Abstract
AIM: To present our experience with endoscopic placement of an esophageal endoprosthesis in 19 patients.
METHODS: A retrospective evaluation was made for the use of 19 stents positioned at the level of the cervical esophagus: 11 for malignant tumours (7 causing obstruction, 4 complicated by an esophago -tracheal or -cutaneous fistula), and 8 for an acquired benign tracheo-esophageal fistula due to prolonged intubation. The covered Ultraflex stent was used in all cases except two. These two patients had an esophagocutaneous fistula following laryngectomy and a Flamingo Wall stent was used.
RESULTS: Stent implantation was technically successful in all patients. Dysphagia score was improved from 3 to 2 in stenosis patients, while sealing of the fistula was achieved in all cases. The median hospital stay was 3 d for malignant tumour patients and 13.5 d for esophagocutaneous fistula patients. One Ultraflex stent and two Flamingo Wall stents were easily removed 33 d and 3 months respectively after implantation when the fistulas had totally occluded.
CONCLUSION: Endoprosthesis implantation for malignancy and/or fistula of malignant or benign origin at the level of the cervical esophagus is an easy, well tolerated, safe and effective procedure with no complications or mortality.
Keywords: Cervical endoprosthesis, Pharyngo-esophageal stenosis, Dysphagia, Esophageal carcinoma, Esophagotracheal fistula
INTRODUCTION
The insertion of an endoprosthesis is one of the most acceptable means of palliative treatment of patients with obstructing esophageal lesions and/or an existing esophagorespiratory fistula. However, the proximity to the cricopharyngeal sphincter is traditionally regarded as a relative contraindication, because of the potential problems of persistent foreign body sensation, pain, odynophagia, compression of the trachea or proximal migration of the prosthesis[1-3].
Recently, this traditional view has begun to change, as witnessed by an augmented number of case reports or small series of data presentations[1,2,4-11]. In such patients, with the inability to swallow even their own saliva at times, palliative intubation aimed at relief of dysphagia, maintenance of nutrition and avoidance of respiratory complications is the primary treatment goal, while the close relationship between the cricopharynx and the cervical lesion continues to be a challenge for every endoscopist.
Herein, we present our experience with stent placement in the cervical esophagus at the level of hypopharynx and/or the upper esophageal sphincter.
MATERIALS AND METHODS
Patients
Over the last 10 years (1995-2004), 19 patients were referred to our department to be treated endoscopically for a lesion in the cervical esophagus. There were 11 patients with a malignancy and 8 patients who spontaneously developed tracheo-esophageal fistula [TEF] after prolonged tracheal intubation for mechanical ventilatory support in the ICU.
The eleven carcinoma patients (9 males and 2 females) with a median age of 70 years (range: 62 - 82 years) suffered from: laryngeal (3 cases), hypopharyngeal (2 cases) and esophageal inlet carcinoma (2 cases), all causing severe stenosis and obstruction, two cases of high esophagotracheal fistula, one due to radical thyroidectomy and concomitant radiotherapy and the other due to an inoperable hypopharyngeal carcinoma and two cases of esophagocutaneous fistula after total laryngectomy for carcinoma, performed postirradiation (Table 1).
Table 1.
Data of carcinoma patients
| Age | Gender | Disease | |
| 1 | 64 | Male | Laryngeal carcinoma causing obstruction |
| 2 | 72 | Male | Laryngeal carcinoma causing obstruction |
| 3 | 75 | Male | Laryngeal carcinoma causing obstruction |
| 4 | 65 | Male | Hypopharyngeal carcinoma causing obstruction |
| 5 | 68 | Male | Hypopharyngeal carcinoma causing obstruction |
| 6 | 70 | Female | Esophageal inlet carcinoma causing obstruction |
| 7 | 80 | Male | Esophageal inlet carcinoma causing obstruction |
| 8 | 62 | Female | Esophagotracheal fistula after thyroidectomy + radiotherapy |
| 9 | 64 | Male | Esophagotracheal fistula after hypopharyngeal carcinoma |
| 10 | 82 | Male | Esophagocutaneous fistulas after total laryngectomy + radiotherapy |
| 11 | 72 | Male | Esophagocutaneous fistulas after total laryngectomy + radiotherapy |
The seven patients with obstructing-type cancer presented with a median dysphagia score of 3 (unable to swallow liquids) ranging from 2 to 4. Under diazepam-induced conscious sedation they were subjected to a Savary bougie progressive dilatation of the stenosis for one to two sessions depending on the rigidity of the tumour. Dilatation was performed over a guide wire which was advanced into the stenosis through the endoscope up to the point of 12.8 mm in diameter, to facilitate the rapid expansion of the stent.
