham-operated controls (Figure 3D), whereas α-SMA-positive cells, presumed to be activated HSCs, were prominent in the surrounding portal areas 14 d after BO (Figure 3E).
Figure 3.
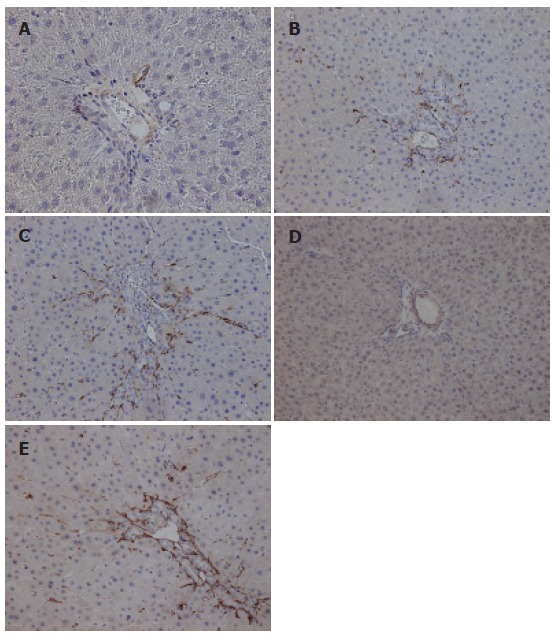
Immunohistochemical staining for desmin A: 14 d after sham operation; B: 14 d after biliary obstruction (BO); C: 21 d after biliary obstruction. Immunohistochemical staining for α-smooth muscle actin (α-SMA); D: 14 d after sham operation; E: 14 d after biliary obstruction.
Remnant liver weight and PCNA labeling index
The ratio of remnant liver to whole liver weight after 70% partial hepatectomy (PH) was approximately 30%. In the sham + PH group, the remnant liver weight ratio started to increase 24 h after hepatectomy (Figure 4A). A significant increase was found from 48 h to 120 h after surgery, as compared with the BO+ PH group. Thereafter, no significant differences were found between the two groups. The remnant liver weight in the BO+ PH group reached the same levels as that in attained by the sham + PH group 240 h after hepatectomy, but required a longer period. Figure 4B shows changes in PCNA labeling index after hepatectomy. In the sham+ PH group, the PCNA labeling index was less than 5% in hepatocytes before hepatectomy. However, a dramatic increase in the PCNA labeling index of hepatocytes was observed 12 h after hepatectomy, reaching a peak 24-48 h after surgery (P < 0.03 vs BO+ PH group). In the BO + PH group, the PCNA labeling index was higher than in the sham+ PH before hepatectomy, and was then increased gradually, with a peak 120 h after hepatectomy. Thereafter, the PCNA labeling index decreased to the baseline value 240 h after surgery.
Figure 4.
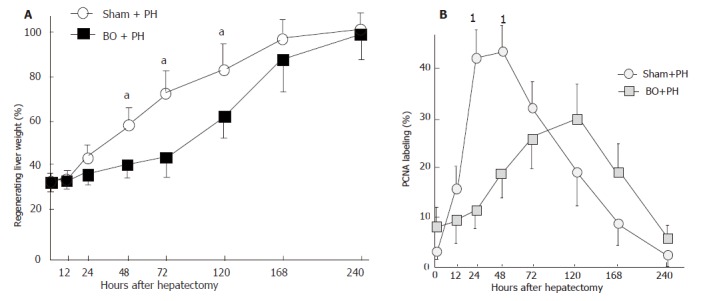
A: Changes in regenerating liver weight ratio after 70% partial patectomy (PH) in the sham-operated (sham) rats and the biliary obstructed (BO) rats (aP <0.05 between the BO + PH group and the sham + PH group). Results are expressed as mean ± SD of n = 10 for each period in each group. B: Changes in the hepatocyte PCNA labeling index after 70% hepatectomy (PH) in the sham-operated (sham) rats and the biliary obstructed (BO) rats (1P < 0.03 between the BO + PH group and the sham + PH group). Results are expressed as mean ± SD of n = 10 for each period in each group.
