Abstract
AIM: To evaluate the effects of osthole on fatty liver, and investigate the possible mechanism.
METHODS: A quail model with hyperlipidemic fatty liver and rat model with alcoholic fatty liver were set up by feeding high fat diet and alcohol, respectively. These experimental animals were then treated with osthole 5-20 mg/kg for 6 wk, respectively. Whereafter, the lipid in serum and hepatic tissue, and coefficient of hepatic weight were measured.
RESULTS: After treatment with osthole the levels of serum total cholesterol (TC), triglyceride (TG), lower density lipoprotein-cholesterol (LDL-C), coefficient of hepatic weight, and the hepatic tissue contents of TC and TG were significantly decreased. The activity of superoxide dismutase (SOD) in liver was improved. In alcohol-induced fatty liver rats, the level of malondialdehyde (MDA) in liver was decreased. In high fat-induced fatty liver quails, glutathione peroxidase (GSH-PX) in liver was significantly improved. The histological evaluation of liver specimens demonstrated that the osthole dramatically decreased lipid accumulation.
CONCLUSION: These results suggested that osthole had therapeutic effects on both alcohol and high fat-induced fatty liver. The mechanism might be associated with its antioxidation.
Keywords: Hyperlipidemic fatty liver, Alcoholic fatty liver, Osthole, Quails, Rats
INTRODUCTION
Fatty liver is the first progression of chronic liver diseases, and without therapy, the disease is apt to develop inflammation, necrosis, fibrosis and finally cirrhosis[1]. There are currently no ideal pharmacological reagents that can prevent or reverse this disease[1-2].
Osthole is an active constituent isolated from the fruit of Cnidium monnieri (L.) Cusson, a Chinese herbal medicine, which has been clinically used as therapy for diseases for many years. It has many anti-inflammatiory, anti-osteoporosis and anti-tumour functions, but there is no report about treatment of fatty liver. We found it could decrease the level of serum triglyceride (TG) in ovariectomized rats occasionally, so we begin to study the therapeutic role of osthole for fatty liver.
The aim of our study was to determine whether osthole could reverse steatosis in the rat model of alcohol and quail model of high fat-induced hepatic fat accumulation, and to investigate potential mechanisms involved in this therapeutic effect. Accordingly, we established alcoholic fatty liver rat model and hyperlipidemic fatty liver quail model, and then these animals were treated with osthole.
MATERIALS AND METHODS
Drugs
Osthole was kindly provided by Dr Jia Zhou of Xi’an Green Fount Natural Product Co. Ltd. (China), the purity of the drug was ≥ 95%. Lipanthyl was procured from Laboratories Fournier SA (France).
The establishment of model[3-4]
Male Sprague-Dawley rats weighing 200 ± 20 g were obtained from Animal Breeding Center of Soochow University, and were housed in regular cages situated in an animal room at 22°C. These experimental rats were fed with standard rat diet and allowed to drink water at will. The animal studies were conducted according to the regulations for the use and care of animal experimentation in Soochow University.
The alcoholic fatty liver rat model was induced by feeding 40% alcohol 1 mL/100 g and corn embryo oil 0.4 mL/100 g. Eight wk after the experiment, three rats were killed and livers were taken out for assessment of fatty hepatic development. After the model developed, the rats were randomly divided into 5 groups (n = 10 for each group): fatty hepatic model, osthole 5 mg/kg, osthole 10 mg/kg, osthole 20 mg/kg groups, and lipanthyl 20 mg/kg groups respectively. A solvent control group (n = 10) was added simultaneously and given 0.5% CMCNa2 solution. These medications lasted for 6 wk. The rats were then sacrificed, blood sample was obtained and hepatic tissues were collected for measurement of designing parameters
Male quails weighing 120 ± 5 g were obtained from Animal Breeding Center of Soochow University, they were housed in regular cages situated in an animal room at 22°C, with a 12 h light/12 h dark cycle. The animals were fed with standard quail diet and allowed to drink water at will.
The hyperlipidemic fatty liver quail model was created by feeding a high-fat diet containing standard diet, 10% lard and 2% cholesterol. After 6 wk the fatty liver was generated, these animals were then randomly divided into 5 groups (n = 10 for each group): model, osthole 5 mg/kg, osthole 10 mg/kg, osthole 20 mg/kg and lipanthyl 20 mg/kg groups respectively. A solvent control group (n = 10) was added as above. These medications lasted for 6 wk. The quails were sacrificed, blood was obtained and hepatic tissues were collected for measurement of designing parameters.
