Abstract
We report a case of liver cell adenoma (LCA) in a 33-year-old female patient with special respect to its clonality status, pathogenic factors and differential diagnosis. The case was examined by histopathology, immunohistochemistry and a clonality assay based on X-chromosomal inactivation mosaicism in female somatic tissues and polymorphism at androgen receptor focus. The clinicopathological features of the reported cases from China and other countries were compared. The lesion was spherical, sizing 2 cm in its maximal dimension. Histologically, it was composed of cells arranged in cords, most of which were two-cell-thick and separated by sinusoids. Focal fatty change and excessive glycogen storage were observed. The tumor cells were round or polygonal in shape, resembling the surrounding parenchymal cells. Mitosis was not found. No portal tract, central vein or ductule was found within the lesion. The tumor tissue showed a positive reaction for cytokeratin (CK) 18, but not for CK19, vimentin, estrogen and progesterone receptors. Monoclonality was demonstrated for the lesion, confirming the diagnosis of an LCA. Clonality analysis is helpful for its distinction from focal nodular hyperplasia.
Keywords: Liver Cell adenoma, Clonality, Literature review
INTRODUCTION
Liver cell adenoma (LCA) is a rare benign liver tumor. It is considered a hepatocellular neoplasm, while some authors hold that LCA originated from liver progenitor cell[1]. A female predominance was noticed for the development of LCA based on the reports from Western countries, with a majority of cases associated with a long-term use of oral contraceptives or other steroids. Other diseases should be excluded before establishing the diagnosis of an LCA. It is indeed a difficult task to distinguish LCA from focal nodular hyperplasia (FNH) when the morphologic features are not prominent and a central scar is absent. Then some molecular approaches, including clonality analysis, may be helpful. In this article, a case of LCA in a female Chinese patient is presented, with its clonality status demonstrated.
CASE REPORT
A hepatic mass was found in the right lobe of a 33-year-old woman during her routine medical check-up. She was then admitted to Tangdu Hospital in Xi’an in January 28, 2003. She had never used oral contraceptives, and she had no history of alcohol abuse or hepatitis. No record of HCC or any hereditary disease was found among her family members. The parameters of routine clinical biochemistry, including values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), γ-glutamyltransferase (γ-GTP) and concentrations of α-fetoprotein (AFP) and plasma proteins, were all within normal ranges. The laboratory tests failed to show any positive signal in her serum for hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus (HCV) antibody.
Ultrasonography revealed a solid mass in the posterior part of the right lobe of liver. Computed tomography (CT) scanning showed reduction of density for the lesion sizing 2.0 cm in diameter (Figure 1), indicating malignant potential. Laparotomy was then performed on February 1, 2003. Size of the liver appeared normal, with a mass found in the right lobe. Partial hepatectomy was then performed. Appearance, color and texture of the surrounding liver were normal, without any indication of cirrhosis, pronounced fibrosis or cholestasis.
Figure 1.
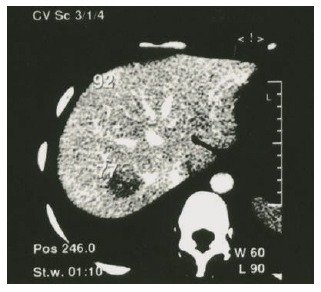
CT scanning showing a lesion with reduction of density in the posterior part of the right lobe of liver.
Histological and immunohistochemical procedures
The sample was fixed in 40 g/L formaldehyde solution, embedded in paraffin. Sections of 4 µm in thickness were prepared and stained by hematoxylin and eosin (HE), Masson trichrome methods and periodic acid-Schiff (PAS) reaction. Immunostaining was carried out using a streptavidin-labeled peroxidase (S-P) kit (KIT9730) as described previously[2]. The primary antibodies used in this study included those against cytokeratin (CK) 18, CK19, vimentin, CD34, estrogen receptor (ER), progesterone receptor (PR), AFP, S-100 protein, HBsAg, hepatitis B core antigen (HBcAg), as well as an anti-HCV antibody. All of the reagents for immunostaining were supplied by Maxim Biotechnology Corporation Limited, Fuzhou, China.
