Abstract
AIM: To assess the correlation between the fibrotic area (FA) as calculated by a digital image analysis (DIA), and to compare the diagnostic accuracy of FibroScan to the other existing Liver fibrosis (LF) markers using the receiver operating curve analysis.
METHODS: We recruited 30 patients who underwent a liver resection for three different etiologies including normal liver, hepatitis B, and hepatitis C. Liver stiffness was measured by using a FibroScan. The FA was then calculated by DIA to evaluate LF in order to avoid any sampling bias.
RESULTS: The FA negatively correlated with Prothrom-bin time (PT), platelet count, lecithin-cholesterol acyltransferase (LCAT), and pre-albumin (ALB). On the other hand, the findings of FibroScan correlated with similar markers. The FA positively correlated with FibroScan, serum hyaluronate level, and type IV collagen level, and aspartate transaminase to platelet ratio index (APRI). The area under the receiver operating curve for FibroScan was higher than that for the other markers, even though the statistical significance was minimal.
CONCLUSION: Our findings suggest that FibroScan can initially be used to assess LF as an alternative to a liver biopsy (LB) and serum diagnosis, because it is a safe method with comparable diagnostic accuracy regarding the existing LF markers.
Keywords: Cirrhosis, Digital image analysis, Fibro-Scan, Fibrotic area, Hyaluronate, Lecithin-cholesterol acyltransferase, Liver fibrosis, Pre-albumin
INTRODUCTION
Liver fibrosis (LF) is characterized by the accumulation of an extracellular matrix, which distorts the hepatic architecture[1,2]. The major etiologies of LF are viral-associated hepatitis, alcohol abuse, non-alcoholic steatohepatitis and autoimmune disease. The progression of LF increases the stiffness of liver and the resistance of liver blood flow[1,3]. An insufficiency of liver blood flow results in liver failure and eventual liver cirrhosis. Once cirrhosis develops, liver transplantation is the only therapy to avoid a fatal condition[4]. Therefore, an accurate assessment LF is very important in order to predict the prognosis and start the appropriate prophylactic therapy to prevent disease progression.
Liver biopsy (LB) is still the gold-standard method for assessing LF[1,2,5]. However, it is difficult to perform LB for all patients who need to be assessed repeatedly due to its invasiveness and prohibitive cost. In addition, biopsy samples are usually too small to diagnose the disease accurately and diagnostic opinions often differ among pathologists[6,7]. As a result, a pathological examination does not always provide an accurate diagnosis. Furthermore, histological quantification of LF is also difficult because of the diagnostic variability[2,8]. Some studies have shown that the diagnostic accuracy of a digital image analysis (DIA) for LF is more reliable than histological scoring systems[8-10] such as METAVIR and Ishak score. Therefore, we assessed LF using DIA, which could accurately calculate the fibrotic area (FA), in order to avoid diagnostic variability and errors.
The liver plays a very important role in maintaining such serum proteins as albumin, cholesterol and coagulation factors. The production of such proteins decreases in liver cirrhosis[11-13]. Although these protein levels decrease in the late stage of liver cirrhosis, it is still not fully understood which markers represent liver damage in the early stage or which liver functional marker is correlated with LF.
Recently, transient elastography (FibroScan®: Echosens, Paris, France) has become available for the assessment of LF as a rapid noninvasive method, which can measure liver stiffness from outside of the body[14-17]. FibroScan has been compared to such classical markers as hematological test [the aspartate transaminase to platelet ratio index (APRI)] and fibrotest, accurately representing the state of liver fibrosis evaluated by METAVIR scoring system. The diagnostic accuracy of FibroScan has thus been found to be comparable with that using traditional markers.