After dilatation the endoscope was advanced to the tumour site. The total length of the stenosis and the distance of the tumor upper orifice from the incisor teeth, were carefully recorded due to their great importance for the correct placement of the stent.
The remaining 4 patients had no need for dilatation, thus immediately following insertion of the endoscope and inspection of the tumourous fistulae, the exact distance of the most proximal end of the lesion from the incisors and the total length of the lesion were recorded as above.
Eight patients had benign TEF (5 males and 3 females with a median age of 71 years, range 21-76 years) due to multiple trauma (2 cases), extended cerebral hemorrhage (2 cases) and post-operative complications (4 cases) implicating cardio-respiratory insufficiency. All patients, previously subjected to a percutaneous endoscopic gastrostomy for feeding, had an overall median intubation time of 30 d (range 15 - 80 d) and had been subjected to percutaneous tracheostomy a median of 16 d (range, 5-62 d) before diagnosis of TEF. All were characterized by poor prognosis and 4 of them were in a septic state (Table 2).
Table 2.
Data of benign disease patients
| Age | Gender | Underlined disease | |
| 1 | 21 | Male | Multiple trauma |
| 2 | 73 | Female | Multiple trauma [in septic state] |
| 3 | 76 | Male | Cerebral hemorrhage |
| 4 | 56 | Male | Cerebral hemorrhage |
| 5 | 47 | Female | Post-operative complications [in septic state] |
| 6 | 70 | Female | Post-operative complications |
| 7 | 72 | Male | Post-operative complications [in septic state] |
| 8 | 72 | Male | Post-operative complications [in septic state] |
The final diagnosis of TEF was made by esophagoscopy, during which the exact characteristics of the fistula, i.e. size and its relationship with the upper esophageal sphincter as well as the distance of the most proximal end of the lesion from the incisors, were carefully recorded.
Stent placement
A self-expandable, covered -proximal release type-Ultraflex stent [Microvasive, Boston Scientific Corp., Natick, Mass] with a proximal flare of 28 mm, a body diameter of 23 mm, and a length of 120 mm or 150 mm, was used in all cases except two patients with esophagocutaneous fistulae after laryngectomy. In these cases, a Flamingo Wall stent [Microvasive Endoscopy, Boston Scientific Corp., Natick, Mass] with a proximal diameter of 24 mm, distal diameter of 16 mm, and length of 120 mm, was considered the most suitable for fistula sealing.
All stents were placed over a guide wire and no fluoroscopy was used in any case. Patients with malignant lesions were treated under diazepam-induced conscious sedation; benign TEF patients were under mechanical ventilatory support due to the underlying illness and had no need for supplementary anesthesia. The whole procedure was performed at the bedside in the ICU.
The stent delivery catheter was passed over the pre-inserted guide wire and advanced so that the proximal end of the stent was at the estimated distance from the incisor teeth. Generally, all stents were gradually deployed in such a position so that at least 2 cm of the prosthesis was over both sides of the lesion. However, our landmark was the upper esophageal sphincter. The stent was thus deployed to achieve the minimal harmful sensation with maximum security regarding stent migration as well as early overlapping by tumour overgrowth, i.e. the upper end of all stents was just within the upper esophageal sphincter or at the hypopharynx.
After stent insertion, its proper position was controlled by endoscopy and under direct vision. In some cases x-ray imaging was additionally performed (Figures 1 and 2).
Figure 1.
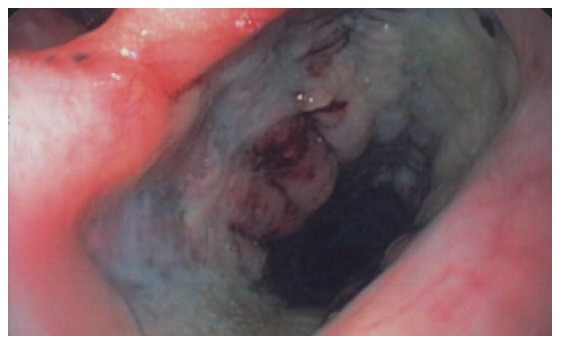
Endoscopic view of the soft funnel of the Ultraflex stent placed in the hypopharynx, posterior to the larynx. The stent was partially compressed in the antero-posterior direction at the level of arytenoid cartilages.
