Abstract
AIM: To study the effect of mucilage obtained from cladodes of Opuntia ficus-indica (Cactaceae) on the healing of ethanol-induced gastritis in rats.
METHODS: Chronic gastric mucosa injury was treated with mucilage (5 mg/kg per day) after it was induced by ethanol. Lipid composition, activity of 5’-nucleotidase (a membrane-associated ectoenzyme) and cytosolic activities of lactate and alcohol dehydrogenases in the plasma membrane of gastric mucosa were determined. Histological studies of gastric samples from the experimental groups were included.
RESULTS: Ethanol elicited the histological profile of gastritis characterized by loss of the surface epithelium and infiltration of polymorphonuclear leukocytes. Phosphatidylcholine (PC) decreased and cholesterol content increased in plasma membranes of the gastric mucosa. In addition, cytosolic activity increased while the activity of alcohol dehydrogenases decreased. The administration of mucilage promptly corrected these enzymatic changes. In fact, mucilage readily accelerated restoration of the ethanol-induced histological alterations and the disturbances in plasma membranes of gastric mucosa, showing a univocal anti-inflammatory effect. The activity of 5’-nucleotidase correlated with the changes in lipid composition and the fluidity of gastric mucosal plasma membranes.
CONCLUSION: The beneficial action of mucilage seems correlated with stabilization of plasma membranes of damaged gastric mucosa. Molecular interactions between mucilage monosaccharides and membrane phospholipids, mainly PC and phosphatidylethanolamine (PE), may be the relevant features responsible for changing activities of membrane-attached proteins during the healing process after chronic gastric mucosal damage.
Keywords: Gastritis, Mucilage, Chronic gastric mucosal injury, Ethanol
INTRODUCTION
The Opuntia ficus-indica (O. ficus-indica) is a plant belonging to the Cactaceae family, located preferentially in arid zones[1]. The main substance produced by this plant is mucilage composed mostly of water and polysaccharides, which may participate in adaptation mechanisms preventing dehydration or freezing[2]. The mucilage obtained from cladodes of O. ficus-indica is soluble in water and produces colloidal solutions of high viscosity[3].
The polysaccharide in mucilage with a high molecular weight[4,5], is produced by specialized cells in the Cactaceae[6]. Chemically, mucilage is composed of α-D-galactopyranosiluronic acid and β-L-rhamnopyranose, forming the main chain and β-D-galactopyranose, β-D-xylopyranose and α-L-arabinofuranose in the side chains[7,8].
In Mexico, mucilage obtained from O. ficus-indica can cure topical inflammation and skin ulcerations[9]. In addition, recent data suggest that mucilage derived from this plant can treat acute gastric damage. The anti-ulcer activity of mucilage from O. ficus-indica has been reported[10]. The beneficial effect of cladodes from O. ficus-indica when simultaneously administered with ethanol seems related to an enhancement of gastric mucus production[11]. Likewise, polygalacturonic acid[4,7] and arabinogalactan protein in mucilage, probably act in combination with some protective factors including macromolecules or small ligands of the gastric mucosa[12]. Although these possibilities remain unproved, mucilage from O. ficus-indica could exert a cytoprotection as an anti-inflammatory agent, which can prevent rat gastric mucosal damage when administered concomitantly with the noxious agent[13].
To solve the problem, we have developed a model of ethanol administration to rats, which could elicits a histological profile of gastric injury characterized by loss of surface epithelium and infiltration of polymorphonuclear leukocytes. This model produces evident alterations in plasma membranes of the gastric mucosa[14]. After ethanol withdrawal[15], these alterations may lead to increased membrane lipid peroxidation accompanying spontaneous restitution of the gastric mucosa epithelium.
Taking advantage of these histological alterations, as well as those in membrane lipid composition present in our model of chronic ethanol-induced rat gastric mucosal damage[14], the present study assessed whether administration of mucilage extracted from O. ficus indica had a therapeutic effect on this experimental model of gastritis in rats.
MATERIALS AND METHODS
Preparation of mucilage
The cladodes were isolated from a cultivation of O. ficus-indica located in Milpa Alta village (near to Mexico City) and the identity of the plant was confirmed by the bibliographic data[1]. Two kg of fresh cladodes free of spines was cut into cubes of 2 cm × 2 cm × 1 cm and placed in one liter of distilled water for 24 h. The supernatant was frozen and lyophilized, from which 41g of mucilage as a yellow-powder was obtained. A 0.25% solution of mucilage in water was prepared.