In the present study, we selected patients who underwent a hepatectomy for this study. In this setting, a large amount of tissue could be used for analysis in order to reduce the risk of sampling bias. In addition, three different backgrounds of liver disease could be evaluated in order to make an accurate diagnostic value of FibroScan for LF without any sampling bias. We first measured the FA precisely using DIA to avoid any diagnostic variability. We next evaluated the correlation between FA and multiple biochemical markers to identify which markers could represent a deterioration of liver function associated with FA. In addition, we also evaluated the correlation between FibroScan and multiple biochemical markers to see whether FibroScan could accurately represent liver function. Furthermore, we also evaluated the correlation between FA and existing LF markers including FibroScan, hyaluronate, type IV collagen, and APRI. The final aim of this study was to assess the correlation of our findings to FA and to compare the clinical diagnostic accuracy of FibroScan and other markers.
MATERIALS AND METHODS
Patients
We examined 30 patients who underwent a liver resection from January 2003 to May 2005. We recruited 10 patients each with three different etiologies including a normal liver, hepatitis B, and hepatitis C. The pathological diagnosis for the liver was made in all 10 samples of normal liver, liver fibrosis, and liver cirrhosis. The indications for a hepatectomy in the normal liver were metastatic liver tumor while the indications for those with viral hepatitis were hepatocellular carcinomas. Informed consent was obtained from each patient included in this study and the study design conformed to the ethical guidelines of the Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Liver stiffness measurement
The principle of elastography using a FibroScan® (EchoSens) has been described previously[14]. All measurements were performed in the right lobe of the liver through the intercostal spaces in patients lying in the dorsal decubitus position with their right arm in maximal abduction. The tip of the probe transducer was covered with coupling gel and placed on the skin between the ribs at level of the right liver lobe. The operator, assisted by ultrasound time-motion and A-mode images provided by the system, located a portion of the liver that was at least 6 cm thick and free of any large vascular structures. Once the area of measurement was located, then the operator pressed the probe button to begin image acquisition. The measurement depth ranged from 25 mm to 45 mm and 10 validated measurements were performed in each patient. The success rate was calculated as the number of validated measurements divided by the total number of measurements.
The results were expressed in kPa. The median value was considered representative of the elastic liver modulus. The whole examination lasted less than 5 min. Only procedures with 10-validated measurements and a success rate of at least 60% were considered reliable.
Digital image analysis of liver fibrotic area
Liver fibrosis was evaluated using a computer program (NIH Image V1.62, National Institutes of Health, Bethesda, MD). Tissue sections were examined with Azan-Mallory staining. The fibrotic area was stained blue and depicted as the only blue signal using Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA). Each patient was examined with 15 different fields in 3 different specimens. One field contained at least 10 portal tracts. The area of blue signals was calculated.
Statistical analysis
A statistical analysis of the relationship between pathological fibrosis and other clinical data was performed with Spearman’s rank correlation coefficient using the StatView 4.5 software package (Abacus Concepts Inc., Berkeley, CA). The area under the receiver operating characteristic curve (AUC) analysis was performed using the MedCalc software package (Ver 8.0.1.0, Mariakerke, Belgium). All results are expressed as mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
We selected patients who underwent a hepatectomy with three different etiologies (Table 1). A large enough specimen for histological assessment could only be obtained from a surgical specimen since biopsy specimens are often insufficient to make an accurate diagnosis. We assessed the exact fibrotic area (FA) of LF using a digital image analysis (DIA), which is a simple and reliable method. We depicted only the blue signals from the full color image, and calculated FA. Representative photographs of the Azan-Mallory stain and the blue signals after depiction are shown in Figure 1. This method could thus be used for evaluating the pathological FA without diagnostic bias.
Table 1.