Clonal analysis
Sections of 10 μm in thickness were prepared, deparaffinized, rehydrated and HE stained. Neoplastic tissues were dissected using a syringe needle from 4 different tumor areas, sizing 0.5 cm × 0.5 cm for each. Normal liver tissue was also isolated from the surrounding parenchyma at 3 different sites of the same size and analyzed in a parallel way as reference samples. Genomic DNA was extracted using a QIAamp kit (Qiagen, Mannheim, Germany). Polymorphism was examined at the androgen receptor (AR) and phosphoglycerokinase (PGK) loci as described previously[3,4], with the former gene proved polymorphic at the CAG short-tandem repeat (STR) located in exon 1. Loss of X-chromosomal inactivation mosaicism was demonstrated by pretreatment of DNA with methylation-sensitive restriction enzyme Hha I and amplification via nested PCR. The CAG STR-polymorphic alleles were resolved on a 100 g/L denaturing polyacrylamide gel at 120 V for 4 h. A reduction of at least 50% in density of either band, as compared to that obtained using the sample not treated with Hha I, is regarded as loss of X-chromosomal inactivation mosaicism [5].
RESULTS
A partial resection liver specimen, sizing 4.0 cm × 3.0 cm × 2.0 cm, presented with a spherical mass of 2.0 cm in its maximal dimention. The mass was beneath the hepatic capsule, yellow brown in color and soft in its texture, without any necrotic focus or fibrotic scar in its cut surface. There was a clear border, but not a fibrotic septum between the lesion and surrounding liver tissue that appeared red brown and apparently normal. Microscopically, the lesion was composed of cells arranged in two-cell-thick cords, with the cell cords separated by sinusoids (Figure 2A). Focal fatty change and excessive glycogen storage were present (Figure 2A and 2B). The tumor cells were round or polygonal, apparently resembling the surrounding liver parenchyma cells in size and shape. Mitosis was not found. There was no portal tract or hepatic venule in the tumor. Ductule or scattered ductular cells (the so-called “oval cells”[6,7] or “liver progenitor cells”[1]) were absent, and immunostaining for the ductular cell markers, including CK19 and S-100 protein[7,8], failed to show any positive cell within the lesion. The tumor cells were positive for CK18 (Figure 2C), but negative for AFP, vimentin and p53 protein. They did not show any positive signal for ER or PR. Both neoplastic and the adjacent parenchymal tissues were negative for HBsAg, HBcAg and HCV antigen.
Figure 2.
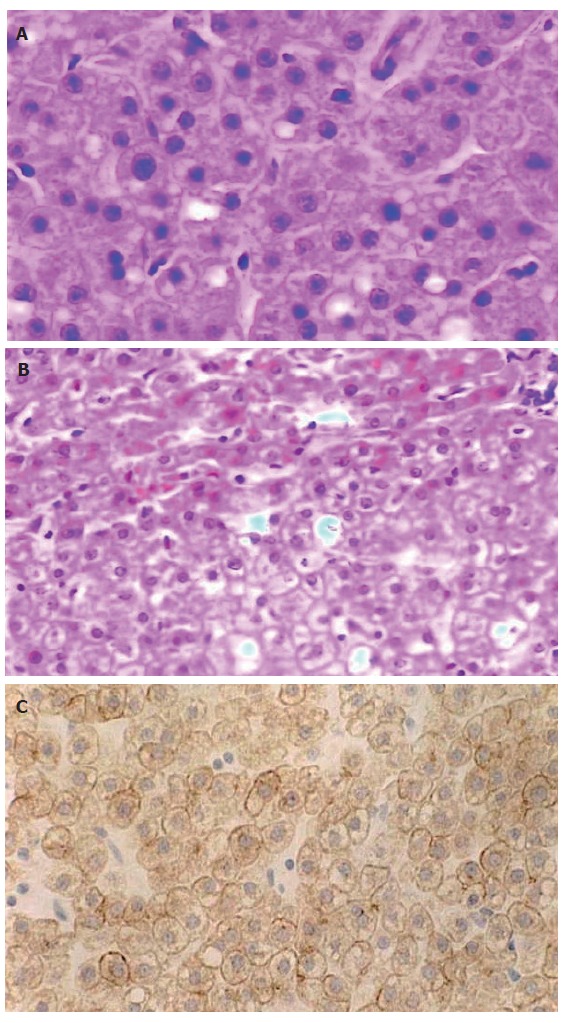
Liver cell adenoma (× 400). A: Similar to normal hepatocytes, arranged in two-cell-thick cords separated by hepatic sinusoids (HE); B: Showing the border between LCA (lower) and surrounding liver parenchyma (upper) (HE); C: CK18 immunoreactivity (S-P).