Animal treatment
A model of gastritis induced by chronic ethanol admini-stration to rats has been described in detail elsewhere[14]. Briefly, two groups of 20 and 15 male Wistar rats weighing 240-260 g, after an overnight fasting, received 1 mL of 50% ethanol through a gastric tube for one day and water containing 5% of ethanol ad libitum to achieve a daily intake of 9-10 g of ethanol per kg of body weight for five days. Then the animals developed gastritis. This was considered as time zero (once histological evidence of gastritis was achieved), at this time the ethanol ingestion was discontinued. The group of 20 animals was considered as the gastritis control group, which was divided into four subgroups of 5 animals. The first subgroup was studied at time zero whereas the second, third and fourth subgroups were studied after 24, 48, and 72 h, respectively. The group of 15 rats served as the gastritis group treated with mucilage (5 mg/kg of body per day, starting at time zero) and divided into three subgroups, 5 animals in each subgroup, and studied after 24, 48, and 72 h, respectively. The complementary control group of 15 healthy animals (divided into three subgroups, 5 animals in each subgroup), received only saline solution and was studied after 24, 48, and 72 h, respectively. The animals were killed by decapitation after administration of an overdose of sodium pentobarbital. All the procedures were performed according to the Federal Regulations for Care and Use of Experimental Animals (Ministry of Agriculture and Animal Breeding; SAGARPA).
Histological assessment and subcellular fractionation of gastric mucosa
The stomach was removed from each rat, dissected along the greater curvature and rinsed in cold saline solution. Strips of gastric wall were embedded in paraffin, stained and analyzed as reported elsewhere[14]. The remnant gastric tissue from the forestomach to the pylorus through the entire glandular mucosa, was homogenized in a buffer containing 0.25 mol/L of sucrose and 10 mmol/L of TRIS-HCl (pH 7.5). Plasma membrane was obtained from the whole homogenate by the method of Loten and Redshaw-Loten[16], while the cytosolic fraction was obtained by differential centrifugation[14]. Identity and purity of the subcellular fractions were routinely assessed by determining the activities of marker enzymes, namely 5’-nucleotidase and lactate dehydrogenase (LDH)[14]. 5’nucleotidase was also used to assess the in vivo plasma membrane fluidity[14].
Analytical procedures
In extracts of total lipids from isolated plasma membranes, phospholipid content was determined as previously described[17]. Total cholesterol was determined with the colorimetric method described by Abell et al[18]. The cytosolic fractions were used as an enzyme source to determine the specific activities of LDH (EC 1.1.1.27) and alcohol dehydrogenase (ADH; EC 1.1.1.1) using the spectrophotometric methods of Vassault[19] and Caballería et al[20] respectively. In all the assays, protein content was determined according to Lowry et al[21].
Statistical analysis
Statistical difference among groups was calculated by the two-way ANOVA test, and expressed as mean ± SE. In case of significance, student’s-t test was applied.
RESULTS
Histological assessment of gastric samples from animals with gastritis and treated with mucilage
When compared to control rats (Figure 1A), at the onset of gastritis (T0), disrupted superficial epithelial cells, loss of specialized cells (glandular), slight submucosal edema, marked margination and infiltration of polymorphonuclear (PMN) leukocytes were found in gastric mucosa, proving clearly an inflammatory process (Figures 1B and 1C). The histological abnormalities generated by ethanol remained unchanged 24 h after spontaneous recovery (Figure 1C), but restoration of the gastric mucosa started 48 h after ethanol withdrawal. Therefore, three days after spontaneous recovery, a histological pattern corresponding to a moderate gastritis was observed in these animals (Figure 1D, Table 1). On the contrary, animals with gastritis and treated with mucilage showed an earlier recovery as reflected in a great amelioration of the inflammatory process (Figure 1E, Table 1).
Figure 1.
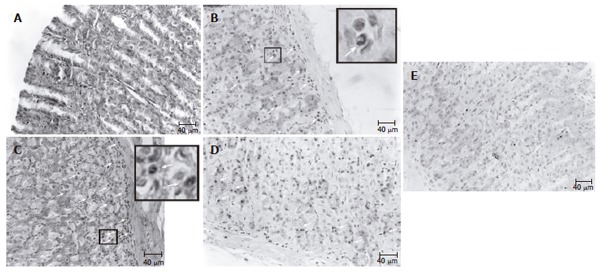
Representative micrographs of gastric mucosa of rats with gastritis and treated with mucilage. A: control animals; B: animals subjected to gastritis (T0); C: animals 24 h after ethanol withdrawal. In micrographs B and C, the presence of PMN infiltrate is shown by large arrows in the insets; D: histological profile of the spontaneous recovery of gastric mucosa 72 h after ethanol withdrawal (arrows: PMN); E: histological profile corresponding to similar conditions after treated with mucilage (3 doses).
Table 1.