Characteristic of the patients
| Characteristics | n =30 | ||
| Etiology (NBNC:B:C) | 10:10:10 | Choline esterase (IU/L) | 262.80 ± 69.34 |
| Background (N:CH:LC) | 10:10:10 | Pre-ALB (mg/dL) | 21.92 ± 7.01 |
| Sex (M:F) | 22:8 | RBP (mg/dL) | 3.19 ± 1.39 |
| Age (yr) | 65.4 ± 10.3 | LCAT (U) | 100.66 ± 25.78 |
| BMI | 24.3 ± 3.1 | Apo-A1 (mg/dL) | 136.73 ± 32.43 |
| ALB (mg/dL) | 4.03 ± 0.60 | ICGR15 (%) | 12.86 ± 6.44 |
| Total Bilirubin (mg/dL) | 0.81 ± 0.28 | Fibrotic area (%) | 15.32 ± 9.69 |
| PT (%) | 98.83 ± 16.85 | APRI | 0.69 ± 0.46 |
| PT-INR | 1.01 ± 0.16 | FibroScan (kPa) | 15.47 ± 13.02 |
| AST (IU/L) | 41.63 ± 21.56 | Hyaluronate (ng/mL) | 120.50 ± 87.71 |
| ALT (IU/L) | 37.03 ± 21.58 | Type IV collagen | 6.85 ± 5.11 |
| Platelet count (104/μL) | 16.14 ± 4.57 | HGF (ng/dL) | 0.30 ± 0.09 |
| ZTT (K-U) | 13.57 ± 9.96 | ||
BMI: Body mass index; ALB: Albumin; PT: Prothrombin time; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ZTT: Zinc turbidity test; TTT: Thymol turbidity test; GGT: Gamma glutamyl transpeptidase; APRI: AST-to platelet ratio index; HGF: Hepatocyte growth factor; RBP: Retinol-binding protein; LCAT: Lecithin-cholesterol acyltransferase; Apo: Apolipoprotein; ICG: Indocyanine.
Figure 1.

Hematoxylin-eosin staining of the surgical specimens (A, C, and E). The images scanned and depicted only blue signals using a computer program. The blue signals were calculated as the area of fibrosis (B, D, and F). The results of such fibrosis were 3.2% for B, 12.2% for D, and 27.7% for F. The bar shows 100 μm.
The characteristics of the patients are shown in Table 1. The liver stiffness was measured by FibroScan in all patients. We also measured serum hyaluronate, type IV collagen, and HGF levels in addition to performing routine chemical laboratory tests. Furthermore, we measured pre-albumin (ALB), retinol-binding protein (RBP), lecithin-cholesterol acyltransferase (LCAT), Apo AI, and indocyanine green (ICG) R15 levels to investigate the relationship between LF and liver function. The patients enrolled for this study showed a mixed etiology and various types of liver damage. However, the mean value of all biochemical data showed a nearly normal range, indicating that the sample bias might be minimal. Among the examined biochemical data, PT-INR, platelet count, RBP, pre-ALB, and LCAT levels correlated with FA (Table 2). On the other hand, ALB, platelet count, pre-ALB, LCAT levels and ICGR15 all correlated with the FibroScan findings (Table 3). A linear regression analysis revealed two types of correlations between FA and the findings of a biochemical analysis. One showed a negative correlation, which was seen for ALB, pre-ALB, LCAT, and platelet count (Figures 2 A-D). The other showed a positive correlation to the FibroScan findings, which was seen for ZTT and ICGR15 (Figures 2 E and F). The pre-ALB levels showed the highest correlation with both FA and FibroScan among the examined biochemistry findings.
Table 2.
Correlation with fibrotic area
| Markers | r | P values |
| ALB | -0.388 | 0.067 |
| T-Bil | 0.13 | 0.559 |
| PT | -0.459 | 0.026a |
| PT-INR | 0.472 | 0.022a |
| Platelet count | -0.58 | 0.003a |
| RBP | -0.515 | 0.011a |
| Pre-ALB | -0.609 | 0.002a |
| CholE | -0.299 | 0.168 |
| LCAT | -0.447 | 0.032a |
| ApoA | -0.045 | 0.839 |
| ICGR15 (%) | 0.364 | 0.088 |
| AST | 0.295 | 0.173 |
| ALT | 0.243 | 0.266 |
| ZTT | 0.373 | 0.079 |
| G-GT | 0.068 | 0.762 |
r > 0.4 or r < -0.4 was considered to indicate a correlation with the fibrotic area.
P < 0.05 was considered to be significantly different.
Table 3.