Measurement of serum TC, TG, lower density lipoprotein-cholesterol (LDL-C) and free fatty acid (FFA)
Blood was obtained after 12 h overnight fasting. The assay kits were purchased from Kexin Biology-Technology Company of Shanghai, China. Serum TC, TG, HDL-C and FFA were determined by colorimetric methods according to the procedures provided, LDL-C was obtained by Friedewald calculation, namely, LDL-C = TC- (TG/2.2 + HDL-C).
Measurement of TC and TG in liver
The hepatic TC and TG was extracted from liver tissue using chloroform/methanol mixed solution (1:1, vol:vol), the prepared sample was then centrifuged at 1200× g for 10 min, the obtained supernatant was used for measurement of TC and TG according to the colorimetric methods.
Measurement of SOD, MDA, GSH-PX and protein in liver
Liver tissue was taken at the time of animal sacrifice and rapidly put in ice-cold saline; the tissue homogenate (10% wt/vol) was prepared. Contents of SOD, MDA and GSH-PX were determined by colorimetric methods according to the procedure provided, respectively. Protein in liver was also measured by colorimetric method.
Histological observation
Liver specimens were fixed in 10% formaldehyde and embedded in paraffin for HE staining. The degree of fatty degeneration was graded by estimating the proportion of hepatocytes containing fat droplets and expressed as “-, +, ++, +++”. “-” means no fat present, “+” less than 1/3 of the hepatic lobule, “++” 1/3 to 2/3, and “+++” more than 2/3. The histological evaluation of the liver sections was blindly performed.
Statistical analysis
Data are expressed as mean ± SD, t-test was used for comparisons between groups, χ2-test was used for histopathological evaluation. P ≤ 0.05 was considered statistically significant.
RESULTS
Results from rats
After 6 wk of osthole administration, rat serum TC, TG and LDL-C levels were significantly lower in osthole groups than in model group, the relative hepatic weight in osthole groups was also decreased, but no significant difference was found in serum FFA and HDL-C levels between osthole and model groups (Table 1). The hepatic tissue levels of TC, TG and MDA in osthole groups were significantly lower than those in the model group, and the activity of SOD in osthole groups was increased significantly. The osthole had no obvious effect on GSH-PX activity (Table 2). Histological evaluation of liver specimens demonstrated that osthole could decrease lipid accumulation (Table 3 and Figure 1). The results of the present study suggested osthole could decrease the alcohol-induced liver injury in rats.
Table 1.
Serum levels of TC, TG, HDL-C, LDL-C, FFA and the relative hepatic weight after treatment with osthole for 6 wk in alcohol-induced fatty liver rats (n = 10)
| Group | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | FFA(μmol/L) | Relative hepatic weight (g/100 g) |
| Control | 2.14 ± 0.28 | 0.40 ± 0.11 | 1.16 ± 0.16 | 0.80 ± 0.21 | 429.93 ± 90.28 | 2.36 ± 0.33 |
| Model | 2.52 ± 0.30a | 0.51 ± 0.09a | 0.96 ± 0.24a | 1.32 ± 0.51b | 535.04 ± 105.44a | 2.82 ± 0.28b |
| Osthole 5 mg/kg | 1.72 ± 0.18d | 0.38 ± 0.08d | 0.91 ± 0.30 | 0.64 ± 0.34d | 477.37 ± 109.47 | 2.30 ± 0.14d |
| Osthole 10 mg/kg | 1.69 ± 0.29d | 0.36 ± 0.11d | 1.02 ± 0.17 | 0.50 ± 0.17d | 473.79 ± 196.55 | 2.30 ± 0.12d |
| Osthole 20 mg/kg | 1.60 ± 0.36d | 0.37 ± 0.06d | 1.05 ± 0.15 | 0.43 ± 0.21d | 487.59 ± 130.79 | 2.37 ± 0.19d |
| Lipanthyl 20 mg/kg | 1.82 ± 0.28d | 0.42 ± 0.09c | 1.01 ± 0.20 | 0.62 ± 0.31d | 654.74 ± 136.86c | 3.45 ± 0.18d |
P < 0.05,
P < 0.01 vs control animal.
P < 0.05,
P < 0.01 vs model animal.