Incidence of microscopic lesions in stomach of rats treated with ethanol and mucilage from O. ficus-indica
| Experimental groups | Parameters | |||
| Time (h) | S. epithelium (disruption) | Specialized cells (loss) | PMN infiltration | |
| Controls | -- | --- | --- | --- |
| Gastritis + saline | 0 | +++ | ++/+++ | +++ |
| Gastritis + saline | 24 | +++ | ++ | +++ |
| Gastritis + saline | 48 | ++/+++ | +/++ | +++ |
| Gastritis + saline | 72 | +/++ | + | +/++ |
| Gastritis + mucilage | 24 | +++ | ++ | +++ |
| Gastritis + mucilage | 48 | ++ | + | +++ |
| Gastritis + mucilage | 72 | + | --/+ | + |
Controls corresponding to rats treated with saline. Scale for injury degree: (-) absent, (+) slight, (++) moderate and severe (+++). PMN: Polymorphonuclear leukocytes; S.: Surface.
Effects of mucilage on plasma membrane lipid compo-sition and cytosolic activities of soluble enzymes in rats with chronic gastric mucosal injury
Along with the histological findings, the lipid compo-sition of isolated mucosa plasma membranes in the experimental groups changed significantly. Table 2 depicts the phospholipid species found in plasma membranes obtained from mucosa of rats with gastritis and treated with mucilage. Chronic ethanol treatment (T0) had a dual effect on phospholipid distribution. Both phosphatidylinositol (PI) and phosphatidylcholine (PC) decreased significantly accompanying a progressive enhancement of phosphatidylethanolamine (PE) in plasma membrane. The less abundant phospholipid in plasma membranes of gastric mucosa was phosphatidic acid (PA). Likewise, the level of phosphatidylserine (PS) had no significant change due to the presence of gastritis (Table 2). The ethanol treatment-induced alteration of PI and PC in plasma membrane persisted for up to 72 h after ethanol withdrawal. However, PE levels were normalized 48 h after spontaneous recovery. A single dose of mucilage (24 h) did not have any significant effect on ethanol-induced alterations in the lipid composition of gastric mucosal membranes, but subsequent administrations promptly normalized the content of phospholipids and their relative distribution in gastric membranes of animals undergoing gastritis (Table 2). Then, mucilage administration elicited a restoration of PC levels, close to the control levels (Table 2). Although mucilage exerted its effects on distribution of plasma membrane phospholipids during the treatment, neither the degree of mucosal damage nor the treatment with mucilage significantly modified the amount of total membrane phospholipids (Figure 2A). However, plasma membrane cholesterol increased as a response to mucosal injury (Figure 2A). Thus, the group of rats subjected to gastritis showed an early increase of cholesterol content, which remained significantly higher than that in the control group during the experiment. In this context, administration of mucilage successfully restored the normal amount of cholesterol in mucosa plasma membranes (Figure 2A), and consequently, the cholesterol/phospholipids ratio was also modified (Figure 2C). This ratio was greatly enhanced in mucosal plasma membranes obtained from rats undergoing gastritis, and remained higher at all the tested time points. On the contrary, treatment with three doses of mucilage (72 h after ethanol withdrawal) normalized the cholesterol/phospholipids ratio (Figure 2C). The methylation index represented by the PC/PE ratio also decreased in animals with gastritis. This effect induced by ethanol, persisted even after 72 h of ethanol administration (Figure 2B). Interestingly, mucilage also corrected this parameter of the lipid composition of mucosal plasma membranes. This was more evident at the end of its administration to animals undergoing gastritis (Figure 2B). Therefore, alterations in the lipid composition of plasma membranes of injured gastric mucosae, were promptly normalized by the administration of mucilage.
Table 2.
Phospholipid composition of stomach mucosal membranes of gastritis rats treated with mucilage from O. ficus-indica (mean ± SE)
| Experimental groups |
Phospholipids (nmols of phosphate per mg of protein) |
||||
| PA | PS | PI | PC | PE | |
| Controls | 29 ± 4 | 103 ± 12 | 172 ± 9 | 464 ± 25 | 206 ± 20 |
| Plus saline | |||||
| Gastritis (Time zero) | 35 ± 5 | 123 ± 14 | 111 ± 16a | 333 ± 52 | 219 ± 22 |
| Gastritis (24 h) | 34 ± 5 | 121 ± 12 | 139 ± 10a | 314 ± 37a | 321 ± 32a |
| Gastritis (48 h) | 38 ± 5 | 135 ± 11 | 126 ± 11a | 332 ± 20a | 266 ± 23 |
| Gastritis (72 h) | 40 ± 5 | 141 ± 19 | 126 ± 15a | 372 ± 30a | 247 ± 18 |
| Plus mucilage | |||||
| Gastritis (24 h) | 38 ± 4 | 136 ± 11 | 148 ± 21 | 321 ± 35a | 347 ± 33a |
| Gastritis (48 h) | 36 ± 5 | 128 ± 9 | 137 ± 12 a | 384 ± 39 | 232 ± 16 |
| Gastritis (72 h) | 29 ± 4 | 102 ± 19 | 160 ± 15 b | 453 ± 31b | 196 ± 18 |
PA: Phosphatidic acid; PS: Phosphatidylserine; PI: Phosphatidylinositol; PC: Phosphatidylcholine; PE: Phosphatidylethanolamine.
