Abstract
We presented an unusual case with coexistence of carcinoid, signet-ring cell carcinoma (SRC) and heterotopic pancreatic tissue in stomach. Gastroscopic examination of this 63-year-old male patient showed multiple protrusions in gastric corpus near the greater curvature, identified by subsequent biopsy as carcinoid. Distal subtotal gastrectomy was performed. Histological and immunohistochemical examinations showed a carcinoid tumor in gastric corpus near the greater curvature, an intramucosal SRC at the lesser curvature of corpus and heterotopic pancreatic tissue in muscularis propria of the antrum at the lesser curvature with hyperplasia of peripheral endocrine cells producing multiple pancreatic hormones. We reviewed the literatures on clinicopathological characteristics and the differential diagnosis of the above three abnormalities, and concluded that the carcinoid in corpus near the greater curvature and SRC in the lesser curvature are independent lesions; the foci of endocrine cells in the muscularis propria and serosa are hyperplastic lesions from the heterotopic pancreatic tissue, rather than dissemination of carcinoid in corpus.
Keywords: Heterotopic pancreas, Carcinoid, Signet- ring cell carcinoma, Gastric tumour, Endocrine cell hyperplasia
INTRODUCTION
Gastric carcinoid is rare, accounting for 0.4%-1.8% in tumors of the gastrointestinal tract[1], while signet-ring cell carcinoma (SRC) is frequent[2]. Both of them occur often in an occult way, with different behavior and prognosis. Pancreatic heterotopia in stomach is often incidentally encountered during surgery or autopsy, with its incidence ranging from 0.6% to 13.7%[3]. We presented a case with synchronous occurrence of these three lesions in stomach, and reviewed related literatures.
CASE REPORT
A 63-year-old male patient visited this hospital with the complaint of “cauterized discomfort in the upper abdomen for more than 2 years, and aggravated for 2 mo”. By gastroscopy, multiple flat nodules (Lesion 1) were found in the front wall near the greater curvature, ranging from 0.2-0.8 cm in diameter. Mucosal biopsy was taken, and the lesions were considered a malignant neuroendocrine cell tumor with the help of immunohistochemistry. Findings from physical examination were unremarkable except for deep tenderness under xiphoid. Chest X-ray, upper alimentary canal contrast, abdominal ultrasonography failed to detect any abnormalities, while a slight increase in thickness of the antrum wall was observed by computed tomography (CT) scanning. Levels of circulating CA199, CEA, CA724 and CA242 were in normal ranges. Serum antibody against parietal cell was positive, and serum gastrin was 93.2 mg/L. No family history of cancer was recorded. Distal subtotal gastrectomy specimen was routinely resected. The samples were fixed in 4% buffered formaldehyde, and embedded in paraffin. Sections of 5 μm in thickness were prepared and stained with hematoxylin and eosin (HE) and Alcin blue/periodic acid Schiff reaction (AB/PAS). Immunohistochemical staining was conducted using a polymer-peroxidase detection kit (PV-9000) following the instructions of the manufacturer. Polyclonal antibodies were used to detect glucagon, gastrin, somatostatin, vasoactive intestinal peptide (VIP), pancreatic polypeptide (PP), and monoclonal antibodies were used for demonstration of CK18, insulin, ACTH, synaptophysin (SY), chromogranin A (CgA), NSE, vimentin, p53 protein (DO-7) and Ki-67 antigen. All of the reagents were supplied by Zhongshan Golden Bridge Biotech Co. Ltd., Beijing. Samples of a gastric SRC and a pancreatic endocrine tumor were used as positive controls, and the immunoglobins from a preimmune rabbit and mouse were used to substitute for the primary antibodies as negative controls. Slides were scored semi-quantitatively according to the percentage of positive cells in all counted cells from 5 randomly selected representative fields based on staining distribution and intensity as below: positive rate < 5% was defined as negative (-); weakly positive (+): 5%-25%; moderately positive (++): 25%-50%; strongly positive (+++): > 50%.