Table 2.
Levels of TC, TG, SOD, MDA and GSH-PX in liver after treatment with osthole for 6 wk in alcohol-induced fatty liver rats (n = 10)
| Group | TC (mg/g wet tissue) | TG (mg/g wet tissue) | SOD (U/mg prot) | MDA (nmol/mg prot) | GSH-PX (vigor units) |
| Control | 1.94 ± 0.24 | 15.27 ± 3.35 | 58.33 ± 4.28 | 4.14 ± 1.34 | 174.86 ± 49.56 |
| Model | 2.25 ± 0.36a | 18.88 ± 3.81a | 50.04 ± 8.20a | 5.36 ± 0.82a | 206.80 ± 66.07 |
| Osthole 5 mg/kg | 1.82 ± 0.38d | 16.19 ± 4.21 | 61.11 ± 3.26d | 3.90 ± 1.35d | 203.80 ± 41.24 |
| Osthole 10 mg/kg | 1.80 ± 0.25d | 14.25 ± 3.10d | 61.45 ± 3.62d | 3.74 ± 0.99d | 182.30 ± 31.42 |
| Osthole 20 mg/kg | 1.76 ± 0.21d | 13.67 ± 4.30d | 58.91 ± 3.72d | 2.88 ± 0.92d | 171.89 ± 32.44 |
| Lipanthyl 20 mg/kg | 1.61 ± 0.20d | 12.84 ± 3.22d | 53.80 ± 2.49 | 2.59 ± 0.54d | 200.95 ± 55.26 |
P < 0.05, bP < 0.01 vs control animal. cP < 0.05,
P < 0.01 vs model animal.
Table 3.
Histopathological changes of hepatic fatty degeneration after treatment with osthole 5-20 mg/kg for 6 wk in alcohol-induced fatty liver rats (n = 10)
| Degree of fatty degeneration | Control | Model |
Osthole |
Lipanthyl 20 mg/kg | ||
| 5 mg/kg | 10 mg/kg | 20 mg/kg | ||||
| — | 9 | 0 | 2 | 2 | 4 | 6 |
| + | 1 | 5 | 7 | 8 | 6 | 4 |
| ++ | 0 | 5 | 1 | 0 | 0 | 0 |
| +++ | 0 | 0 | 0 | 0 | 0 | 0 |
| P | < 0.01 | < 0.05 | < 0.05 | < 0.01 | < 0.01 | |
P value for medicine-treated or control animal vs model animal.
Figure 1.
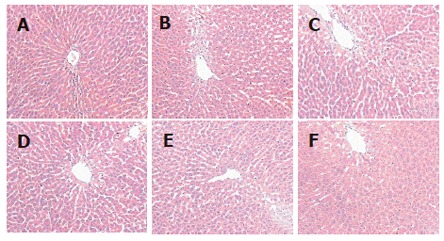
Histopathological changes of rat liver (hematoxylin and eosin staining, 25 ×). No steatosis was observed in control group (A) and the fatty degeneration was seen in the model group (B). Fatty degeneration of the liver was alleviated after treated with osthole 5 mg/kg (C), 10 mg/kg (D), 20 mg/kg (E) and lipanthyl 20 mg/kg (F) for 6 wk.
The serum levels of TC, TG, LDL-C, FFA and the relative hepatic weight in model group were significantly higher than those in control group, whereas the level of HDL-C was decreased markedly. After treatment with osthole 5-20 mg/kg for 6 wk, serum levels of TC, TG, LDL-C and the relative hepatic weight were decreased by 31.7%-36.5%, 25.4%-29.4%, 51.5%-67.4% and 16.0%-18.4%, respectively. Serum levels of FFA and HDL-C were decreased or increased to some degree, but the effects were not significant compared with those in the model group. In lipanthyl group, serum levels of TC, TG and LDL-C were decreased, but the level of HDL-C was not increased, the level of FFA and the relative hepatic weight were inversely increased.
The tissue levels of TC, TG and MDA in the model group were significantly higher than those in the control group, while the activity of SOD in model group was depressed significantly. Treatment of rats with osthole 5-20 mg/kg for 6 wk, resulted in a significant decline in levels of TC, TG and MDA, and increase in activity of SOD. In lipanthyl group, the levels of TC, TG and MDA were decreased, but the SOD activity was not increased. There was no obvious effect on GSH-PX activity.