P < 0.01 vs controls;
P < 0.01 vs rats subjected to gastritis (untreated with mucilage).
Figure 2.
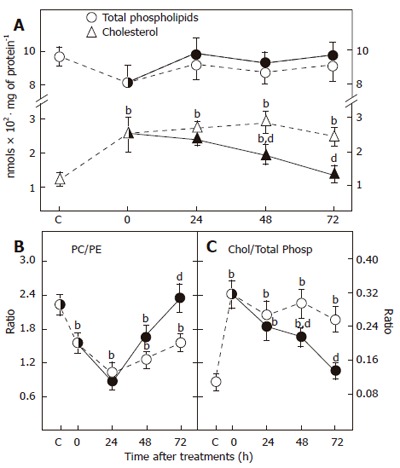
Total phospholipids and cholesterol in plasma membranes of mucilage-treated rats with gastritis (A) and the phosphatidylcholine (PC)/phosphatidylethanolamine (PE) methylation ratio (B) and cholesterol (chol)/total phospholipids (total phosp) ratio (C). In all cases mean ± SE, n = 5. Animals subjected to gastritis are represented by empty symbols while those treated with mucilage are represented by solid symbols. bP < 0.01 vs healthy animal control. dP < 0.01 vs animals with gastritis not treated with mucilage.
Due to the effect of mucosal damage induced by ethanol, some cytosolic enzyme activities were significantly affected (Figure 3). As previously reported[14], animals with gastritis showed a significant drop in ADH activity (54%). Regardless of the ethanol withdrawal, the activity of ADH remained abnormal during the tested recovery period (up to 72 h). However, mucilage promoted an early recovery of the ADH activity when administered to rats undergoing gastritis (Figure 3). The opposite to the LDH activity was observed. This cytosolic enzyme with a very high specific activity increased after chronic ethanol administration (133% over controls) and its activity remained significantly higher even 72 h after ethanol withdrawal. In this case, administration of mucilage also normalized LDH activity, but only after 3 doses (Figure 3), suggesting that administration of mucilage from O. ficus-indica to animals with ethanol-induced gastritis, could not only correct the alterations found in plasma membranes, but also practically normalize the activities of some soluble enzymes present in the cytosolic fraction obtained from the gastric mucosa.
Figure 3.
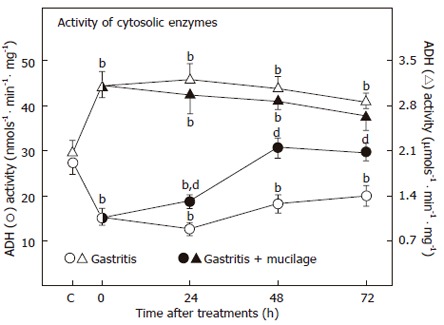
Effects of gastritis and mucilage from O. ficus-indica on cytoplasmic specific ADH and LDH activities. The results are expressed as mean ± SE of five individual observations per experimental group for the alcohol (ADH) and lactate (LDH) dehydrogenases in the cytosolic fraction. Statistical significance is shown in Figure 2.
Effects of gastritis and mucilage on membrane 5’-nucleotidase activity
The effects of gastritis and mucilage on the activity of 5’-nucleotidase were also evident. This ectoenzyme could respond to changes in the lipidic microenvironment, thus its activity could indirectly indicate the fluidity status of gastric mucosa plasma membranes. This enzyme presented a decreased activity at the onset of gastritis (T0) and was still lower 24 h after ethanol withdrawal (Figure 4, left panel). Thereafter, the activity of 5’-nucleotidase was suddenly enhanced and normalized after 72 h of spontaneous recovery. When rats undergoing gastritis were administered with mucilage, an early recovery of the plasma membrane activity of 5’-nucleotidase (24 h) was noted, which was followed by a sustained increase in the 5’-nucleotidase activity during the whole treatment period (Figure 4, left panel). The influence of the lipidic composition of plasma membranes on 5’-nucleotidase activity was evident when this enzymatic activity was plotted against indicative parameters of plasma membrane fluidity (Figures 2B and 2C). As shown in Figure 4B (right panel), the activity of 5’-nucleotidase correlated directly with the PC/PE ratio (r = 0.65, P < 0.005), while increased cholesterol/phospholipids ratio negatively affected the activity of plasma membrane 5’-nucleotidase (r = -0.51, P < 0.01; Figure 4B, right panel).