Clonality status of the lesion was determined by an assay based on the CAG-STR polymorphism at exon 1 of AR gene. Pretreatment with Hha I resulted in pronounced reduction or loss of the upper band for all of the tissue samples from 4 different areas of the lesion (Figure 3), demonstrating loss of the X-chromosomal inactivation mosaicism. The adjacent liver parenchyma, however, did not show the change (Figure 4). The data proved the monoclonal, neoplastic nature of the lesion, confirming the diagnosis of LCA. The patient has survived for 30 mo after the operation without any indication of recurrence.
Figure 3.
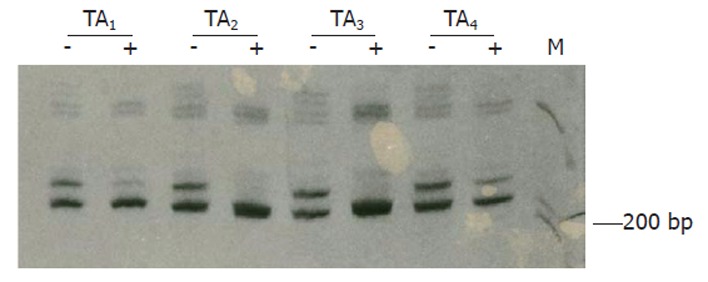
Clonal analysis on tissues from 4 tumor areas (TAs). The pretreatment with Hha I results in loss of the upper band for TA2 and TA3, and intensity reduction by factors of 71.8% and 57.1% for TA1 and TA4, respectively. +, with Hha I pretreatment; -, without Hha I treatment.
Figure 4.
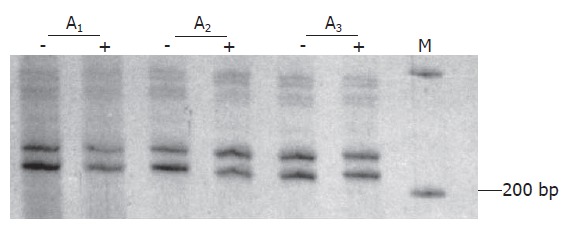
Data of clonality test of three areas of liver parenchymal tissues (A1-A3). Neither of the bands on gel shows marked intensity change for the samples pretreated with Hha I (+) compared to those not treated (-).
DISCUSSION
LCA is a rare benign hepatic neoplasm, accounting for less than 2% of all hepatic tumors[9]. A pronounced female predominance was noted mainly by authors from Western countries. It often occurs in women of 20 to 40 years during their child-bearing period[10,11], being closely associated with long-term use of steroids, mainly oral contraceptives[12-14]. In fact, it was rarely reported before the introduction of oral contraceptives in 1960s. Edmondson et al[15] found only two cases in 48 900 necropsies performed in Los Angeles General Hospital during the period from 1918 to 1954. In 1973, Baum et al[16] pointed out the possible link between the use of oral contraceptives and LCA development. Data from several reports has confirmed the etiologic association. Leese et al [17] reported 24 cases of HA, 16 (66.7%) of them with a history of using oral contraceptives. Tumor regression was observed in some cases after withdrawal of the hormones[18,19], and then the tumor remained silent or grew slowly for many years, or even progressed to HCC[20], albeit infrequently. Complete remission was observed in an LCA patient, who had used oral contraceptives for 8 years, after the hormone withdrawal for 9 months[21].