Figure 2.
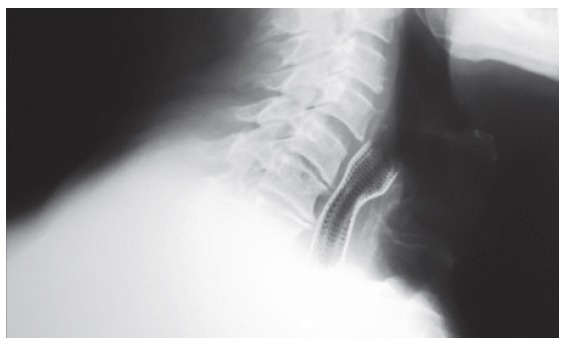
Plain radiograph of Ultraflex stent in situ, shows the fully expanded stent. Its proximal edge slightly protrudes in the hypopharynx (posterior endplate of C4).
RESULTS
Prosthesis implantation was technically successful in all patients and there was no procedure-related mortality. No complications occurred and no patient experienced severe pain at the site of stent placement, lasting more than 24 h and needed narcotic analgesics. Dysphagia score was improved from a median value of 3 (range, 2-4) to a value of 2 (range,1-3) in esophageal stenosis patients. Fistula sealing was achieved in all cases, both benign and malignant (Table 3).
Table 3.
Results after stent placement
| Obstruction due to malignancy | 7 cases |
| Stenting related mortality | 0 |
| Improvement of dysphagia [score] | From 3 to 2 |
| Median hospital stay after stenting | 3 d |
| Malignant esophagotracheal fistula | 2 cases |
| Stenting related mortality | 0 |
| Sealing of fistula | 2/2 (100%) |
| Median hospital stay after stenting | 3 d |
| Malignant esophagocutaneous fistula | 2 cases |
| Stenting related mortality | 0 |
| Sealing of fistula | 2/2 (100%) |
| Median hospital stay after stenting | 13.5 d |
| Benign esophagotracheal fistula | 8 cases |
| Stenting related mortality | 0 |
| Sealing of fistula | 8/8 (100%) |
| Disease related mortality | 7 cases |
Regarding foreign body sensation, all conscious patients could well tolerate the stent placement in the first few days with no further difficulties. The two patients with esophagocutaneous fistula stented by Flamingo tubes experienced a foreign body sensation when the head/neck was bent since the proximal end of the stent was at the level of the mesopharynx, easily visible through the mouth opening (Figure 3). Both stents remained in place for three months until the fistula was totally closed, and were then removed by being grasped with retrieval forceps and pulled out without difficulty.
Figure 3.
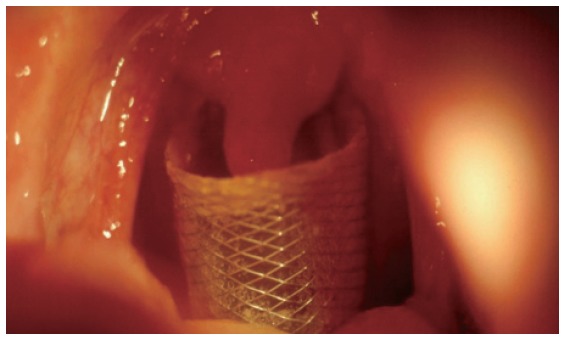
Direct view through the mouth opening of the upper part of the Flamingo stent placed temporarily at the cervical esophagus in the patient suffering from esophagocutaneous fistula after laryngectomy.
The median hospital stay was 3 d (range 2 - 4 d) for the 9 patients with stenosis and/or malignant esophagotracheal fistula. The other two patients with esophagocutaneous fistula remained hospitalised for 12 and 15 d, respectively due to cutaneous trauma debridement. Seven out of the eight TEF patients remained hospitalized in the ICU until their death, after 10 to 60 d, due to sepsis in 4, respiratory insufficiency in 2 and heart failure in one. The remaining patient, a 21-year-old multiple trauma victim, was weaned from a ventilator 33 d later and scheduled to be operated on for tracheoplasty. The Ultraflex stent was easily removed by simply grasping it with a pair of retrieval forceps under direct vision, just before the operation.