HGF, TGF-β1, VEGF, and c-Met mRNA expression after 70% hepatectomy
The expression of HGF mRNA in the sham + PH group started to increase 6 h after hepatectomy, with a peak at 12 h and 24 h (Figure 5A). Meanwhile, in the BO+ PH group, a small increase of HGF mRNA was observed up to 72 h after surgery, but no earlier peak was observed. The expression of c-Met mRNA in the BO+ PH group was lower, when compared with the sham+ PH control between 0 and 72 h after hepatectomy, but there was no significant difference between the groups (Figure 5B). The expression of VEGF mRNA in the BO+ PH group was somewhat higher up to 24 h after surgery, as compared to the sham+ PH (Figure 5C), but no significant differences were found at any point in this experiment. Meanwhile, the expression of TGF-β1 mRNA (Figure 5D) in the BO+ PH group was significantly up-regulated up to 48 h after hepatectomy, as compared to the sham+ PH group (P < 0.05). Thereafter, TGF-β1 mRNA expression was not significantly different between the groups.
Figure 5.
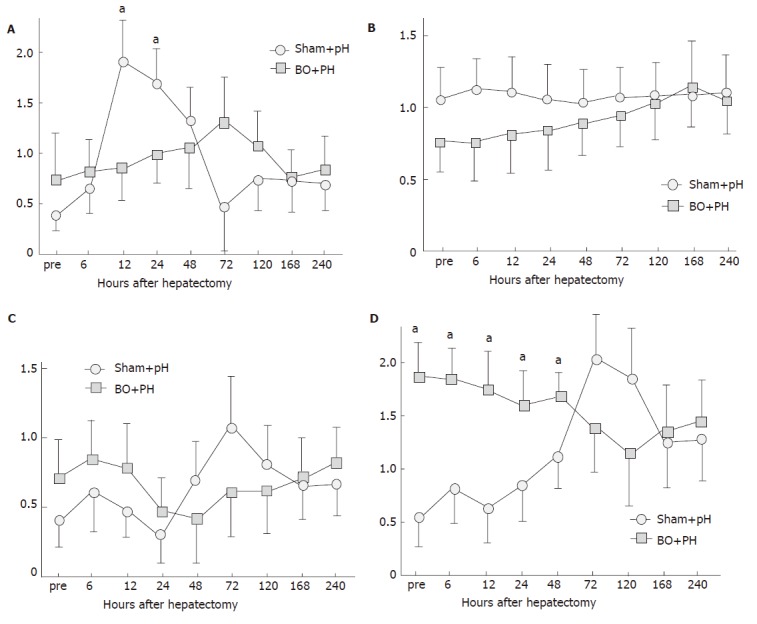
Changes in A: hepatocyte growth factor (HGF); B: c-Met; C: vascular endothelial growth factor (VEGF); D: transforming growth factor-β1 (TGF-β1) mRNA expression after partial hepatectomy (PH) in the sham-operated (sham) rats and the biliary obstructed (BO) rats (aP <0.05 between the BO + PH group and the sham + PH group). Results are expressed as mean ± SD of n = 10 for each period in each group.
DISCUSSION
In the present study, we have clearly shown that hepatic TGF-β1 mRNA levels increased with length of cholestasis. Moreover, in cell isolates from BO liver tissue, TGF-β1 mRNA expression was found mainly in the HSC fraction. Immunohistochemical study also revealed an increased number of HSCs (desmin-positive cells) and activated HSCs (α-SMA-positive cells) in portal areas in proportion to the length of cholestasis. In the hepatectomy model, liver regeneration rate in the BO rats was delayed, as compared to sham-operated rats. TGF-β1 mRNA was also significantly up-regulated up to 48 h after hepatectomy in the BO rats, and no earlier peak of HGF mRNA expression was observed, despite a small increase in HGF mRNA during extrahepatic cholestasis. These findings suggest that increased TGF-β1 secreted from activated HSCs, and earlier suppression of HGF production, may greatly contribute to delayed liver regeneration in a paracrine manner. Earlier suppression of HGF after hepatectomy may be also related to HSCs activation, because activated HSCs reportedly lose their ability to express HGF mRNA [37].