Through literature review, the clinicopathological data of 127 cases of LCA reported from Chinese patients were collected and compared to those of 130 patients from Western countries, with the male/female ratios being 1.8/1 and 1/2.9, respectively. The ages of the Chinese patients ranged from 2 to 73 years, with their mean and average values estimated to be 31.0 and 35.8 years, respectively. For patients from Western countries, the ages ranged from 11 months to 82 years, with their mean and average values 30.0 and 31.6 years, respectively. Only 3.9% (5/127) of Chinese patients had a history of using oral contraceptives, the percentage being much lower than that for the patients from Western countries (54/130, 41.5%). Difference is evident, therefore, between the LCAs occurring in China and in Western countries in their etiologic associations, indicating possibly different pathogenic pathways. This, at least partly, provides an explanation for the distinct gender distribution pattern in Chinese patients.
Other factors are also linked to LCA development, including glycogen storage diseases [22] and administration of danazol[23], phenobarbital[24] and androgenic/anabolic steroids[9,25-33]. Among the 130 cases from Western countries, 4 (3.1%) were found to have glycogen-storage diseases, but only one (0.8%), in the Chinese group, was with the association. For some LCA cases, there seemed no identifiable pathogenic factor. The majority of the cases from the Chinese group fell into this category. Among the 127 Chinese patients, 13 (10.2%) were seropositive for HBsAg, and 8 of them were with chronic hepatitis[34-37]. Among the patients from Western countries, however, only one (0.8%) was shown to be an HBsAg-carrier. In consideration of the high prevalence (up to 10%[38]) of HBsAg-carrier state in China, the data does not provide support for the role of persistent HBV infection in development of the solitary LCA.
Most of LCAs grow slowly and are asymptomatic or cause only mild symptoms, while rupture and hemorrhage may occur in some tumors (15%-33%[39]), and malignant transformation was also observed in a minority of the cases (5/39, 12.8%[20]). Ultrasonography, CT scanning and magnetic resonance imaging (MRI) are useful for detecting hepatic occupation and determining its location and size. Imaging approaches may be helpful for identifying LCA from other hepatic lesions, and a peritumorous halo, demonstrated by CT scanning, was considered indicative of LCA[40,41]. These features, however, are not specific[40], and the accurate preoperative diagnosis of LCA, particularly the distinction from HCC, is frequently problematic. This is even more serious as a surgical consideration in China where incidence of HCC is overwhelmingly high compared to that of LCA. For the pathologists, well differentiated HCC and FNH are among the hepatic lesions that should be excluded before making a diagnosis of LCA.
HCC is diagnosed usually at ages between 40 and 60 years, with a pronounced male predominance. In China, including Hong Kong and Taiwan, about 80% of HCCs were found in patients with chronic hepatitis B and cirrhosis or advanced liver fibrosis. An elevated level of circulating AFP is indicative of HCC, but this change may not be evident in patients with an early-stage, often well differentiated, HCC. Liver-cell plates more than three cells thick, acinar structures, increased nuclear/cytoplasmic ratio, prominent nucleoli, mitoses, increased cytoplasmic basophilia, loss of the reticulin fibers, absence of Kupffer cells, presence of vascular invasion or immunoreactivity for AFP, all indicate HCC. However, none of these features can be relied upon with certainty, as some are also seen in the hepatic lesions with high-grade SCC, including the premalignant nodules of altered hepatocytes[2], adenomatous hyperplasia[42-46] and LCA. The most helpful parameters, in our consideration, are thickness of the liver-cell plates (more than three cells), cell density (an increase of two folds compared to the surrounding liver parenchyma) and vascular invasion[38]. It should be noted that male, cirrhosis, and chronic HBV infection strongly indicate that a hepatic neoplasm is malignant. Conversely, female and a history of oral contraceptive use support the diagnosis of LCA. Exceptional difficulties may be encountered in some of the LCAs associated with long-term use of anabolic steroids or metabolic disorders. Some of them may show pronounced architectural disturbance and cellular atypia, which make their distinction impossible from well-differentiated HCCs based on histological grounds alone. Malignant transformation is proposed for such cases[13,20,47,48], but they often show more favorable clinical courses, or even regression after withdrawal of the steroid[21]. Such lesions may represent the borderline hepatocellular neoplasm, and their behaviors should be determined by careful postoperative observations.