Figure 4.
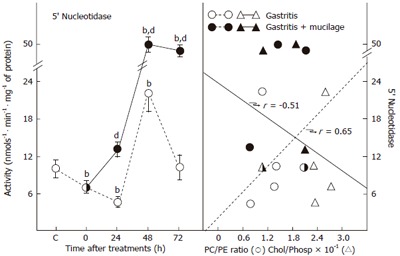
Activity of 5’-nucleotidase and its relation with the cholesterol/ phospholipid ratio in plasma membranes of mucilage-treated rats with gastritis. Left panel represents the mean ± SE of five individual observations per experimental group for 5’-nucleotidase activity. The correlation between activity of this enzyme and the PC/PE ratio or the cholesterol (chol)/phospholipid (phos) ratio is shown in the right panel of Figure 2. Statistical significance is indicated in the left panel of Figure 2.
DISCUSSION
Administration of concentrated ethanol solutions could induce damage to the gastric mucosa, including a disruption of up to 80% of the surface epithelial cells throughout the glandular stomach of rats[22,23]. Indeed, ethanol administration could result in a reduction in resistance, transmucosal potential difference, H+ secretion, and increased appearance of Na+ in the lumen at neutral pH[24,25], indicating that ethanol can induce profound changes in membrane permeability.
The present model resembles an active gastritis, similar to that found in humans. In fact, this model has been already validated by histological and biochemical findings[14,15]. Furthermore, the main histological findings, such as inflammation and reduction of surface epithelial cells, as well as alterations in mucosal glands (Figure 1) and parietal cells[26] are described in another model of chronic gastritis in rats[27]. Our experimental model of ethanol-induced gastritis in rats is characterized by decreased PI and PC, and increased PE in plasma membranes of the gastric mucosa. PC is abundant in the gastric mucosa of rats, and the PC/PE ratio represents the lipid methylation activity[28]. Additionally, the cholesterol/phospholipid ratio significantly decreased, strongly suggesting that ethanol-induced gastritis diminishes the membrane permeability[14]. In fact, the cholesterol/phospholipid ratio influences the fluidity of a variety of membranes, and increases during the occurrence of altered membrane permeability[29,30]. All these modifications in the membrane lipid composition of gastric mucosa obtained from animals with gastritis are due to the changes in the rate of lipid peroxidation[15].
Lipid peroxidation and its effects on the composition and function of plasma membranes seem to play their part in chronic ethanol-induced gastric mucosa injury, promoting diminished binding of ligands to membrane histaminergic H2-receptors, which is inversely correlated with the rate of restoration of the surface epithelium in gastric mucosa[15]. In this regard, the effects of administration of mucilage obtained from O. ficus-indica on gastric mucosal lesions need to be further studied.
In the present model, mucilage administration was capable of correcting the alterations found in plasma membrane, especially its lipid composition, suggesting that mucilage can accelerate the repair of gastric mucosal lesions in rats undergoing gastritis (Figures 1-3). Histological examination revealed that the main action of mucilage in injured gastric mucosa was an anti-inflammatory effect, which seemed to be involved in the further restitution of the mucosal integrity. Along with this, mucilage contains polygalacturonic acid[4] and arabinogalactan, which could interact with macromolecules or small ligands of the gastric mucosa, enhancing gastric mucus production[11].
In addition to the partial blocking effect on the PMN infiltrate, the specific activity of cytosolic enzymes ADH and LDH also normalised after the treatment with mucilage. These enzymes seem related to the condition of the gastric mucosa in some extent, since altered LDH and ADH activities seem to be associated with processes of gastric carcinogenesis and intestinal metaplasia[31,32].
The change of phase from gel to liquid of membrane phospholipids can result from an abnormal increase of permeability and loss of cellular functionality. In this sense, polysaccharides may stabilize and protect the biological membranes, allowing the transitional phase remaining within the range of biological activity, and avoiding the damage induced by dehydration or freezing[2]. Polysaccharide interaction between κ-carrageenan[33] or thehalose[34] or fructanes[35] and the polar heads of phospholipids, plays a role in stabilizing membranes. Saccharides interact with proteins such as mucin, and/or the polar heads of membrane phospholipids. Therefore, they could originate a protective effect once they replace hydrogen bonds of water molecules, generating and increasing local viscosity[36]. This can avoid dehydration such as that produced by alcohol. The changes observed in cell membrane after chronic ethanol treatment, such those in the lipid composition, could decrease or even induce a complete loss of the selective membrane barrier function which could eventually lead to cell death.