Although several previous studies regarding liver regeneration after hepatectomy with obstructive jaundice have been reported, it is not clear how BO affects liver regeneration. Aronson et al [4] reported that extrahepatic cholestasis inhibits liver regeneration after hepatectomy, but they studied liver regeneration in the presence of postoperative BO. In this study, restoration of bile flow was performed simultaneously with hepatectomy, as in clinical cases. According to our results, the PCNA labeling index of hepatocytes, HGF and VEGF mRNA were somewhat higher in BO livers than in sham-operated livers. These results are consistent with previous studies [4,6,38,39] indicating that BO induces DNA replication of hepatocytes. Furthermore, we clearly demonstrated that TGF-β1 mRNA was significantly up-regulated after BO. This phenomenon might be related to the hepatic repair process that compensates for hepatocyte injury caused by BO. This is also suggested by the fact that serum AST levels increased shortly after BO (data not shown). In cell isolates from BO liver, TGF-β1 producing cells were found to be mainly HSCs. Furthermore, an increased number of desmin-positive cells (HSCs) were found in portal areas, and α-SMA-positive cells, presumably activated HSCs, were progressively extended around portal areas in proportion to the length of BO. Activated HSCs reportedly lose their ability to express HGF mRNA, but produce a large amount of TGF-β1. These findings suggest that HSCs in portal areas were gradually activated into myofibroblast-like cells during BO. Activated HSCs produce TGF-β1, and TGF-β1 stimulates activated HSCs to produce more TGF-β1 in an autocrine manner, whereas activated HSCs hardly produce any HGF in the BO liver. HGF mRNA increased, to some extent, after BO, but HGF producing cells were mainly SECs, rather than HSCs. This may be related to the proliferative response of hepatocytes and bile duct cells during cholestatic liver injuries.
We clearly demonstrated that liver regeneration was significantly delayed after hepatectomy in the BO rats, as compared to the sham-operated rats. Moreover, TGF-β1 mRNA was significantly up-regulated up to 48 h after hepatectomy in the BO rats, and high levels of HGF mRNA were not induced in the earlier phase of hepatectomy. Meanwhile, c-Met expression in the BO liver was not significantly different from the sham-operated rats. These results suggest that delayed regeneration may be associated with the initial high expression of TGF-β1, and the suppression of HGF induction after hepatectomy. It is obvious that initial up-regulation of TGF-β1 has a nonbeneficial effect on liver regeneration, because TGF-β1 is the most important cytokine controlling the inhibition of hepatocyte proliferation. Furthermore, although HGF mRNA increased to some extent in BO rats after hepatectomy, the amount may be insufficient to induce the initial stimulus for DNA synthesis of in hepatocytes for adequate liver regeneration.
In conclusion, BO induces proliferation and activation of HSCs, resulting in up-regulation of TGF-β1 and negative regulation of HGF expression. The altered expression patterns may be involved to a considerable degree in delayed liver regeneration after hepatectomy in rats with obstructive jaundice. These findings may provide clues for the treatment of impaired hepatic regeneration after major hepatectomy with obstructive jaundice. That is, regulation of HSCs activation or restoration of an altered growth/inhibitory expression pattern might have beneficial effects on liver regeneration following major hepatectomy with obstructive jaundice.