The 11 patients with malignancies were followed up every month. One patient with esophagocutaneus fistula died 7 months later, i.e. 4 months after fistula closure and stent removal. having developed total dysphagia due to recurrence of the disease. Unfortunately, this patient had totally refused a second stent; the second patient with esophagocutaneus fistula was alive 8 months after stenting. 5 months after fistula closure and stent removal, He is able to eat (dysphagia score 3) and no leakage has been reported; Seven patients remained alive for a median of 8 months (range 4 to18 months) and two patients were alive 10 months and 3 months after stenting. All these patients could eat semi-solid food and needed no other nutritional support.
DISCUSSION
The cervical esophagus is accepted as a segment between C6 at the pharyngoesophageal junction and the thoracic inlet at T1. It is endoscopically between 15 cm and 19cm from the incisor teeth and radiologically projects above the sternoclavicular joint[8,10]. At that level, any endoscopic procedure is more problematic even in the presence of a normal anatomical situation, since flexible endoscopy of the hypopharynx and upper esophageal sphincter is technically difficult, due to the reduced efficacy of insufflation and movements-related swallowing.
Generally, there is no report supporting placement of esophageal prosthesis for cervical lesions because of concerns about the increased risk of proximal migration of the stent into the hypopharynx and most importantly, the intolerable sensation of a foreign body[11,12]. However, there is no other acceptable means of palliating a terminal-stage tumor disease patient. This is because of the rapid decline of the patient’s general condition upon the development of an esophagotracheal fistula due to aspiration pneumonia and malnutrition. On the other hand, as tumor stage is generally advanced and life expectancy is short, the major interest of any therapeutic procedure must be a rapid and successful palliation, ensuring acceptable quality of life, reducing the duration of hospital stay and cost.
There exists a general hesitation about applying stents in patients with benign diseases because of concerns regarding short-term complications and the absence of information regarding long term sequelae[3,13-15]. In our study, the patients with TEF of benign origin were all critically ill with a short term expectation of life if left untreated.
We question the risk of stent migration. In benign cases the risk is reported to be as high as 24%[15], which is probably related to a smaller mucosal surface area with less inward force anchoring the device. We experienced no migration in our cases, which might be attributed to the upper conical configuration of the stent, the external pressure induced by the cuff of the endotracheal tube, the constriction of the upper part of the stent by the upper esophageal sphincter, and mainly the absence of head-neck movements as well as swallow movements, due to the deep sedation of the patients. Moreover, the small number of patients could explain the absence of this complication in our series.
In the malignant cases, the risk of cervical stenting relates to the possibility of proximal migration, which shares the danger of sudden upper respiratory tract occlusion[3,8,10,16]. We experienced no migration in our patients, since the tumour masses were hard and protrusive and occluded the esophagus, which kept the stent in place, while in the two laryngectomy cases, it was impossible for the stent to impair breathing.
The second main concern for stenting at the level of cervical esophagus is the theoretical probability of a foreign body sensation[3,10-12]. Although such a sensation was experienced by some of our tumour-bearing patients, this was minimal and well tolerated. Most likely, by the time when a prosthesis becomes necessary, such patients especially those after laryngestomy with a Flamingo stent no longer have normal sensation, probably due to local infiltration of the nerves innervating the hypopharynx, cricopharyngeal sphincter and upper esophagus resulting in hypo/anesthesia. This propensity to infiltrate local neural structures is reflected by the frequent occurrence of unilateral or bilateral vocal cord paralysis. In addition, previous radiotherapy or surgery also impairs normal sensation. The two patients who were placed Flamingo stents for sealing the esophagocutaneous fistula after laryngectomy experienced a foreign body sensation every time they moved their heads forward. However, in these cases the stent was high at the level of the mesopharynx and easily visible even though the mouth was opened.