Correlation with FibroScan
| Markers | r | P values |
| ALB | -0.446 | 0.013a |
| T-Bil | 0.127 | 0.506 |
| PT | -0.144 | 0.452 |
| PT-INR | 0.088 | 0.647 |
| Platelet count | -0.431 | 0.017a |
| RBP | -0.351 | 0.057 |
| Pre-ALB | -0.535 | 0.002a |
| CholE | -0.179 | 0.347 |
| LCAT | -0.413 | 0.023a |
| ApoA | -0.25 | 0.184 |
| ICGR15 (%) | 0.45 | 0.012a |
| AST | 0.21 | 0.268 |
| ALT | -0.001 | 0.998 |
| ZTT | 0.577 | 0.006a |
| G-GT | 0.118 | 0.538 |
r > 0.4 or r < -0.4 was considered to indicate a correlation with the fibrotic area.
P < 0.05 was considered to be significantly different.
Figure 2.
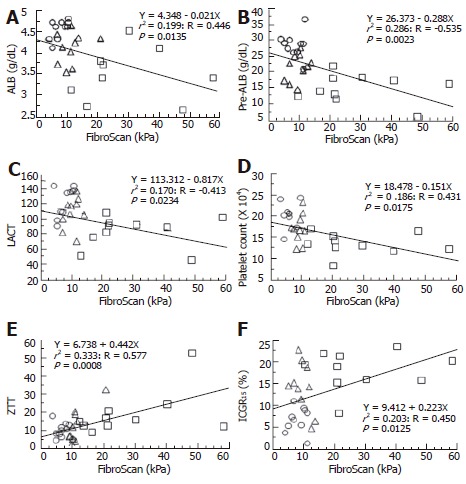
A linear regression analysis of FibroScan and clinical laboratory tests including liver functional markers. A negative correlation was seen for ALB (A), pre-ALB (B), lecithine-cholesterol acyltransferase (LCAT) (C), and platelet count (D). On the other hand, a positive correlation was seen for ZTT (E) and ICGR15 (F). ○: NBNC and normal liver; △: HBV and chronic hepatitis; □: HCV and liver cirrhosis.
We compared the correlation between FA and existing LF markers including FibroScan, hyaluronate, type IV collagen, and APRI (Figure 3). All markers correlated with FA. The correlation with the FibroScan findings was much higher than that with any other markers, even though the serum hyaluronate level was formerly believed to be the best available marker for evaluating LF[8,18,19].
Figure 3.
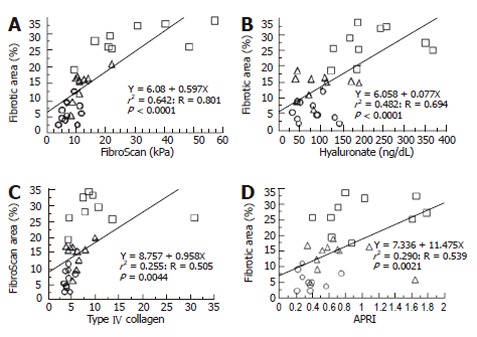
A linear regression analysis of the fibrotic area and liver fibrotic markers including FibroScan. A positive correlation was seen for all the markers such as FibroScan (A), hyaluronate (B), type IV collagen (C), and aspartate transaminase levels in comparison to the platelet ratio index (APRI) (D). ○: NBNC and normal liver; △: HBV and chronic hepatitis; □: HCV and liver cirrhosis.
We investigated the area under the receiver operating curve (AUC) in order to compare the diagnostic accuracy of FibroScan and other markers (Table 4). The diagnostic specificity of FibroScan increased when the FA increased. The AUC of FibroScan was the highest among the markers at any level of FA, even though the statistical difference was minimal.
Table 4.