FNH occurs most commonly in young women, having similar etiological associations and clinical manifestations to LCA[49-51]. It is a localized lesion, frequently solitary, within an otherwise normal or nearly normal liver. The lesion is similar to cirrhosis by its histology, and a central stellate fibrous region containing large vessels can often be found. Its development has been attributed to the vascular malformation[52,53]. Usually, FNH is readily distinguished from LCA by its central scar, multinodularity and presence of proliferating bile ductules in the fibrous septa. It may become a diagnostic problem, however, when the central fibrous region is not evident. Data from different laboratories have demonstrated the polyclonal cell composition and indicated non-neoplastic nature for the lesion[54,55]. In contrast, an LCA was shown to be monoclonal[56], and our data confirm the conclusion that LCA is a neoplastic lesion. The clonality assays, therefore, are helpful for the differential diagnosis between LCA and some FNH lesions without an identifiable central scar.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30171052 and No. 30572125
S- Editor Pan BR L- Editor Zhu LH E- Editor Zhang Y
References
- 1.Libbrecht L, De Vos R, Cassiman D, Desmet V, Aerts R, Roskams T. Hepatic progenitor cells in hepatocellular adenomas. Am J Surg Pathol. 2001;25:1388–1396. doi: 10.1097/00000478-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Su Q, Benner A, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Human hepatic preneoplasia: phenotypes and proliferation kinetics of foci and nodules of altered hepatocytes and their relationship to liver cell dysplasia. Virchows Arch. 1997;431:391–406. doi: 10.1007/s004280050116. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Su Q, Zhu S, Liu J, Hu L, Li D. Clonality of multiple uterine leiomyomas. Zhonghua Bing Li Xue Za Zhi. 2002;31:107–111. [PubMed] [Google Scholar]
- 4.Diao XL, Su Q, Wang SF, Feng YM, Liu J. Non-isotopic clonality analysis on uterine leiomyomas based on AR gene polymorphism. Disi Junyi Daxue Xuebao. 2002;23:1969–1973. [Google Scholar]
- 5.Wang SF, Liu Q, Zhang W, Liu J, Su Q. Clonality of uterine leiomyomas, an assay using X chromosome polymorphism at the phosphoglycerate kinase locus. Disi Junyi Daxue Xuebao. 2001;22:1576–1582. [Google Scholar]
- 6.Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER, Thorgeirsson SS. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- 7.Su Q, Liu YF, Zhang JF, Zhang SX, Li DF, Yang JJ. Expression of insulin-like growth factor II in hepatitis B, cirrhosis and hepatocellular carcinoma: its relationship with hepatitis B virus antigen expression. Hepatology. 1994;20:788–799. doi: 10.1002/hep.1840200404. [DOI] [PubMed] [Google Scholar]
- 8.Su Q, Zerban H, Otto G, Bannasch P. Cytokeratin expression is reduced in glycogenotic clear hepatocytes but increased in ground-glass cells in chronic human and woodchuck hepadnaviral infection. Hepatology. 1998;28:347–359. doi: 10.1002/hep.510280209. [DOI] [PubMed] [Google Scholar]
- 9.Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Semin Liver Dis. 1981;1:116–128. doi: 10.1055/s-2008-1040724. [DOI] [PubMed] [Google Scholar]
- 10.Hytiroglou P, Theise ND. Differential diagnosis of hepatocellular nodular lesions. Semin Diagn Pathol. 1998;15:285–299. [PubMed] [Google Scholar]
- 11.Li LG, Cui XJ, Li HN. Hepatocellular Adenoma. Zhonghua Waike Zazhi. 1995;10:613. [Google Scholar]
- 12.Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 13.Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF 2nd. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 14.Shortell CK, Schwartz SI. Hepatic adenoma and focal nodular hyperplasia. Surg Gynecol Obstet. 1991;173:426–431. [PubMed] [Google Scholar]