The procedure of stenting itself is totally uneventful. The use of local anesthesia and conscious sedation, in conjunction with a slim endoscope, can facilitate the procedure, which is time consuming only in the case of a very rigid and narrow malignant stricture requiring a guidewire to be advanced blindly through the stricture for bougie dilatation. Otherwise, the total endoscopic procedure takes only a few minutes, that is, the time needed for passing the endoscope through the lesion, making the appropriate measurements of total length of the lesion and distances of its proximal margin from upper esophageal sphincter and from incisor teeth, and advancing a guidewire through the endoscope into the stomach. The endoscope is then withdrawn and the stent is advanced ‘blindly’ over the guidewire, using only the numerical marks on the stent’s sheath (centimeters from its proximal edge) for correct positioning. The use of general anesthesia does not facilitate the endoscopic maneuvering. However, insertion of the scope under direct inspection of the esophageal opening, by means of a laryngoscope as for tracheal intubation, is easier, but is not a reason to give general anesthesia to an otherwise conscious patient.
Thus, the eight mechanically ventilated TEF patients were under general anesthesia, while the remaining malignant lesion patients were simply given midazolam for conscious sedation. We experienced no problem with the latter group, but we could not give any favor to the former. However, this fact may be partially related to the benign nature of the disease in this group of patients.
With regard to the technical problems of proper positioning, the theoretical point of difficulty is the proximity of the mesopharynx and epiglottis. Profili
et al[10] and Conio et al[17] have advised peroral administration of iodinated contrast medium for exclusive fluoroscopy guidance throughout the procedure. However, we found it was not useful and adds excessive difficulties, either because of the inability of a sedated patient to swallow or simply because of the increased time required to complete the procedure, or just because the procedure should be done on the ICU bed. We consider that the main difficulty is the accurate deployment of the proximal end of the stent close to the cricopharynx, because of the retraction of the expandable stent on deployment. The radiopaque markers used for stent placement under fluoroscopic assistance are not always reliable, because they are intended to indicate the position of a fully expanded stent. The problem was overridden when the proximally released type of Ultraflex prosthesis was used, allowing more accurate placement because we carefully measured the distances of the orifice of the upper esophageal sphincter and the proximal edge of the lesion from the incisor teeth, thus enabling us to know exactly where the proximal end of the stent should begin to deploy.
Additionally, the use of the Ultraflex stent has the advantages of being less rigid, thus reducing pain during movements of the head and neck, and has smooth edges, making it atraumatic when positioned in the hypopharynx, despite continuous soliciting during swallowing. This prosthesis can exert a constant, gentle radial force on the esophageal wall, but withstand angulation forces better than the Song and Gianturco stents, as well as the Wallstent which is stiffer and thus less suitable for lesions in this area[12,17].
A further advantage is the rapid expansion to the full diameter, enabling all patients to ingest well-chewed food two days after intervention. The rapid expansion also results in tight fixation to the esophageal wall without any tendency to dislocate and an immediate and complete sealing. In the case of tracheoesophageal fistula, it has successful rate of 73%-100%[5,16,18-20], referring both to malignant and benign fistulae. Moreover, its funnel-shape facilitates the collection of saliva and maintains proper positioning.
For the esophagocutaneus fistula patients we preferred the Flamingo Wall stent due to a number of distinct characteristics: conical shape with proximal flaring and lange braiding angle in the upper part and small in the distal part of the stent. When swallow movements and oesophageal peristalsis propel the stent downwards, its upper end becomes trapped in the laryngoplasty area, whereas the lower end becomes stretched, thus resisting distal migration. Moreover, its polyethylene cover is on the inside of the stent, thus increasing the possibility of anchoring the metal mesh to the esophageal mucosa, since there is no stricture to hold it in place[12,21]. To the best of our knowledge, there are no comparative studies on types of stents except two which relate to the distal esophagus, suggesting that all stents offer the same degree of palliation while Ultraflex and Flamingo stents are both equally less atraumatic than the Gianturco stent[21,22].
Stent placement in the setting of chemoradiotherapy may be associated with life-threatening complications such as esophageal perforation or bleeding. Kinsman
et al[23] reported that the complication rate is 36.4% and the mortality rate is 23% in patients receiving radiation and/or chemotherapy, compared with 2.5% and 0% of those without prior therapy. Sumiyoshi et al[24] have recorded 6 sudden massive hemorrhages in a group of 22 patients. Additionally, it is likely that T4 cancers are susceptible to pressure necrosis from stent with a consequent increase in the risk of perforation into adjacent structures[24]. Fortunately, we did not experience such complications in our patients. This could be partially explained by the fact that by using the Ultraflex stent, excessive dilatation of the malignant stricture is avoided.
