Abstract
Spontaneous rupture is a rare complication of splenic hamartoma. A review of the literature revealed only four such cases. To the best of our knowledge, this is the first report of spontaneous rupture of splenic hamartoma associated with liver cirrhosis and portal hypertension. A 53-year-old woman, who was followed up for aortic dissection and hepatitis C virus (HCV)-related liver cirrhosis, was referred with sudden left chest and shoulder pain. An abdominal ultrasound showed intraabdominal bleeding, and computed tomography indicated rupture of a splenic tumor. Emergent splenectomy was carried out. The postoperative course was uneventful, and the patient was discharged on the 13th postoperative day. Pathology revealed the tumor to be a ruptured splenic hamartoma. The non-tumorous splenic parenchyma revealed congestive changes. We consider that the presence of liver cirrhosis and portal hypertension are risk factors for spontaneous rupture of the splenic hamartoma.
Keywords: Splenic hamartoma, Spontaneous rupture, Hepatitis C virus, Cirrhosis, Portal hypertension
INTRODUCTION
Splenic hamartoma is a rare benign tumor, and is usually asymptomatic. Its spontaneous rupture is an uncommon, but life-threatening, complication. A review of the literature revealed only four such cases [1-4]. Furthermore, there has been little discussion of underlying disease and no previous reports of a ruptured splenic hamartoma in a patient with chronic liver disease. Here we report a case of spontaneous rupture of splenic hamartoma associated with hepatitis C virus (HCV)-related cirrhosis and portal hypertension.
CASE REPORT
A 53-year-old woman was referred to our emergency care center with sudden left chest and shoulder pain without a history of trauma. She was being followed up for aortic dissection (DeBakey typeIIIb) and HCV-related liver cirrhosis. The aortic dissection had been stable for seven years and the pseudo-lumen was also patent. Portal hypertension and moderate splenomegaly was associated with the liver cirrhosis. During the clinical examination, the patient lost consciousness and her blood pressure dropped below 60 mmHg, though rapid infusion of saline prompted a swift recovery of consciousness and blood pressure. Initially, ischemic heart disease was suspected, but a chest X-ray and electrocardiogram showed no abnormal findings. Laboratory data were: hemoglobin, 8.2 g/dL; platelets, 5.9 × 104/m3; total bilirubin, 1.2 mg/dL; asparate aminotransferase, 63 IU/L; alanine aminotransferase, 53 IU/L, albumin, 2.8 g/dL; prothrombin time, 74% to control. An abdominal ultrasound revealed fluid collection suggestive of intraabdominal bleeding. Computed tomography indicated rupture of a splenic tumor, which had been not detected by the previous screening for the aortic dissection (Figure 1). The patient was transferred to our intensive care unit, where conservative treatment was started, beginning with a blood transfusion. Interventional treatment was not initially selected because of the aortic dissection. However, her blood pressure dropped again, necessitating an emergency operation.
Figure 1.
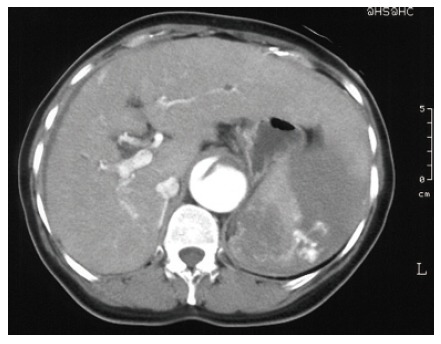
Enhanced computed tomography showed intraabdominal fluid collection near the spleen, and contrast material outflow from the splenic tumor.
At laparotomy, there was a massive hemorrhage with recovered blood weighing 2 880 g. Active bleeding continued from the ruptured spleen, and a splenectomy was performed. The spleen weighed 520 g and measured 15 × 10 cm with subcapsular hemorrhage. Macroscopically, there were four nodules on the cut surface of the specimen. The nodule near the upper pole was the largest, 4.5 × 3.0 cm in diameter, and ruptured, causing intraabdominal hemorrhage (Figure 2A). Microscopically, the nodules were not encapsulated and composed of sinusoidal spaces and cords of Billroth as in normal red pulp without lymphatic follicles or fibrous trabeculae (Figure 2B). The vessel spaces linked by plump endothelial cells were filled with a mixed population of erythrocytes and lymphoreticular cells. Pathology revealed the tumor to be a ruptured splenic hamartoma, of the red pulp type. The non-tumorous splenic parenchyma showed proliferation of splenic sinuses and cords with extramedullary hematopoiesis, indicating congestive changes. These findings corresponded to the splenomegaly caused by portal hypertension. The postoperative course was uneventful, and the patient was discharged on the 13th postoperative day.
Figure 2.
