Abstract
AIM: To study the dynamic changes of hepatits B virus (HBV) DNA in serum and peripheral blood mononuclear cells (PBMCs) of patients after lamivudine therapy.
METHODS: A total of 72 patients with chronic HBV infection were included in this study. All patients were confirmed to have the following conditions: above 16 years of age, elevated serum alanine amonotransferase (ALT), positive hepatitis B e antigen (HBeAg), positive HBV DNA in serum and PBMCs, negative antibodies against HAV, HCV, HDV, HEV. Other possible causes of chronic liver damages, such as drugs, alcohol and autoimmune diseases were excluded. Seventy-two cases were randomly divided into lamivudine treatment group (n = 42) and control group (n = 30). HBV DNA was detected both in serum and in PBMCs by fluorescence quantitative polymerase chain reaction (PCR), during and after lamivudine treatment.
RESULTS: In the treatment group, HBV DNA became negative both in serum and in PBMC, of 38 and 25 out of 42 cases respectively during the 48 wk of lamivudine treatment, the negative rate was 90.5% and 59.5% respectively. In the control group, the negative rate was 23.3% and 16.7% respectively. It was statistically significant at 12, 24 and 48 wk as compared with the control group (P < 0.005). The average conversion period of HBV DNA was 6 wk (2-8 wk) in serum and 16 wk (8-24 wk) in PBMC.
CONCLUSION: Lamivudine has remarkable inhibitory effects on HBV replication both in serum and in PBMCs. The inhibitory effect on HBV DNA in PBMCs is weaker than that in serum.
Keywords: Lamivudine, Hepatitis B virus, DNA, Peripheral blood mononuclear cells
INTRODUCTION
Lamivudine (LAM) is a new antiviral agent against hepatitis B virus (HBV) infection. It has been shown that lamivudine therapy can rapidly reduce HBV DNA levels in serum and improve liver histology[1-3]. However, the dynamic changes of HBV DNAs in serum and peripheral mononuclear cells (PBMCs) in patients after lamivudine therapy are not clear, especially in PBMCs.
PBMCs contain various kinds of active immune cells. The activities of immune cells affect directly the results and efficacy of antiviral therapy[4,5]. Few studies are available on the dynamic changes of HBV DNA in serum and PBMCs. The aim of this study was to investigate the inhibition of HBV DNA in serum and PBMCs in patients after LAM therapy.
MATERIALS AND METHODS
Patients and materials
A total of seventy-two patients with chronic hepatitis B were from Out-patient and In-patient Departments of our hospital during February 2003-February 2004. There were 50 males and 22 females aged 18-60 years (average 32.4 years). Diagnosis of hepatitis B was made according to the revised standard of the diagnosis established at the Tenth National Symposium on Viral Hepatitis in Xi’an in 2000[6]. All patients were confirmed to have the following conditions: above 16 years of age, normal or abnormal ALT, positive HbsAg and HBeAg in serum, negative anti-HCV, anti-HDV and anti-HEV in serum, positive HBV DNA in serum and PBMCs.
Methods
Seventy-two patients with chronic hepatitis B were randomly divided into two groups: lamivudine treatment group (n = 42) receiving 100 mg lamivudine daily for 48 wk, control group (n = 30) receiving routine medication with vitamin C and inosine, etc. All samples were prepared and stored at -20°C for further examination. HBV DNA in serum and PBMCs was detected after 4, 12, 24 and 48 wk of treatment.
Quantitative determination of HBA DNA in serum and PBMCs
HBV DNA in serum was detected by fluorescence quantit-ative PCR assay, strictly according to the manufacturer,s instructions (Da An Gene Institute, Shenzhen, No: 20030041).
Lymphocyte separation medium (Second Reagent Factory of Shanghai, No. 200304) was used to separate PBMCs. After isolation, the cells were washed three times with PBS containing 1% brine. The cells were diluted to 1× 106 /mL with RPMI before detection.