Histological evaluation of liver specimens demonstrated that administration of osthole dramatically decreased lipid accumulation. Thus, our results demonstrated a therapeutic effect of osthole in alcohol-induced fatty liver of the rats.
Results from quails
In quails treated with osthole for 6 wk, serum TC, TG, LDL-C and FFA levels, the relative hepatic weight in osthole groups, particularly in 20 mg/kg group, were significantly lower as compared with model group. No significant difference was observed in HDL-C level between osthole and model groups (Table 4). The hepatic tissue levels of TC and TG in osthole groups were significantly lower than those in model group, and the activities of SOD and GSH-PX were significantly improved. There were no obvious effects on FFA and MDA levels (Table 5). Histological evaluation of liver specimens demonstrated that osthole could decrease lipid accumulation (Table 6 and Figure 2). These results suggested that osthole could decrease the high fat-induced liver injury in quails.
Table 4.
Serum levels of TC, TG, HDL-C, LDL-C, FFA and the relative hepatic weight after treatment with osthole for 6 wk in high fat-induced fatty liver quails (n = 10)
| Group | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | FFA(μmol/L) | Relative hepatic weight (g/100 g) |
| Control | 5.74 ± 0.51 | 0.68 ± 0.14 | 5.12 ± 0.48 | 0.31 ± 0.23 | 963.21 ± 231.70 | 1.89 ± 0.16 |
| Model | 9.53 ± 3.92b | 0.89 ± 0.21a | 5.35 ± 0.98 | 3.78 ± 3.36b | 1303.77 ± 372.58a | 2.13 ± 0.19b |
| Osthole 5 mg/kg | 7.34 ± 0.88 | 0.49 ± 0.08d | 5.50 ± 0.58 | 1.62 ± 0.73 | 938.68 ± 146.77d | 1.83 ± 0.11d |
| Osthole 10 mg/kg | 7.32 ± 1.53 | 0.57 ± 0.16d | 5.56 ± 0.93 | 1.50 ± 1.41 | 794.34 ± 225.05d | 1.83 ± 0.25d |
| Osthole 20 mg/kg | 6.89 ± 0.84c | 0.45 ± 0.05d | 5.72 ± 0.75 | 0.98 ± 1.19c | 783.96 ±1 04.05d | 1.78 ± 0.13d |
| Lipanthyl 20 mg/kg | 6.74 ± 0.73c | 0.55 ± 0.10d | 5.87 ± 0.66 | 0.62 ± 0.53d | 1170.75 ± 425.34 | 1.76 ± 0.19d |
P < 0.05,
P < 0.01 vs control animal.
P < 0.05,
P < 0.01 vs model animal.
Table 5.
Levels of TC, TG, FFA, SOD, MDA and GSH-PX in liver after treatment with osthole for 6 wk in high fat-induced fatty liver quails (n = 10)
| Group | TC (mg/g wet tissue) | TG (mg/g wet tissue) | FFA (mg/g wet tissue) | SOD (U/mg prot) | MDA (nmol/mg prot) | GSH-PX (vigor units) |
| Control | 2.26 ± 0.56 | 11.42 ± 4.37 | 499.14 ± 90.73 | 368.10 ± 21.60 | 10.93 ± 2.44 | 121.53 ± 20.88 |
| Model | 6.94 ± 3.25b | 17.34 ± 5.90a | 418.97 ± 181.75 | 314.24 ± 17.91b | 11.37 ± 1.71 | 111.23 ± 29.85 |
| Osthole 5 mg/kg | 4.32 ± 0.69c | 16.05 ± 4.74 | 508.62 ± 180.05 | 343.20 ± 33.71c | 10.73 ± 2.30 | 130.00 ± 38.82 |
| Osthole 10 mg/kg | 4.05 ± 1.82c | 13.48 ± 2.55 | 478.45 ± 134.74 | 344.16 ± 10.25d | 10.86 ± 3.56 | 151.40 ± 41.34c |
| Osthole 20 mg/kg | 3.66 ± 1.14d | 12.97 ± 2.38c | 419.83 ± 106.21 | 353.70 ± 33.95d | 10.73 ± 0.95 | 174.35 ± 46.43d |
| Lipanthyl 20 mg/kg | 4.05 ± 1.06c | 14.87 ± 6.21 | 731.03 ± 237.13d | 357.98 ± 16.08d | 11.02 ± 1.90 | 150.05 ± 46.54c |
P < 0.05,
P < 0.01 for medicine-treated animal versus model animal.