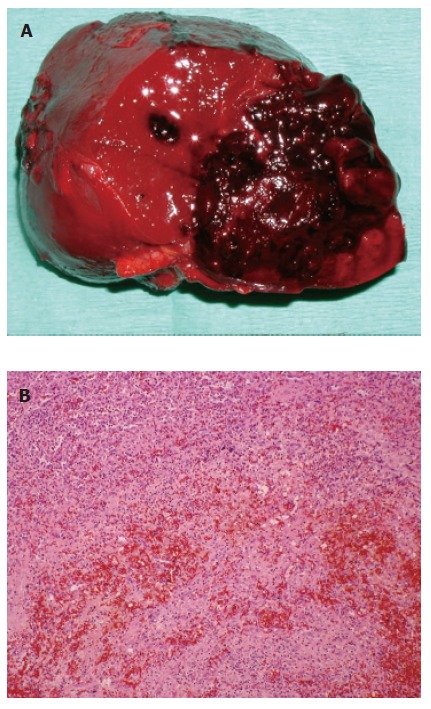
A: Macroscopic findings for the ruptured splenic hamartoma. The hamartoma is well demarcated from the surrounding parenchyma and significant hemorrhage is shown; B: Microscopy showed a red pulp with no lymphatic structures, or trabeculae of the spleen. (hematoxylin-eosin, original magnification, ×200).
DISCUSSION
Splenic hamartoma is a rare benign tumor composed of all the normal constituents of splenic parenchyma arranged in a disorganized fashion. The incidence has been reported as 0.024-0.13% from autopsy cases [5,6]. Clinical symptoms include mass effect due to splenomegaly, pancytopenia, hematological disorders, and spontaneous rupture [5-7]. The average spleen weight and size of all reported hamartomas were about 600 g and 5.1 cm, respectively [6,7].
Spontaneous rupture of splenic hamartoma is rare. The cause of rupture has not been specified in any of the small number of cases reported to date (Table 1). The cases discussed in these reports showed mild to moderate splenomegaly, however, the weight of the spleen (117-459 g) was below the average, and the size of the hamartomas (2.5 cm-5.4 cm in diameter) did not far exceed the average. There were also no underlying diseases, except for one case, in which the patient had lung cancer. These cases indicate that the weight of the spleen, the size of the hamartoma, or any underlying diseases, is not closely related to rupture of the hamartoma. In contrast to these cases, our case was characterized by moderate splenomegaly in association with hepatitis C virus-related cirrhosis. The patient had no other risks of spontaneous splenic rupture, such as a history of trauma.
Table 1.
Literature review of spontaneous rupture of splenic hamartoma
| Author | Year | Age/gender | Spleen weight/size | No. of tumors | Tumor size | Underlying disease |
| Morgenstern et al [1] | 1984 | 73 yr/F | 117 g/11×7×4.5 cm | 1 | 2.5 cm | no |
| Ferguson et al [2] | 1993 | 48 yr/F | 459 g/15.5×8.0×4.5 cm | 1 | 5.4 cm | no |
| Yoshizawa et al [3] | 1999 | 5 mo/F | 230 g/6×9×6 cm | 1 | 5.0 cm | no |
| Ballardini et al [4] | 2004 | 60 yr/M | NA | 1 | NA | lung cancer (chemotherapy) |
| Present case | 2005 | 53 yr/F | 520 g/15×10×4 cm | 4 | 4.5 cm | liver cirrhosis, portal hypertension |
Whether or not hepatitis C virus-related cirrhosis contributed to the rupture of the splenic hamartoma in our case is unclear. Spontaneous rupture of the spleen is a rare complication of liver cirrhosis [8-14]. In these reported cases, portal hypertension associated with liver cirrhosis has been suspected of playing a role in splenic rupture. In our case, portal hypertension and splenomegaly due to cirrhosis was apparent, and microscopy revealed congestive changes in the non-tumorous splenic parenchyma. Since the hamartoma nodules are not encapsulated and composed of sinusoidal spaces and cords of Billroth as in normal red pulp, it is quite likely that the hamartoma itself was affected by portal hypertension. Although an association of splenic hamartoma with liver cirrhosis and portal hypertension has been demonstrated in several studies [1,15-17], to the best of our knowledge, this is the first report of spontaneous rupture of splenic hamartoma associated with liver cirrhosis and portal hypertension.
Recent progress in imaging enables us to detect splenic tumors, and elective surgery is sometimes conducted [18-20]. Operative indications for splenic hamartoma include the possibility of malignant disease and the risk of spontaneous rupture. If splenic hamartoma is suspected by image findings, elective splenectomy should be taken into consideration to make a definitive diagnosis and to prevent rupture [21]. We consider that the presence of liver cirrhosis and portal hypertension are risk factors for spontaneous rupture of the splenic hamartoma.