Alterations of gastric mucosal cell membranes seem correlated with the activity of 5’-nucleotidase, which permits monitoring the in vivo changes in the membrane lipid composition after mucilage treatment. The effects of gastritis and mucilage on the 5’-nucleotidase activity deserve some comments. Changes in the lipid composition and the cholesterol/phospholipid molar ratio differentially affect the activities of 5’-nucleotidase, Mg2+-ATPase, and γ-glutamyltransferase[37]. Since 5’-nucleotidase is attached to the membrane bilayer through a glycosylphosphatidylinositol anchor, PC and PE can induce the highest degree of activation of 5’-nucleotidase[38]. Ethanol alone (up to 400 mM) does not affect the 5’-nucleotidase activity, but its derivative phosphatidylethanol, enhances 5'-nucleotidase activity[39]. The present data indicate that changes found in the activity of this enzyme reflect changes in the lipidic microenvironment of gastric plasma membranes.
On the other hand, the activity of ecto-5’-nucleotidase constitutes a key enzyme responsible for adenosine production in rat hearts[40]. In the rat stomach, adenosine has been demonstrated to inhibit gastric acid secretion probably by indirectly inhibiting gastrin release, which seems to be regulated through participation of A(1)-adenosine receptors[41]. Adenosine also increases somatostatin release by acting on A(2A) receptors[42] and may have a putative gastroprotective effect against several types of inductors of gastric mucosal injury such as deficient circulatory conditions[43] and indomethacin-induced gastric lesions[44], as well as against stress- and ethanol-evoked gastric lesion formation[45]. Whether endogenous increase of gastric mucosal levels of adenosine, mediated by mucilage-induced activation of 5’-nucleotidase, participates in the beneficial action of mucilage, remains to be clarified.
In any case, the association between mucilage poly-saccharides and membranes may contribute to the repair of damaged membranes[2]. This may occur following the insertion of polysaccharides into membranes, originating a lateral spacing among the phospholipid polar heads, reducing the van der Waals interactions within the hydrocarbon chains, increasing viscosity and reducing mobility around the disturbed membrane. All these changes accompany capturing and associating water molecules[2].
In conclusion, mucilage may exert its anti-inflammatory effect by promoting the healing process of gastritis in at least three ways: formation of a viscous protective cover against the damage induced by ethanol or other noxious substances; restoration of stomach epithelial surface and stabilization of plasma membranes; participation in enzyme recovery, restoration of both the cholesterol/phospholipid ratio and membrane fluidity.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
References
- 1.Bravo-Hollis H. Las cactáceas de México. Vol. I. Editorial UNAM. 1978. [Google Scholar]
- 2.Demel RA, Dorrepaal E, Ebskamp MJ, Smeekens JC, de Kruijff B. Fructans interact strongly with model membranes. Biochim Biophys Acta. 1998;1375:36–42. doi: 10.1016/s0005-2736(98)00138-2. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas A, Higuera-Ciapara I, Goycoolea FM. Rheology and aggregation of Cactus (Opuntia ficus- e indica) mucilagin solution. J PACD. 1997;2:152–157. [Google Scholar]
- 4.Trachtenberg S, Mayer A. Biophysical properties of Opuntia ficus-indica mucilage. Phytochem. 1980;21:2835–2843. [Google Scholar]
- 5.Medina-Torres L, Brito de la Fuente E, Torrestiana-Sánchez B, Katthain R. Rheological properties of the mucilage gum (Opuntia ficus-indica) Food Hydrocoll. 2000;14:417–424. [Google Scholar]
- 6.Trachtenberg S, Fahn A. The mucilage cells of Opuntia ficus-indica (L) Mill. Development, ultrastructure, and mucilage secretion. Bot Gaz. 1981;142:206–213. [Google Scholar]
- 7.McGarvie D, Haralambos P. Methylation analysis of the mucilage of Opuntia ficus- indica. Carbohydr Res. 1981;88:305–314. [Google Scholar]
- 8.McGarvie D, Parolis H. The acid-labile, peripheral chains of the mucilage of Opuntia ficus-indica. Carbohydr Res. 1981;94:57–65. [Google Scholar]