Comparison of diagnostic accuracy of FibroScan and other markers
| Fibrotic Area >10% | ||||||
| OC | Sens | Speci | SE | 95% CI | AUC | |
| FibroScan | 9.1 | 100 | 76.9 | 0.047 | 0.777 - 0.999 | 0.932 |
| HA | 96.0 | 76.5 | 84.6 | 0.080 | 0.618 - 0.924 | 0.803 |
| Col | 6.6 | 52.9 | 100 | 0.081 | 0.615 - 0.923 | 0.801 |
| APRI | 0.57 | 76.5 | 84.6 | 0.081 | 0.615 - 0.923 | 0.801 |
| Fibrotic Area > 20% | ||||||
| OC | Sens | Speci | SE | 95% CI | AUC | |
| FibroScan | 13.6 | 100 | 95.5 | 0.024 | 0.868 - 1.000 | 0.991 |
| HA | 106.0 | 100 | 77.3 | 0.061 | 0.797 - 0.993 | 0.946 |
| Col | 6.6 | 87.5 | 90.9 | 0.071 | 0.758 - 0.985 | 0.918 |
| APRI | 0.58 | 87.5 | 68.2 | 0.098 | 0.642 - 0.937 | 0.824 |
OC: Optimal cut off level; Sens: Sensitivity; Speci: Specificity; SE: Standard error; CI: Confidence interval; AUC: Area under the curve; HA: Hyaluronate; Col: Type IV collagen; APRI: AST-to platelet ratio index.
DISCUSSION
We found that the biochemical data correlated with FA based on DIA. Among the examined markers, pre-ALB showed a higher negative correlation to FA and FibroScan than the other markers. The diagnostic accuracy of FibroScan was higher than that of the other existing markers although the statistical significance was minimal. FibroScan can therefore be used to evaluate LF and liver function without any unnecessary invasiveness.
Biochemical assessment of LF
There are many ways to assess LF by serum biomarkers and pathological evaluations[1,8,18-22]. Most such studies have been designed to evaluate fibrosis in chronic hepatitis C patients. Simple biomarkers such as those for aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AAR) and AST-to platelet ratio index (APRI), and commercial tests such as Fibrotest® (Biopredictive, Paris, France) have been validated to distinguish between patients with severe LF and a normal liver. However, it is unclear whether they represent FA accurately regarding liver disease with various etiologies. APRI correlated with FA in our study. However, the relative factor was much less than observed for the other markers. It is easy to examine APRI in clinical examinations. However, it may not accurately represent FA until the liver disease has already reached a severely advanced stage. Therefore, the clinical benefit of the existing markers or their combinations may be limited by the etiology or stage of the disease. On the other hand, hyaluronate and type IV collagen were significantly correlated with FA in our results. Hyaluronate and type IV collagen are deposited in perisinusoidal lesions and can be used as specific markers to detect liver fibrosis[8,18-20]. In addition, the serum levels both in liver cirrhosis and in fibrosis have been found to correlate with severity of the disease[21,22]. In fact, they demonstrated a good correlation with FA in our results although AUC of them decreased in fewer FAs. Our findings suggest that hyaluronate and type IV collagen can thus be used to assess severe LF or cirrhosis. However, it would be difficult to assess LF at its early stage.
Liver stiffness as a novel marker for assessment of LF
Recently, FibroScan has been tested for assessment of LF in comparison to the classical markers[14-17]. The diagnostic accuracy of FibroScan increases when it is used for severe fibrosis, whereas it decreases when it is used for a nearly normal liver. In our study, the diagnostic accuracy of FibroScan for livers with more than 20% FA was higher than that for livers with 10% FA. Our optimal cut-off level for FibroScan was 13.6 kPa, which could thus distinguish livers with more than 20% FA. This result is consistent with those in recent reports[15,16] investigating viral hepatitis C patients with severe fibrosis or cirrhosis. Although AUC of the tested markers for more than 20% FA was higher than that for more than 10% FA, AUC of FibroScan for more than 10% FA maintained a farely high level. Therefore, our results suggest that FibroScan is useful and reliable for assessment of earlier fibrotic stage compared to other existing markers and can be used for assessment of LA. However, these findings should be tested in prospective studies using a large number of cases because our results are based on a preliminary study. Especially, reduction of AUC for fewer FAs implies limitation of the diagnostic value of FibroScan in early LF stage.