Cellular DNA was extracted according to NaI method. Briefly, 100 μL 1 × 106/mL cell suspension and 200 μL NaI were well mixed, inverted for 20 s, then mixed with 400 μL chloroform/iso-amy alcohol (24:1) and spun by centrifugation at 10 000 r/min for 12 min. Three hundred microlitre supernatant was mixed with 200 μL pure dimethyl carbinol, spun by centrifugation for 12 min at 14 000 r/min. Supernatant was collected and mixed with 1 mL 70% ethanol, the DNA was precipitated at -20°C.
HBV DNA in PBMCs was also detected by fluorescence quatitative PCR assay. The reaction conditions were: pre-denaturation at 93°C for 3 min, followed by 40 cycles of denaturation at 93°C for 45 s, extension at 55°C for 60 s and a final extension at 93°C for 30 s, at 55°C for 60 s. The reference graph was drawn according to standard content by a computer. The amount of HBV DNA was calculated. During PCR, a strict control was performed. All results were negative.
Statistical analysis
Statistical analysis was carried out by chi square test and t-test. P < 0.05 was considered statistically significant.
RESULTS
The content of HBV DNA in serum and PBMC was decreased after lamivudine treatment (Table 1, Figure 1).
Table 1.
Change of HBV DNA in serum and PBMC of chronic hepatitis B patients after lamivudine treatment n (%)
| HBVDNA in serum |
HBVDNA in PBMC |
|||||||
| Group | 4 wk | 12 wk | 24 wk | 48 wk | Group | 4 wk | 12 wk | 24 wk |
| Lamivudine Treatment 42 | 9 (21.1) | 26 (61.9)a | 34 (80.9)a | 38 (90.5)a | 4 (9.5) | 15 (35.7)a | 20 (47.6)a | 25 (59.5)a |
| Routine Treatment 30 | 2 (6.7) | 3 (10.0) | 5 (16.7) | 7 (23.3) | 1 (3.3) | 2 (6.7) | 4 (13.3) | 5 (16.7) |
P < 0.005 vs control group.
Figure 1.
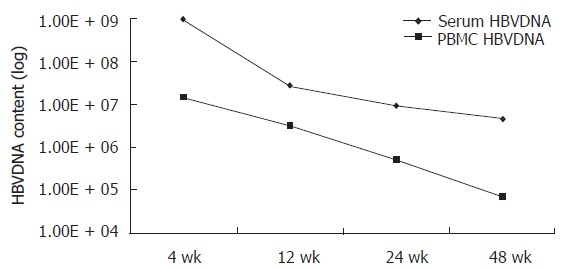
Content of HBV DNA in serum and PBMC of chronic hepatitis B patients after lamivudine treatment.
DISCUSSION
Lamivudine is a HBV polymerase inhibitor and acts on HBV replication both in vitro and in vivo. It has been shown that lamivudine can suppress HBV replication, decrease transaminase levels and improve liver histology[7-10]. However, the main goal of treatment in chronic hepatitis B is to eliminate or persistently inhibit the replication of HBV. After long-term application of lamivudine, antiviral resistance may occur due to HBV polymerase mutation, manifested as increased HBV DNA and serum transaminase, suggesting that the content of HBV DNA can be used to evaluate the efficacy of antiviral therapy. Lamivudine is not able to eliminate HBV ccc DNA in hepatocytes[11], which is the main reason for HBV DNA rebound after withdrawal. Studies have shown that patients with hepatitis B usually accompany disorder of immune function caused by immunological injury[12-25]. PBMCs contain various immune active cells, and play a significant role in immune responses during antiviral therapy. It has been shown that HBV infection may exist in PBMCs[4,26,27]. When PBMCs are infected with HBV, they not only affect the function of immunity, but also weaken the efficacy of antiviral drugs. Therefore, the dynamic change of HBV DNA in PBMCs may be a reliable index for curative antiviral efficacy.