- 9.Morton JF. Mucilaginous plants and their uses in medicine. J Ethnopharmacol. 1990;29:245–266. doi: 10.1016/0378-8741(90)90036-s. [DOI] [PubMed] [Google Scholar]
- 10.Galati EM, Monforte MT, Tripodo MM, d'Aquino A, Mondello MR. Antiulcer activity of Opuntia ficus indica (L.) Mill. (Cactaceae): ultrastructural study. J Ethnopharmacol. 2001;76:1–9. doi: 10.1016/s0378-8741(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 11.Galati EM, Pergolizzi S, Miceli N, Monforte MT, Tripodo MM. Study on the increment of the production of gastric mucus in rats treated with Opuntia ficus indica (L.) Mill. cladodes. J Ethnopharmacol. 2002;83:229–233. doi: 10.1016/s0378-8741(02)00243-x. [DOI] [PubMed] [Google Scholar]
- 12.Clarke AE, Anderson RL, Stone BA. Form and function of arabinogalactans and arabinogalactan-proteins. Phytochem. 1979;18:521–540. [Google Scholar]
- 13.Park EH, Kahng JH, Paek EA. Studies on the pharmacological action of cactus: identification of its anti-inflammatory effect. Arch Pharm Res. 1998;21:30–34. doi: 10.1007/BF03216749. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Muñoz R, Montiel-Ruíz F. Reversion by histamine H2-receptor antagonists of plasma membrane alterations in ethanol-induced gastritis. Dig Dis Sci. 1996;41:2156–2165. doi: 10.1007/BF02071395. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Muñoz R, Montiel-Ruíz C, Vázquez-Martínez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest. 2000;80:1161–1169. doi: 10.1038/labinvest.3780124. [DOI] [PubMed] [Google Scholar]
- 16.Loten EG, Redshaw-Loten JC. Preparation of rat liver plasma membranes in a high yield. Anal Biochem. 1986;154:183–185. doi: 10.1016/0003-2697(86)90512-9. [DOI] [PubMed] [Google Scholar]
- 17.García-Sáinz JA, Fain JN. Effect of insulin, catecholamines and calcium ions on phospholipid metabolism in isolated white fat-cells. Biochem J. 1980;186:781–789. doi: 10.1042/bj1860781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abel LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–366. [PubMed] [Google Scholar]
- 19.Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer J, Grabl M, eds , editors. Methods of Enzymatic Analysis. Verlag-Chemie, Deerfield Beach: Florida; 1983. pp. 119–126. [Google Scholar]
- 20.Caballeria J, Baraona E, Rodamilans M, Lieber CS. Effects of cimetidine on gastric alcohol dehydrogenase activity and blood ethanol levels. Gastroenterology. 1989;96:388–392. doi: 10.1016/0016-5085(89)91562-x. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Konturek SJ, Radecki T, Brzozowski T, Piastucki I, Dembińska-Kieć A, Zmuda A. Gastric cytoprotection by prostaglandins, ranitidine, and probanthine in rats. Role of endogenous prostaglandins. Scand J Gastroenterol. 1981;16:7–12. [PubMed] [Google Scholar]
- 23.Lacy ER, Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982;83:619–625. [PubMed] [Google Scholar]
- 24.Biggerstaff RJ, Leitch GJ. Effects of ethanol on electrical parameters of the in vivo rat stomach. Am J Dig Dis. 1977;22:1064–1068. doi: 10.1007/BF01072858. [DOI] [PubMed] [Google Scholar]
- 25.Ohno T, Ohtsuki H, Okabe S. Effects of 16,16-dimethyl prostaglandin E2 on ethanol-induced and aspirin-induced gastric damage in the rat. Scanning electron microscopic study. Gastroenterology. 1985;88:353–361. doi: 10.1016/s0016-5085(85)80189-x. [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Rincón I, Olguín-Martínez M, Hernández-Muñoz R. Enhanced intracellular calcium promotes metabolic and secretory disturbances in rat gastric mucosa during ethanol-induced gastritis. Exp Biol Med (Maywood) 2003;228:315–324. doi: 10.1177/153537020322800311. [DOI] [PubMed] [Google Scholar]
- 27.Xiang Z, Si JM, Huang HD. Chronic gastritis rat model and role of inducing factors. World J Gastroenterol. 2004;10:3212–3214. doi: 10.3748/wjg.v10.i21.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata F, Viveros OH, Diliberto EJ Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978;75:1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Incerpi S, Jefferson JR, Wood WG, Ball WJ, Schroeder F. Na pump and plasma membrane structure in L-cell fibroblasts expressing rat liver fatty acid binding protein. Arch Biochem Biophys. 1992;298:35–42. doi: 10.1016/0003-9861(92)90090-j. [DOI] [PubMed] [Google Scholar]