Why AUC of FibroScan for fewer FAs decreases may be due to the system itself. Elasticity of the liver can be calculated by the different velocities between ultrasound (5MHz) and low-frequency (50 Hz) elastic waves[17]. Wave velocity in liver was affected by fibrosis and watery distributions in liver[23]. In general, watery distributions in the organ could be altered in the general systemic condition of the body such as inflammation which induces organ edema[24]. In addition, watery contents in earlier LF stage may alter due to active hepatitis. On the other hand, watery distributions in late LF stage may be more stable than that in its earlier stage. Liver atrophies in severe LF and cirrhosis may reduce the space to store watery contents. One dimensional transient method could not eliminate the bias of watery distributions in liver, which may affect velocity and elasticity. However, we found that FibroScan could be used for primary assessment of LF among the existing non-invasive markers.
DIA for accurate assessment of LF
Quantification of LF is very important for detecting severity of the disease. There are several scoring systems, such as histology activity index of Knodell et al, and its modification by Ishak et al, and METAVIR system[5]. However, such diagnostic variability cannot be ignored in pathological examinations due to the bias of pathologists and small samples[7,8]. Therefore, digital image analysis should be performed for quantification of LF[8]. On the other hand, the clinical benefits of digital image analysis remain controversial[25]. The problem of this approach is the methodology used to depict fibrous signals. Once original pathological images are converted into gray scale, it becomes very difficult to distinguish between stained and non-stained areas for specific types of staining. In fact, a histogram of the depicted images can show overlapping curves between them[25]. Therefore, specific fibrotic signals should be depicted in a full color image in order to eliminate any other colored signals, which could thus be other cellular components. In this setting, the area of LF could be assessed precisely reflecting the actual state of FA. Whether this strategy can be used for small pathological specimens from LB remains unclear. We used large samples after surgical removal of the tissue to eliminate any sampling errors due to a small size. Therefore, the usefulness of DIA for LB specimens should be evaluated in other settings in future.
Biochemical markers for the liver function show a negative correlation to LF
Liver function deteriorates during progression of LF and cirrhosis. However, whether serum proteins decrease with severity of the disease has not been fully understood and whether they are markers for LF remain unclear. Child-Turcotte-Pugh (CTP) score is a classical liver functional indicator, which comprises serum albumin level, bilirubin, prothrombin time, and other clinical signs[26]. However, CTP score in early stage of the disease does not show alteration[26,27] along with the disease progression, suggesting that factors in CTP score are stable in early LF stage. On the other hand, we found that LCAT and pre-ALB significantly correlated with LF. Previous reports indicate that LCAT[28,29] and pre-ALB[30] correlate with severity of the disease and galactose elimination test, respectively. Our results showed that LCAT and pre-ALB were significantly correlated with FA assessed by DIA for LF, suggesting that LCAT and pre-ALB are markers for detecting LF. Although our study was a preliminary one using only a small number of patients, the results nevertheless support the findings of previous reports.
In conclusion, we assessed FA using DIA to eliminate the diagnostic variability often observed in pathological specimens. We examined the diagnostic accuracy of FibroScan, which was compared to that of simple biomarkers used in routine laboratory tests and extracellular matrix markers. FibroScan therefore showed a better correlation with FA than the existing LF markers, suggesting that FibroScan can be used as an alternative to LB in assessment of LF, as it is safe and has a sufficient diagnostic accuracy.
ACKNOWLEDGMENTS
The authors thank Mr. Brian Quinn for his help in preparing this manuscript.