Footnotes
S- Editor Guo SY L- Editor Pravda J E- Editor Zhang Y
References
- 1.Morgenstern L, McCafferty L, Rosenberg J, Michel SL. Hamartomas of the spleen. Arch Surg. 1984;119:1291–1293. doi: 10.1001/archsurg.1984.01390230057013. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson ER, Sardi A, Beckman EN. Spontaneous rupture of splenic hamartoma. J La State Med Soc. 1993;145:48–52. [PubMed] [Google Scholar]
- 3.Yoshizawa J, Mizuno R, Yoshida T, Kanai M, Kurobe M, Yamazaki Y. Spontaneous rupture of splenic hamartoma: a case report. J Pediatr Surg. 1999;34:498–499. doi: 10.1016/s0022-3468(99)90512-2. [DOI] [PubMed] [Google Scholar]
- 4.Ballardini P, Incasa E, Del Noce A, Cavazzini L, Martoni A, Piana E. Spontaneous splenic rupture after the start of lung cancer chemotherapy. A case report. Tumori. 2004;90:144–146. doi: 10.1177/030089160409000129. [DOI] [PubMed] [Google Scholar]
- 5.Silverman ML, LiVolsi VA. Splenic hamartoma. Am J Clin Pathol. 1978;70:224–229. doi: 10.1093/ajcp/70.2.224. [DOI] [PubMed] [Google Scholar]
- 6.Lam KY, Yip KH, Peh WC. Splenic vascular lesions: unusual features and a review of the literature. Aust N Z J Surg. 1999;69:422–425. doi: 10.1046/j.1440-1622.1999.01550.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg JJ, Suhrland M, Valensi Q. The spleen in the spleen syndrome: the association of splenoma with hematopoietic and neoplastic disease--compendium of cases since 1864. J Surg Oncol. 1991;47:193–202. doi: 10.1002/jso.2930470311. [DOI] [PubMed] [Google Scholar]
- 8.WOOD DA. Pathologic aspects of acute epidemic hepatitis, with especial reference to early stages; report of a series of ten cases, including a case in which there was spontaneous rupture of the spleen and six cases of fulminating disease in patients who had been wounded several months previously. Arch Pathol (Chic) 1946;41:345–375. [PubMed] [Google Scholar]
- 9.Thijs JC, Schneider AJ, van Kordelaar JM. Spontaneous rupture of the spleen complicating portal hypertension. Intensive Care Med. 1983;9:299–300. doi: 10.1007/BF01691260. [DOI] [PubMed] [Google Scholar]
- 10.Van Landingham SB, Rawls DE, Roberts JW. Pathological rupture of the spleen associated with hepatitis A. Arch Surg. 1984;119:224–225. doi: 10.1001/archsurg.1984.01390140080014. [DOI] [PubMed] [Google Scholar]
- 11.DeSitter L, Rector WG Jr. The significance of bloody ascites in patients with cirrhosis. Am J Gastroenterol. 1984;79:136–138. [PubMed] [Google Scholar]
- 12.Horie Y, Suou T, Hirayama C, Nagasako R. Spontaneous rupture of the spleen secondary to metastatic hepatocellular carcinoma: a report of a case and review of the literature. Am J Gastroenterol. 1982;77:882–884. [PubMed] [Google Scholar]
- 13.Chien RN, Liaw YF. Pathological rupture of spleen in hepatitis B virus-related cirrhosis. Am J Gastroenterol. 1993;88:1793–1795. [PubMed] [Google Scholar]
- 14.Sugahara K, Togashi H, Aoki M, Mitsuhashi H, Matsuo T, Watanabe H, Abe T, Ohno S, Saito K, Saito T, et al. Spontaneous splenic rupture in a patient with large hepatocellular carcinoma. Am J Gastroenterol. 1999;94:276–278. doi: 10.1111/j.1572-0241.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhagwat AG, Datta DV, Mitra S, Aikat BK. Splenoma with portal hypertension. Br Med J. 1975;3:520. doi: 10.1136/bmj.3.5982.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spalding RM, Jennings CV, Yam LT. Splenic hamartoma. Br J Radiol. 1980;53:1197–1200. doi: 10.1259/0007-1285-53-636-1197. [DOI] [PubMed] [Google Scholar]
- 17.Singh K, Subbramaiah A, Choudhary SR, Bhasin DK, Wig JD, Radotra B, Nagi B. Splenic hamartoma with portal hypertension: a case report. Trop Gastroenterol. 1992;13:155–159. [PubMed] [Google Scholar]
- 18.Ohtomo K, Fukuda H, Mori K, Minami M, Itai Y, Inoue Y. CT and MR appearances of splenic hamartoma. J Comput Assist Tomogr. 1992;16:425–428. doi: 10.1097/00004728-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Yu RS, Zhang SZ, Hua JM. Imaging findings of splenic hamartoma. World J Gastroenterol. 2004;10:2613–2615. doi: 10.3748/wjg.v10.i17.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang S, Shimizu T, Kikuchi Y, Shinya S, Kishimoto R, Fujioka Y, Miyasaka K. Color Doppler sonographic findings in splenic hamartoma. J Clin Ultrasound. 2000;28:249–253. doi: 10.1002/(sici)1097-0096(200006)28:5<249::aid-jcu7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi T, Iso Y, Yasunaga C, Kitano S, Sugimachi K. Laparoscopic splenectomy for splenic hamartoma. Surg Endosc. 1997;11:848–849. doi: 10.1007/s004649900469. [DOI] [PubMed] [Google Scholar]