- 15.EDMONDSON HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–929. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 17.Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558–564. doi: 10.1097/00000658-198811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edmondson HA, Reynolds TB, Henderson B, Benton B. Regression of liver cell adenomas associated with oral contraceptives. Ann Intern Med. 1977;86:180–182. doi: 10.7326/0003-4819-86-2-180. [DOI] [PubMed] [Google Scholar]
- 19.Tao LC. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 1991;68:341–347. doi: 10.1002/1097-0142(19910715)68:2<341::aid-cncr2820680223>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129:712–717. doi: 10.1001/archsurg.1994.01420310044007. [DOI] [PubMed] [Google Scholar]
- 21.Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, Osio C, Vertemati M, Forti D. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33:234–236. doi: 10.1097/00004836-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bartley J, Loddenkemper C, Lange J, Mechsner S, Radke C, Neuhaus P, Ebert AD. Hepatocellular adenoma and focal nodular hyperplasia after long-term use of danazol for endometriosis: a case report. Arch Gynecol Obstet. 2004;269:290–293. doi: 10.1007/s00404-002-0435-z. [DOI] [PubMed] [Google Scholar]
- 24.Ferko A, Bedrna J, Nozicka J. [Pigmented hepatocellular adenoma of the liver caused by long-term use of phenobarbital] Rozhl Chir. 2003;82:192–195. [PubMed] [Google Scholar]
- 25.Paradinas FJ, Bull TB, Westaby D, Murray-Lyon IM. Hyperplasia and prolapse of hepatocytes into hepatic veins during longterm methyltestosterone therapy: possible relationships of these changes to the developement of peliosis hepatis and liver tumours. Histopathology. 1977;1:225–246. doi: 10.1111/j.1365-2559.1977.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 26.Westaby D, Portmann B, Williams R. Androgen related primary hepatic tumors in non-Fanconi patients. Cancer. 1983;51:1947–1952. doi: 10.1002/1097-0142(19830515)51:10<1947::aid-cncr2820511034>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Chandra RS, Kapur SP, Kelleher J Jr, Luban N, Patterson K. Benign hepatocellular tumors in the young. A clinicopathologic spectrum. Arch Pathol Lab Med. 1984;108:168–171. [PubMed] [Google Scholar]
- 28.Carrasco D, Prieto M, Pallardó L, Moll JL, Cruz JM, Muñoz C, Berenguer J. Multiple hepatic adenomas after long-term therapy with testosterone enanthate. Review of the literature. J Hepatol. 1985;1:573–578. doi: 10.1016/s0168-8278(85)80001-5. [DOI] [PubMed] [Google Scholar]
- 29.Grangé JD, Guéchot J, Legendre C, Giboudeau J, Darnis F, Poupon R. Liver adenoma and focal nodular hyperplasia in a man with high endogenous sex steroids. Gastroenterology. 1987;93:1409–1413. doi: 10.1016/0016-5085(87)90273-3. [DOI] [PubMed] [Google Scholar]
- 30.Ishak KG, Zimmerman HJ. Hepatotoxic effects of the anabolic/androgenic steroids. Semin Liver Dis. 1987;7:230–236. doi: 10.1055/s-2008-1040579. [DOI] [PubMed] [Google Scholar]
- 31.Søe KL, Søe M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12:73–79. doi: 10.1111/j.1600-0676.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 32.Creagh TM, Rubin A, Evans DJ. Hepatic tumours induced by anabolic steroids in an athlete. J Clin Pathol. 1988;41:441–443. doi: 10.1136/jcp.41.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klava A, Super P, Aldridge M, Horner J, Guillou P. Body builder's liver. J R Soc Med. 1994;87:43–44. doi: 10.1177/014107689408700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Chen LZ, Li XH. Six cases of hepatocellular adenoma. Zhonghua Gandan Waike Zazhi. 2003;9:142–147. [Google Scholar]
- 35.Guan CN. Liver cell adenoma. Zhongliu Fangzhi Yanjiu. 2001;28:156. [Google Scholar]
- 36.Xiao KY, Li LQ, Peng MH, Chen B, Shang LM. Misdiagnosis analysis of hepatocellular adenoma in older man. Linchuang Wuzhen Wuzhi. 2004;17:191. [Google Scholar]
- 37.Pan SB, Ye GR, Che SY. Analysis of nine cases of hepatocellular adenoma misdiagnosed as HCC. Ling Nan Xiandai Linchuang Waike. 2004;4:96. [Google Scholar]
- 38.Su Q. Preneoplastic lesions in human liver. Zhenduan Binglixue Zazhi. 2003;10:112–115. [Google Scholar]