Footnotes
S- Editor Guo SY L-Editor Pravda J E- Editor Zhang Y
References
- 1.Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, Kato A, Nakamura S, Omoto H, Nakajima N, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection. Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- 2.Bengmark S, Ekberg H, Evander A, Klofver-Stahl B, Tranberg KG. Major liver resection for hilar cholangiocarcinoma. Ann Surg. 1988;207:120–125. doi: 10.1097/00000658-198802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108:572–580. [PubMed] [Google Scholar]
- 4.Aronson DC, Chamuleau RA, Frederiks WM, Bosman DK, Oosting J. The effect of extrahepatic cholestasis on liver regeneration after partial hepatectomy in the rat. Liver. 1995;15:242–246. doi: 10.1111/j.1600-0676.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno S, Nimura Y, Suzuki H, Yoshida S. Portal vein branch occlusion induces cell proliferation of cholestatic rat liver. J Surg Res. 1996;60:249–257. doi: 10.1006/jsre.1996.0039. [DOI] [PubMed] [Google Scholar]
- 6.Polimeno L, Azzarone A, Zeng QH, Panella C, Subbotin V, Carr B, Bouzahzah B, Francavilla A, Starzl TE. Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology. 1995;21:1070–1078. [PMC free article] [PubMed] [Google Scholar]
- 7.Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–1536. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27–54. [PubMed] [Google Scholar]
- 9.Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology. 1992;15:149–155. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- 10.Schirmacher P, Geerts A, Pietrangelo A, Dienes HP, Rogler CE. Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. [DOI] [PubMed] [Google Scholar]
- 11.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244–2252. doi: 10.1172/JCI116451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 13.Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557–562. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- 14.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- 15.Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34:683–689. doi: 10.1016/s0168-8278(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- 17.Tucker RF, Shipley GD, Moses HL, Holley RW. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984;226:705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- 18.Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46:2330–2334. [PubMed] [Google Scholar]
- 19.Russell WE, Coffey RJ Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun L, Mead JE, Panzica M, Mikumo R, Bell GI, Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988;85:1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda H, Mine T, Shibata H, Eto Y, Hasegawa Y, Takeuchi T, Asano S, Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993;92:1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakowlew SB, Mead JE, Danielpour D, Wu J, Roberts AB, Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991;2:535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsue S, Hamanoue M, Tanabe G, Ogura Y, Yoshidome S, Aikou T, Nakamura T. Expression of HGF and TGF-beta 1 mRNA after partial hepatectomy in rats with liver cirrhosis. Surg Today. 1995;25:237–243. doi: 10.1007/BF00311534. [DOI] [PubMed] [Google Scholar]
- 25.De Bleser PJ, Niki T, Rogiers V, Geerts A. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 1997;26:886–893. doi: 10.1016/s0168-8278(97)80257-7. [DOI] [PubMed] [Google Scholar]
- 26.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 27.Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells) Hepatology. 1984;4:709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- 28.Ramadori G, Veit T, Schwögler S, Dienes HP, Knittel T, Rieder H, Meyer zum Büschenfelde KH. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- 29.Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 30.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 31.Gumucio JJ, May M, Dvorak C, Chianale J, Massey V. The isolation of functionally heterogeneous hepatocytes of the proximal and distal half of the liver acinus in the rat. Hepatology. 1986;6:932–944. doi: 10.1002/hep.1840060521. [DOI] [PubMed] [Google Scholar]
- 32.Knook DL, Sleyster EC. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976;99:444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi M, Takada A, Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987;7:277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- 34.Kaplow LS. Manual of macrophage methodology. Herscowitz HB, Holden HT, Bellanti JA, Ghaffar A, Eds. New York: Marcel Dekker;; 1981. pp. 199–227. [Google Scholar]
- 35.Chijiiwa K, Nakano K, Kameoka N, Nagai E, Tanaka M. Proliferating cell nuclear antigen, plasma fibronectin, and liver regeneration rate after seventy percent hepatectomy in normal and cirrhotic rats. Surgery. 1994;116:544–549. [PubMed] [Google Scholar]
- 36.Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K, Tajima H, Okazaki H, Nakamura T. Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-beta 1 and glucocorticoids. J Biol Chem. 1992;267:24917–24920. [PubMed] [Google Scholar]
- 38.Kimura F, Miyazaki M, Itoh H. Effects of biliary obstruction on hepatic deoxyribonucleic acid and protein synthesis after partial hepatectomy. Hepatogastroenterology. 1997;44:501–507. [PubMed] [Google Scholar]
- 39.Terasaki M, Kuriki H, Nimura Y, Shionoya S, Kojima K, Yoshida S. Induction of DNA replication and cell growth in rat liver by obstructive jaundice. Jpn J Cancer Res. 1991;82:170–175. doi: 10.1111/j.1349-7006.1991.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