Table 6.
Histopathological changes of hepatic fatty degeneration after treatment with osthole 5-20 mg/kg for 6 wk in high fat-induced fatty liver quails (n = 10)
| Degree of fatty degeneration | Control | Model |
Osthole |
Lipanthyl |
||
| 5 mg/kg | 10 mg/kg | 20 mg/kg | 20 mg/kg | |||
| — | 10 | 0 | 3 | 4 | 7 | 5 |
| + | 0 | 4 | 7 | 6 | 3 | 4 |
| ++ | 0 | 4 | 0 | 0 | 0 | 1 |
| +++ | 0 | 2 | 0 | 0 | 0 | 0 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
P value for medicine-treated or control animal vs model animal.
Figure 2.
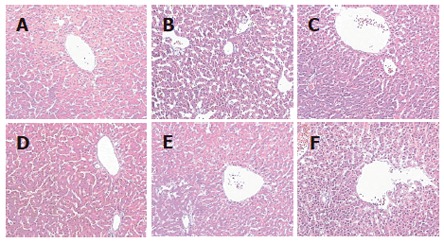
Histopathological changes of quail liver (hematoxylin and eosin staining, 25 ×). No steatosis was observed in control group (A) and the fatty degeneration was seen in the model group (B). Fatty degeneration of liver was alleviated after treated with osthole 5 mg/kg (C), 10 mg/kg (D), 20 mg/kg (E) and lipanthyl 20 mg/kg (F) for 6 wk.
The serum levels of TC, TG, LDL-C, FFA and the relative hepatic weight in model group were significantly higher than those in the control group. Serum levels of TG, FFA and the relative hepatic weight in osthole groups were decreased, while serum levels of TC and LDL-C only in 20 mg/kg group were significantly decreased. In lipanthyl group, serum levels of TC, TG, LDL-C and the relative hepatic weight were also decreased, but serum FFA level was not.
The hepatic tissue levels of TC and TG in model group were significantly higher than those in control group, and the activity of SOD was significantly depressed. Levels of TC and TG in liver in osthole groups were decreased by 37.6%-47.3% and 7.4%-25.2%. Notably, continuous administration of the drug for 6 wk significantly increased the activities of SOD and GSH-PX in hepatic tissue. In lipanthyl group, level of TC was decreased, activities of SOD and GSH-PX in liver were increased, level of FFA was inversely increased, but it had no obvious effects on TG levels.
Histological evaluation of liver specimens demonstrated that administration of osthole dramatically decreased lipid accumulation. Thus, our results demonstrated that osthole possessed a therapeutic effect on high fat-induced fatty liver in quails.
DISCUSSION
Fatty liver can be induced by alcoholic and non-alcoholic aetiologies. It commonly occurs in the general population and has the potential to progress to fibrosis and cirrhosis[5-6]. The spectrum of pathology is wide, ranging from benign steatosis to cirrhosis, hepatocellular carcinoma and hepatic failure[6]. The prevalence of hepatic damage in fatty liver is difficult to determine accurately. However, it has been estimated that amongst a population of obese/diabetic individuals approximately 50%-70% will have fatty change, 20%-30% will progress to steatohepatitis/fibrosis and 2%-5% will eventually become cirrhosis[7-9]. But fatty liver is reversible with abstinence or medicine therapy. To prevent the occurrence of hepatic fibrosis and cirrhosis, treatment of fatty liver is an important step.
Non-alcoholic fatty liver (NAFL) has been extensively reported over the world. In general population studies, screening with ultrasound or CT has shown that a prevalence ranging of NAFL is from 16% to 23%. In liver biopsy studies, the prevalence ranges are 15%-39%[10-12]. Obesity type 2 diabetes mellitus (DM), female gender and hyperlipidaemia are frequently associated with NAFL[6,13]. Because the typical dyslipdaemia of NAFL is also characteristic of the commonly occurring insulin resistance syndrome, and there is a strong link between hyperlipidemia and obesity which associate with visceral fat accumulation[14], so our study emphasized particularly on the hyperlipidemic fatty liver.
Alcohol is also a major cause of fatty liver. Alcoholic fatty liver (AFL) has a common pathophysiology with NAFL[15]. At least 80% of heavy drinkers develop fatty liver. It is reversible with abstinence, whereas it may progress to cirrhosis without abstinence.