- 39.Tan M, Di Carlo A, Robinson P, Tchervenkov JI, Barkun JS, Metrakos P. Successful outcome after transplantation of a donor liver with focal nodular hyperplasia. Liver Transpl. 2001;7:652–655. doi: 10.1053/jlts.2001.23910. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu D, Bruneton JN, Drouillard J, Pointreau CC, Vasile N. Hepatic adenomas and focal nodular hyperplasia: dynamic CT study. Radiology. 1986;160:53–58. doi: 10.1148/radiology.160.1.3520655. [DOI] [PubMed] [Google Scholar]
- 41.Welch TJ, Sheedy PF 2nd, Johnson CM, Stephens DH, Charboneau JW, Brown ML, May GR, Adson MA, McGill DB. Focal nodular hyperplasia and hepatic adenoma: comparison of angiography, CT, US, and scintigraphy. Radiology. 1985;156:593–595. doi: 10.1148/radiology.156.3.3895291. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863–877. doi: 10.1002/1097-0142(19830301)51:5<863::aid-cncr2820510520>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Ferrell LD, Crawford JM, Dhillon AP, Scheuer PJ, Nakanuma Y. Proposal for standardized criteria for the diagnosis of benign, borderline, and malignant hepatocellular lesions arising in chronic advanced liver disease. Am J Surg Pathol. 1993;17:1113–1123. doi: 10.1097/00000478-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Le Bail B, Belleannée G, Bernard PH, Saric J, Balabaud C, Bioulac-Sage P. Adenomatous hyperplasia in cirrhotic livers: histological evaluation, cellular density, and proliferative activity of 35 macronodular lesions in the cirrhotic explants of 10 adult French patients. Hum Pathol. 1995;26:897–906. doi: 10.1016/0046-8177(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 45.Hytiroglou P, Theise ND, Schwartz M, Mor E, Miller C, Thung SN. Macroregenerative nodules in a series of adult cirrhotic liver explants: issues of classification and nomenclature. Hepatology. 1995;21:703–708. [PubMed] [Google Scholar]
- 46.Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 47.Ferrell LD. Hepatocellular carcinoma arising in a focus of multilobular adenoma. A case report. Am J Surg Pathol. 1993;17:525–529. doi: 10.1097/00000478-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Scott FR, el-Refaie A, More L, Scheuer PJ, Dhillon AP. Hepatocellular carcinoma arising in an adenoma: value of QBend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology. 1996;28:472–474. doi: 10.1046/j.1365-2559.1996.t01-3-297345.x. [DOI] [PubMed] [Google Scholar]
- 49.Stocker JT, Ishak KG. Focal nodular hyperplasia of the liver: a study of 21 pediatric cases. Cancer. 1981;48:336–345. doi: 10.1002/1097-0142(19810715)48:2<336::aid-cncr2820480220>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Brady MS, Coit DG. Focal nodular hyperplasia of the liver. Surg Gynecol Obstet. 1990;171:377–381. [PubMed] [Google Scholar]
- 51.Pain JA, Gimson AE, Williams R, Howard ER. Focal nodular hyperplasia of the liver: results of treatment and options in management. Gut. 1991;32:524–527. doi: 10.1136/gut.32.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- 53.Kondo F, Nagao T, Sato T, Tomizawa M, Kondo Y, Matsuzaki O, Wada K, Wakatsuki S, Nagao K, Tsubouchi H, et al. Etiological analysis of focal nodular hyperplasia of the liver, with emphasis on similar abnormal vasculatures to nodular regenerative hyperplasia and idiopathic portal hypertension. Pathol Res Pract. 1998;194:487–495. doi: 10.1016/S0344-0338(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 54.Paradis V, Laurent A, Flejou JF, Vidaud M, Bedossa P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997;26:891–895. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- 55.Zhang SH, Cong WM, Wu MC. Focal nodular hyperplasia with concomitant hepatocellular carcinoma: a case report and clonal analysis. J Clin Pathol. 2004;57:556–559. doi: 10.1136/jcp.2003.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradis V, Benzekri A, Dargère D, Bièche I, Laurendeau I, Vilgrain V, Belghiti J, Vidaud M, Degott C, Bedossa P. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126:1323–1329. doi: 10.1053/j.gastro.2004.02.005. [DOI] [PubMed] [Google Scholar]