Finally, the quality of life becomes an overriding issue in patients with inoperable cancer, therefore the ability to swallow their saliva and maintain oral intake is important to most patients with esophageal tumours[25]. Since the most realistic goal of any palliative therapy is maximal relief of symptoms with minimal risk, the insertion of a stent with no or little risk of dilation and a minimum of post-procedure complications are the optimum. Good symptom relief of dysphagia and successful occlusion of fistulae are clearly possible by the use of a stent as shown in our malignant and benign cases and more importantly, many patients with malignant strictures or fistulae are able to swallow their saliva after stent placement.
In conclusion, the results of the present series support the thesis that the presence of a lesion within 2 cm of the cricopharyngeal muscle should no longer be considered a contraindication for the palliative or temporary use of an endoprosthesis.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
References
- 1.Loizou LA, Rampton D, Bown SG. Treatment of malignant strictures of the cervical esophagus by endoscopic intubation using modified endoprostheses. Gastrointest Endosc. 1992;38:158–164. doi: 10.1016/s0016-5107(92)70382-4. [DOI] [PubMed] [Google Scholar]
- 2.Goldschmid S, Boyce HW Jr, Nord HJ, Brady PG. Treatment of pharyngoesophageal stenosis by polyvinyl prosthesis. Am J Gastroenterol. 1988;83:513–518. [PubMed] [Google Scholar]
- 3.Gislason GT, Pasricha PJ. Crossing the upper limit: esophageal stenting in the proximal esophagus. Dysphagia. 1997;12:84–85. [PubMed] [Google Scholar]
- 4.Bethge N, Sommer A, Vakil N. A prospective trial of self-expanding metal stents in the palliation of malignant esophageal strictures near the upper esophageal sphincter. Gastrointest Endosc. 1997;45:300–303. doi: 10.1016/s0016-5107(97)70275-x. [DOI] [PubMed] [Google Scholar]
- 5.May A, Ell C. Palliative treatment of malignant esophagorespiratory fistulas with Gianturco-Z stents. A prospective clinical trial and review of the literature on covered metal stents. Am J Gastroenterol. 1998;93:532–535. doi: 10.1111/j.1572-0241.1998.160_b.x. [DOI] [PubMed] [Google Scholar]
- 6.Lörken A, Krampert J, Kau RJ, Arnold W. Experiences with the Montgomery Salivary Bypass Tube (MSBT) Dysphagia. 1997;12:79–83. doi: 10.1007/PL00009523. [DOI] [PubMed] [Google Scholar]
- 7.Law S, Tung PH, Chu KM, Wong J. Self-expanding metallic stents for palliation of recurrent malignant esophageal obstruction after subtotal esophagectomy for cancer. Gastrointest Endosc. 1999;50:427–436. doi: 10.1053/ge.1999.v50.97948. [DOI] [PubMed] [Google Scholar]
- 8.Spinelli P, Cerrai FG, Meroni E. Pharyngo-esophageal prostheses in malignancies of the cervical esophagus. Endoscopy. 1991;23:213–214. doi: 10.1055/s-2007-1010659. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald S, Edwards RD, Moss JG. Patient tolerance of cervical esophageal metallic stents. J Vasc Interv Radiol. 2000;11:891–898. doi: 10.1016/s1051-0443(07)61807-7. [DOI] [PubMed] [Google Scholar]
- 10.Profili S, Meloni GB, Feo CF, Pischedda A, Bozzo C, Ginesu GC, Canalis GC. Self-expandable metal stents in the management of cervical oesophageal and/or hypopharyngeal strictures. Clin Radiol. 2002;57:1028–1033. doi: 10.1053/crad.2002.0988. [DOI] [PubMed] [Google Scholar]