Footnotes
Supported by the Grants-in-Aid from the Society for the Promotion of Science, Sapporo Medical University for T. Mizuguchi, and Grants-in-Aid from the Ministry of Education, Culture, Sports Science and Technology, Japan. No. 18591519 for T. Mizuguchi, No. 17591420 for T. Katsuramaki, and No. 15390403 for K. Hirata
S- Editor Wang J L- Editor Wang XL E- Editor Ma N
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol. 2005;42 Suppl:S22–S36. doi: 10.1016/j.jhep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 4.Everson GT. Management of cirrhosis due to chronic hepatitis C. J Hepatol. 2005;42 Suppl:S65–S74. doi: 10.1016/j.jhep.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36:S152–S160. doi: 10.1053/jhep.2002.36381. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, Calès P. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 8.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaitoun AM, Al Mardini H, Awad S, Ukabam S, Makadisi S, Record CO. Quantitative assessment of fibrosis and steatosis in liver biopsies from patients with chronic hepatitis C. J Clin Pathol. 2001;54:461–465. doi: 10.1136/jcp.54.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui AY, Liew CT, Go MY, Chim AM, Chan HL, Leung NW, Sung JJ. Quantitative assessment of fibrosis in liver biopsies from patients with chronic hepatitis B. Liver Int. 2004;24:611–618. doi: 10.1111/j.1478-3231.2004.0957.x. [DOI] [PubMed] [Google Scholar]
- 11.Tessari P. Protein metabolism in liver cirrhosis: from albumin to muscle myofibrils. Curr Opin Clin Nutr Metab Care. 2003;6:79–85. doi: 10.1097/00075197-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Habib A, Mihas AA, Abou-Assi SG, Williams LM, Gavis E, Pandak WM, Heuman DM. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin Gastroenterol Hepatol. 2005;3:286–291. doi: 10.1016/s1542-3565(04)00622-6. [DOI] [PubMed] [Google Scholar]
- 13.Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83–96. doi: 10.1055/s-2002-23205. [DOI] [PubMed] [Google Scholar]
- 14.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 17.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Zheng M, Cai WM, Weng HL, Liu RH. ROC curves in evaluation of serum fibrosis indices for hepatic fibrosis. World J Gastroenterol. 2002;8:1073–1076. doi: 10.3748/wjg.v8.i6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36:S57–S64. doi: 10.1053/jhep.2002.36800. [DOI] [PubMed] [Google Scholar]
- 21.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis A, Mangia A, Leandro G, Clemente R, Festa V, Attino V, Ricciardi R, Giacobbe A, Facciorusso D, Andriulli A. External validation of biochemical indices for noninvasive evaluation of liver fibrosis in HCV chronic hepatitis. Am J Gastroenterol. 2005;100:868–873. doi: 10.1111/j.1572-0241.2005.40881.x. [DOI] [PubMed] [Google Scholar]
- 23.Sarvazyan AP, Lyrchikov AG, Gorelov SE. Dependence of ultrasonic velocity in rabbit liver on water content and structure of the tissue. Ultrasonics. 1987;25:244–247. doi: 10.1016/0041-624x(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18:385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Wright M, Thursz M, Pullen R, Thomas H, Goldin R. Quantitative versus morphological assessment of liver fibrosis: semi-quantitative scores are more robust than digital image fibrosis area estimation. Liver Int. 2003;23:28–34. doi: 10.1034/j.1600-0676.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- 26.Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- 27.Mizuguchi T, Katsuramaki T, Nobuoka T, Kawamoto M, Oshima H, Kawasaki H, Kikuchi H, Shibata C, Hirata K. Serum hyaluronate level for predicting subclinical liver dysfunction after hepatectomy. World J Surg. 2004;28:971–976. doi: 10.1007/s00268-004-7389-1. [DOI] [PubMed] [Google Scholar]
- 28.Horton RC, Owen JS. LCAT activity as a prognostic liver function test. Lancet. 1990;336:249–250. doi: 10.1016/0140-6736(90)91777-8. [DOI] [PubMed] [Google Scholar]
- 29.Tahara D, Nakanishi T, Akazawa S, Yamaguchi Y, Yamamoto H, Akashi M, Chikuba N, Okuno S, Maeda Y, Kusumoto Y. Lecithin-cholesterol acyltransferase and lipid transfer protein activities in liver disease. Metabolism. 1993;42:19–23. doi: 10.1016/0026-0495(93)90166-l. [DOI] [PubMed] [Google Scholar]
- 30.Rondana M, Milani L, Merkel C, Caregaro L, Gatta A. Value of prealbumin plasma levels as liver test. Digestion. 1987;37:72–78. doi: 10.1159/000199471. [DOI] [PubMed] [Google Scholar]