- 30.Daveloose D, Linard A, Arfi T, Viret J, Christon R. Simultaneous changes in lipid composition, fluidity and enzyme activity in piglet intestinal brush border membrane as affected by dietary polyunsaturated fatty acid deficiency. Biochim Biophys Acta. 1993;1166:229–237. doi: 10.1016/0005-2760(93)90102-f. [DOI] [PubMed] [Google Scholar]
- 31.Carda-Abella P, Perez-Cuadrado M, Mate-Jimenez J. LDH isoenzyme patterns in human gastric mucosa with precancerous changes. Cancer. 1978;42:490–494. doi: 10.1002/1097-0142(197808)42:2<490::aid-cncr2820420217>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Baraona E, Yokoyama A, Ishii H, Hernández-Muñoz R, Takagi T, Tsuchiya M, Lieber CS. Lack of alcohol dehydrogenase isoenzyme activities in the stomach of Japanese subjects. Life Sci. 1991;49:1929–1934. doi: 10.1016/0024-3205(91)90295-m. [DOI] [PubMed] [Google Scholar]
- 33.Girod S, Cara L, Maillols H, Salles JP, Devoisselle JM. Relationship between conformation of polysaccharides -in the dilute regime and their interaction with a phospholipid bilayer. Luminescence. 2001;16:109–116. doi: 10.1002/bio.642. [DOI] [PubMed] [Google Scholar]
- 34.Crowe JH, Crowe LM, Chapman D. Infrared spectroscopic studies on interactions of water and carbohydrates with a biological membrane. Arch Biochem Biophys. 1984;232:400–407. doi: 10.1016/0003-9861(84)90555-1. [DOI] [PubMed] [Google Scholar]
- 35.Vereyken IJ, Chupin V, Demel RA, Smeekens SC, De Kruijff B. Fructans insert between the headgroups of phospholipids. Biochim Biophys Acta. 2001;1510:307–320. doi: 10.1016/s0005-2736(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 36.Ryden P, MacDougall AJ, Tibbits CW, Ring SG. Hydration of pectic polysaccharides. Biopolymers. 2000;54:398–405. doi: 10.1002/1097-0282(200011)54:6<398::AID-BIP40>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Galán AI, Muñoz ME, Jiménez R. S-Adenosylmethionine protects against cyclosporin A-induced alterations in rat liver plasma membrane fluidity and functions. J Pharmacol Exp Ther. 1999;290:774–781. [PubMed] [Google Scholar]
- 38.Lehto MT, Sharom FJ. Release of the glycosylphosphatidylinositol-anchored enzyme ecto-5'-nucleotidase by phospholipase C: catalytic activation and modulation by the lipid bilayer. Biochem J. 1998;332(Pt 1):101–109. doi: 10.1042/bj3320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omodeo-Salé F, Lindi C, Palestini P, Masserini M. Role of phosphatidylethanol in membranes. Effects on membrane fluidity, tolerance to ethanol, and activity of membrane-bound enzymes. Biochemistry. 1991;30:2477–2482. doi: 10.1021/bi00223a026. [DOI] [PubMed] [Google Scholar]
- 40.Obata T. Adenosine production and its interaction with protection of ischemic and reperfusion injury of the myocardium. Life Sci. 2002;71:2083–2103. doi: 10.1016/s0024-3205(02)01993-8. [DOI] [PubMed] [Google Scholar]
- 41.Yip L, Leung HC, Kwok YN. Role of adenosine A1 receptor in the regulation of gastrin release. J Pharmacol Exp Ther. 2004;310:477–487. doi: 10.1124/jpet.104.066654. [DOI] [PubMed] [Google Scholar]
- 42.Yip L, Kwok YN. Role of adenosine A2A receptor in the regulation of gastric somatostatin release. J Pharmacol Exp Ther. 2004;309:804–815. doi: 10.1124/jpet.103.061986. [DOI] [PubMed] [Google Scholar]
- 43.Gislason H, Varhaug P, Sørbye H, Waldum HL, Svanes K. Role of adenosine and nitric oxide in the hyperemic response to superficial and deep gastric mucosal injury and H+ back-diffusion in cats. Scand J Gastroenterol. 1996;31:14–23. doi: 10.3109/00365529609031621. [DOI] [PubMed] [Google Scholar]
- 44.Bozkurt A, Yüksel M, Haklar G, Kurtel H, Yeğen BC, Alican I. Adenosine protects against indomethacin-induced gastric damage in rats. Dig Dis Sci. 1998;43:1258–1263. doi: 10.1023/a:1018859824926. [DOI] [PubMed] [Google Scholar]
- 45.Cho CH. Adenosine: a novel ulcer modulator in stomachs. Acta Physiol Hung. 1992;80:175–180. [PubMed] [Google Scholar]