In our study, lamivudine had remarkable inhibitory effects on HBV DNA both in serum and in PBMCs. When the treatment time was prolonged, the negative rate of HBV DNA in serum and PBMCs was gradually increased. The eliminate effect of lamivudine on HBV DNA in PBMCs was lower than that in serum. The negative rate of HBV DNA in serum was 90.48%, but only 59.52% in PBMCs after lamivudine treatment. HBV DNA was positive in 17 cases of chronic hepatitis B after 48 wk of treatment, which may be associated with the fact that mutations occurred in HBV after LAM therapy[28], HBV DNA in PBMCs interferes with the immune activity of cells after HBV integrates with PBMCs, and decreases the contents of Ig, C3, TNF and activity of NK cells as well as the ratio of CD4+/CD8+ [4,5,29]. Therefore, the function of cell-mediated immunity and humoral immunity are reduced and affect the curative effect of lamivudine.
In conclusion, HBV DNA exists in PBMCs even after 48 wk of lamivudine treatment. The presence of HBV DNA in PBMCs may infect hepatocytes again and cause the relapse of hepatitis.
Footnotes
Supported by the Innovation Foundation of Wuhan University, No.301270054
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
References
- 1.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 3.Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562–568. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang R, Feng X, Guo Y, Lu Q, Hou J, Luo K, Fu N. T helper cells in patients with chronic hepatitis B virus infection. Chin Med J (Engl) 2002;115:422–424. [PubMed] [Google Scholar]
- 5.Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, Fujiyama S, Morimoto M, Tanaka K, Takiguchi M. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol. 2002;36:105–115. doi: 10.1016/s0168-8278(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhonghua Yixuehui Chuanranbing Yu Jishengchongbingxue Fenhui, Ganbingxue Fenhui. The Standard of Diagnosis of Viral Hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324–329. [Google Scholar]
- 7.Lai CL, Ching CK, Tung AK, Li E, Young J, Hill A, Wong BC, Dent J, Wu PC. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology. 1997;25:241–244. doi: 10.1002/hep.510250144. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ari Z, Shmueli D, Mor E, Shapira Z, Tur-Kaspa R. Beneficial effect of lamivudine in recurrent hepatitis B after liver transplantation. Transplantation. 1997;63:393–396. doi: 10.1097/00007890-199702150-00011. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 10.Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000;32:847–851. doi: 10.1053/jhep.2000.17915. [DOI] [PubMed] [Google Scholar]
- 11.Mason WS, Cullen J, Saputelli J, Wu TT, Liu C, London WT, Lustbader E, Schaffer P, O'Connell AP, Fourel I. Characterization of the antiviral effects of 2' carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994;19:398–411. [PubMed] [Google Scholar]
- 12.Helvaci M, Ozkaya B, Ozbal E, Ozinel S, Yaprak I. Efficacy of interferon therapy on serum fibronectin levels in children with chronic hepatitis B infection. Pediatr Int. 1999;41:270–273. doi: 10.1046/j.1442-200x.1999.01066.x. [DOI] [PubMed] [Google Scholar]
- 13.Park YN, Han KH, Kim KS, Chung JP, Kim S, Park C. Cytoplasmic expression of hepatitis B core antigen in chronic hepatitis B virus infection: role of precore stop mutants. Liver. 1999;19:199–205. doi: 10.1111/j.1478-3231.1999.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 14.Khettry U, Anand N, Gordon FD, Jenkins RL, Tahan SR, Loda M, Lewis WD. Recurrent hepatitis B, hepatitis C, and combined hepatitis B and C in liver allografts: a comparative pathological study. Hum Pathol. 2000;31:101–108. doi: 10.1016/s0046-8177(00)80205-1. [DOI] [PubMed] [Google Scholar]