At present the treatment for fatty liver mainly includes dietary intervention, treatment of associated insulin resistance, lipid-lowering medications, antioxidants and ursodeoxycholic acid and so on. An ideal and effective drug has not been found to prevent and treat this disease[6].
Our experimental results show that after treatment with osthole for 6 wk, the levels of serum TC, TG, LDL-C, the relative hepatic weight and the hepatic tissue contents of TC and TG were decreased significantly in alcohol-induced fatty liver rats or in high fat-induced fatty liver quails. The histological evaluation of liver specimens demonstrated that osthole dramatically decreased lipid accumulation. These results suggest that osthole has a new function to protect the liver from fat accumulation. It decreased high fat-induced liver injury in quails and alcohol-induced liver injury in rats.
To develop fatty liver, it has been recently suggested that one prerequisite condition should exist, i.e., a source of oxidative stress capable of initiating significant lipid peroxidation[16]. Both animal data and human studies have shown a link between fatty liver and oxidative stress and lipid peroxidation. Mitochondria are thought to be the source of the reactive oxygen species (ROS) leading to lipid peroxidation. The increased hepatic influx of FFA that results from the reduced ability of insulin to suppress lipolysis is thought to increase the rate of mitochondrial [beta] oxidation and produce the ROS. There are other potential sources of oxidative stress including the cytochrome P450 enzymes (CYP2E1 and CYP3A4) and increased hepatic iron content. Chronic alcohol exposure may also result in oxidant production[16].
Our experimental results showed in the alcohol-induced fatty liver test that administration of osthole promoted SOD production, depressed MDA production, and lightened the degree of steatosis in liver tissue. In the high fat-induced fatty liver test, administration of osthole had same effects as in the alcohol-induced fatty liver test. Furthermore, osthole also promoted GSH-PX production in liver tissue. These results demonstrated that the therapeutic effect of osthole on fatty liver was partly due to its antioxidation against ROS. From the findings of present studies, we suggested that the mechanism of fatty liver generation was related to the extra production of ROS.
Footnotes
S- Editor Wang J L- Editor Zhao JB E- Editor Ma N
References
- 1.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186–S190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 3.Li SD, Li YM, Yu CH. Establishment of experimental model for liver injury of chronic alcoholism in rats. Zhejiang Yixue. 2002;24:524–530. [Google Scholar]
- 4.Zhao GX, Dai YT, Wang Y, Gao ZF. Effects of natural non-shell seeds on the diet-induced hyperlipidemia and fatty liver in quails. Yingyang Xuebao. 1994;16:101–103. [Google Scholar]
- 5.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 6.Malnick SD, Beergabel M, Knobler H. Non-alcoholic fatty liver: a common manifestation of a metabolic disorder. QJM. 2003;96:699–709. doi: 10.1093/qjmed/hcg120. [DOI] [PubMed] [Google Scholar]
- 7.Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 9.Bahrami H. Nonalcoholic fatty liver disease in developing countries. World J Gastroenterol. 2005;11:3808–3809. doi: 10.3748/wjg.v11.i24.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 11.Nonomura A, Mizukami Y, Unoura M, Kobayashi K, Takeda Y, Takeda R. Clinicopathologic study of alcohol-like liver disease in non-alcoholics; non-alcoholic steatohepatitis and fibrosis. Gastroenterol Jpn. 1992;27:521–528. doi: 10.1007/BF02777789. [DOI] [PubMed] [Google Scholar]
- 12.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 13.el-Hassan AY, Ibrahim EM, al-Mulhim FA, Nabhan AA, Chammas MY. Fatty infiltration of the liver: analysis of prevalence, radiological and clinical features and influence on patient management. Br J Radiol. 1992;65:774–778. doi: 10.1259/0007-1285-65-777-774. [DOI] [PubMed] [Google Scholar]
- 14.Chen QK, Chen HY, Huang KH, Zhong YQ, Han JA, Zhu ZH, Zhou XD. Clinical features and risk factors of patients with fatty liver in Guangzhou area. World J Gastroenterol. 2004;10:899–902. doi: 10.3748/wjg.v10.i6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280–286. doi: 10.1136/pmj.76.895.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CP, James OF. Steatohepatitis: a tale of two "hits". Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]