- 11.Segalin A, Granelli P, Bonavina L, Siardi C, Mazzoleni L, Peracchia A. Self-expanding esophageal prosthesis. Effective palliation for inoperable carcinoma of the cervical esophagus. Surg Endosc. 1994;8:1343–1345. doi: 10.1007/BF00188298. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH. The role of oesophageal stenting in the non-surgical management of oesophageal strictures. Br J Radiol. 2001;74:891–900. doi: 10.1259/bjr.74.886.740891. [DOI] [PubMed] [Google Scholar]
- 13.Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroenterol Hepatol. 2001;16:484–487. doi: 10.1046/j.1440-1746.2001.02367.x. [DOI] [PubMed] [Google Scholar]
- 14.Low DE, Kozarek RA. Comparison of conventional and wire mesh expandable prostheses and surgical bypass in patients with malignant esophagorespiratory fistulas. Ann Thorac Surg. 1998;65:919–923. doi: 10.1016/s0003-4975(98)00081-2. [DOI] [PubMed] [Google Scholar]
- 15.Hramiec JE, O'Shea MA, Quinlan RM. Expandable metallic esophageal stents in benign disease: a cause for concern. Surg Laparosc Endosc. 1998;8:40–43. [PubMed] [Google Scholar]
- 16.Abadal JM, Echenagusia A, Simo G, Camuñez F. Treatment of malignant esophagorespiratory fistulas with covered stents. Abdom Imaging. 2001;26:565–569. doi: 10.1007/s002610000193. [DOI] [PubMed] [Google Scholar]
- 17.Conio M, Blanchi S, Munizzi F, Giacosa A. Metal stents in the cervical esophagus. Gastrointest Endosc. 2002;55:964–995; author reply 965. doi: 10.1067/mge.2002.123909. [DOI] [PubMed] [Google Scholar]
- 18.Lee JG, Hsu R, Leung JW. Are self-expanding metal mesh stents useful in the treatment of benign esophageal stenoses and fistulas An experience of four cases. Am J Gastroenterol. 2000;95:1920–1925. doi: 10.1111/j.1572-0241.2000.02246.x. [DOI] [PubMed] [Google Scholar]
- 19.Saxon RR, Barton RE, Katon RM, Lakin PC, Timmermans HA, Uchida BT, Keller FS, Rösch J. Treatment of malignant esophagorespiratory fistulas with silicone-covered metallic Z stents. J Vasc Interv Radiol. 1995;6:237–242. doi: 10.1016/s1051-0443(95)71104-6. [DOI] [PubMed] [Google Scholar]
- 20.Tomaselli F, Maier A, Sankin O, Woltsche M, Pinter H, Smolle-Jüttner FM. Successful endoscopical sealing of malignant esophageotracheal fistulae by using a covered self-expandable stenting system. Eur J Cardiothorac Surg. 2001;20:734–738. doi: 10.1016/s1010-7940(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 21.Sabharwal T, Hamady MS, Chui S, Atkinson S, Mason R, Adam A. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52:922–926. doi: 10.1136/gut.52.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siersema PD, Hop WC, van Blankenstein M, van Tilburg AJ, Bac DJ, Homs MY, Kuipers EJ. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: a prospective, randomized study. Gastrointest Endosc. 2001;54:145–153. doi: 10.1067/mge.2001.116879. [DOI] [PubMed] [Google Scholar]
- 23.Kinsman KJ, DeGregorio BT, Katon RM, Morrison K, Saxon RR, Keller FS, Rosch J. Prior radiation and chemotherapy increase the risk of life-threatening complications after insertion of metallic stents for esophagogastric malignancy. Gastrointest Endosc. 1996;43:196–203. doi: 10.1016/s0016-5107(96)70315-2. [DOI] [PubMed] [Google Scholar]
- 24.Sumiyoshi T, Gotoda T, Muro K, Rembacken B, Goto M, Sumiyoshi Y, Ono H, Saito D. Morbidity and mortality after self-expandable metallic stent placement in patients with progressive or recurrent esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2003;57:882–885. doi: 10.1016/s0016-5107(03)70024-8. [DOI] [PubMed] [Google Scholar]
- 25.Shim CS, Jung IS, Bhandari S, Ryu CB, Hong SJ, Kim JO, Cho JY, Lee JS, Lee MS, Kim BS. Management of malignant strictures of the cervical esophagus with a newly-designed self-expanding metal stent. Endoscopy. 2004;36:554–557. doi: 10.1055/s-2004-814555. [DOI] [PubMed] [Google Scholar]