- 15.Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 16.Chemin I, Ohgaki H, Chisari FV, Wild CP. Altered expression of hepatic carcinogen metabolizing enzymes with liver injury in HBV transgenic mouse lineages expressing various amounts of hepatitis B surface antigen. Liver. 1999;19:81–87. doi: 10.1111/j.1478-3231.1999.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 17.Chomarat P, Rice JM, Slagle BL, Wild CP. Hepatitis B virus-induced liver injury and altered expression of carcinogen metabolising enzymes: the role of the HBx protein. Toxicol Lett. 1998;102-103:595–601. doi: 10.1016/s0378-4274(98)00254-9. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi N, Mita E. Fas system and apoptosis in viral hepatitis. J Gastroenterol Hepatol. 1997;12:S223–S226. doi: 10.1111/j.1440-1746.1997.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarin SK, Thakur V, Guptan RC, Saigal S, Malhotra V, Thyagarajan SP, Das BC. Profile of hepatocellular carcinoma in India: an insight into the possible etiologic associations. J Gastroenterol Hepatol. 2001;16:666–673. doi: 10.1046/j.1440-1746.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- 21.Shoenfeld Y, Aron-Maor A. Vaccination and autoimmunity-'vaccinosis': a dangerous liaison. J Autoimmun. 2000;14:1–10. doi: 10.1006/jaut.1999.0346. [DOI] [PubMed] [Google Scholar]
- 22.Trobonjaca Z, Kröger A, Stober D, Leithäuser F, Möller P, Hauser H, Schirmbeck R, Reimann J. Activating immunity in the liver. II. IFN-beta attenuates NK cell-dependent liver injury triggered by liver NKT cell activation. J Immunol. 2002;168:3763–3770. doi: 10.4049/jimmunol.168.8.3763. [DOI] [PubMed] [Google Scholar]
- 23.Rapicetta M, Ferrari C, Levrero M. Viral determinants and host immune responses in the pathogenesis of HBV infection. J Med Virol. 2002;67:454–457. doi: 10.1002/jmv.10096. [DOI] [PubMed] [Google Scholar]
- 24.Tanner MS. Mechanisms of liver injury relevant to pediatric hepatology. Crit Rev Clin Lab Sci. 2002;39:1–61. doi: 10.1080/10408360290795439. [DOI] [PubMed] [Google Scholar]
- 25.Rivero M, Crespo J, Fábrega E, Casafont F, Mayorga M, Gomez-Fleitas M, Pons-Romero F. Apoptosis mediated by the Fas system in the fulminant hepatitis by hepatitis B virus. J Viral Hepat. 2002;9:107–113. doi: 10.1046/j.1365-2893.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 26.Pontisso P, Poon MC, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J (Clin Res Ed) 1984;288:1563–1566. doi: 10.1136/bmj.288.6430.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquinelli C, Lauré F, Chatenoud L, Beaurin G, Gazengel C, Bismuth H, Degos F, Tiollais P, Bach JF, Bréchot C. Hepatitis B virus DNA in mononuclear blood cells. A frequent event in hepatitis B surface antigen-positive and -negative patients with acute and chronic liver disease. J Hepatol. 1986;3:95–103. doi: 10.1016/s0168-8278(86)80152-0. [DOI] [PubMed] [Google Scholar]
- 28.Ono-Nita SK, Kato N, Shiratori Y, Masaki T, Lan KH, Carrilho FJ, Omata M. YMDD motif in hepatitis B virus DNA polymerase influences on replication and lamivudine resistance: A study by in vitro full-length viral DNA transfection. Hepatology. 1999;29:939–945. doi: 10.1002/hep.510290340. [DOI] [PubMed] [Google Scholar]
- 29.Shi JP, Shi YQ, Chen HY. Mensuration of HBV DNA in Periphearal Mononuclear Cells in the Patients of chronic hepatitis B and clinical signification. Linchuang Gandanbing Zazhi. 2001;17:227–228. [Google Scholar]