Three non-continuous lesions (Figure 1) with distinct histochemical phenotypes (Table 1) were identified: Lesion 1 (Figure 2), multiple nodules of 0.2-0.8 cm in diameter was seen in the anterior wall near the greater curvature of gastric corpus, and multiple foci of hyperplastic endocrine cells were seen in the deeper part of lamina propria, infiltrating the muscularis mucosa. Immunoreactivities were seen in the cells for CK18, NSE, SY and CgA. Therefore, Lesion 1 was regarded as intramucosal endocrine cell hyperplasia and formation of a type I carcinoid, with muscularis mucosae involved. Lesion 2 was identified beside the lesser curvature of corpus and sized 3.0 cm × 2.0 cm. The cancer cells contained mixed mucin, positive for both Alcin blue and PAS, but were negative for the neuroendocrine markers (Figure 3). Therefore, Lesion 2 was diagnosed as an intramucosal SRC (superficially flat type), rather than a signet-ring-like carcinoid. Lesion 3 (Figure 4) was a yellowish mural mass sizing 3.0 cm × 2.0 cm on cut surfaces, located between the submucosa and lamina muscularis of the antrum. Histologically, the mass was composed of well developed pancreatic acini and ducts, intermingled with hyperplastic smooth muscle tissue, giving rise to the pattern of an adenomyoma. At the peripheral area, multiple nodules of 0.1-0.4 mm in diameter were found. They were composed of well circumscribed small, bland epithelial cells which arranged in solid nests. The nodules resembled normal pancreatic islets in shape, scattered within the muscularis propria and serosa of the gastric wall. No stromal reaction was observed. The epithelial cells were shown to be diffusely positive for CK18, CgA, SY, insulin, glucagons, somatostatin and gastrin, the pattern being similar to that of pancreatic islets. Neither mitosis nor necrosis was identified. Therefore, the mass was diagnosed as pancreatic heterotopia and those peripheral nodules were hyperplastic tissues from endocrine pancreatic heterotopia rather than dissemination of Lesion 1.
Figure 1.
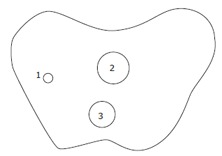
Diagrammatic representation of three lesions. 1: Carcinoid nodule of 0.7 cm in diameter near the greater curvature of gastric corpus; 2: SRC sizing 3.0 cm × 2.0 cm within mucosa at the lesser curvature of gastric corpus; 3: Ectopic pancreas nodule of 2.5 cm in diameter between submucosa and serosa in gastric corpus-antrum.
Table 1.
Immunohistochemical phenotypes of different mucosal lesions and various components of heterotopic pancreatic tissue in gastric wall
| Markers |
Mucosal lesions |
Heterotopic pancreas (Lesion 3) |
|||||
| Carcinoid (Lesion 1) | NE cell hyperplasia | SRC (Lesion 2) | Endocrine cell hyperplasia | Islets | Acini | Ducts | |
| CK18 | +++ | +++ | +++ | + | + | ++ | ++ |
| Vimentin | - | - | - | - | - | - | - |
| NSE | ++ | +-++ | - | +++ | +++ | - | + |
| CgA | +++ | +++ | - | +++ | +++ | - | + |
| Synaptophysin | +++ | +++ | - | +++ | +++ | - | + |
| Insulin | - | - | - | +++ | +++ | - | ± |
| Glucagon | - | - | - | ++ | ++ | - | + |
| Somatostatin | - | + | - | ++ | ++ | ± | ± |
| Gastrin | - | ++ | - | ++ | ++ | - | ± |
| VIP | ± | + | - | ± | ± | - | - |
| ACTH | - | - | - | - | - | - | - |
| PP | - | - | - | - | - | + | ± |
| p53 protein | 2% | - | - | - | - | - | - |
| Ki-67-LI | 2% | < 1% | 20% | 1% | < 1% | 3% | 5% |
ACTH: adrenocorticotropin; CK18: cytokeratin 18; CgA: chromogranin A; Ki-67-LI: Ki-67-lebelling index; PP: pancreatic peptide; NE: neuroendocrine; NSE: neuron-specific enolase; SRC: signet-ring cell carcinoma; VIP: vasoactive intestinal peptide.
Figure 2.
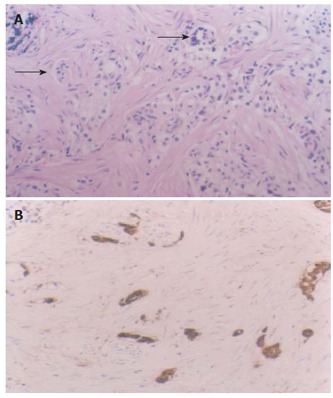
Lesion 1, intramucosal carcinoid with the muscularis mucosa infiltrated (A) (arrows, HE × 20), positively expresses CgA (B) (S-P method, conterstained with hematoxylin, × 20).
Figure 3.
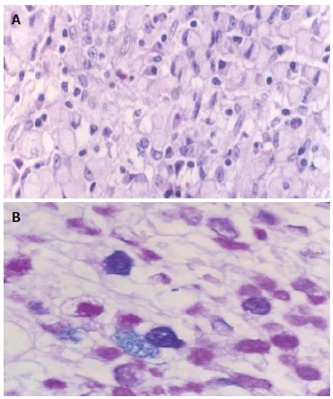
Lesion 2, intramucosal signet-ring cell carcinoma (A) (HE × 40) with cytoplasmic mixed mucin (B) (AB/PAS reaction, × 40).
Figure 4.
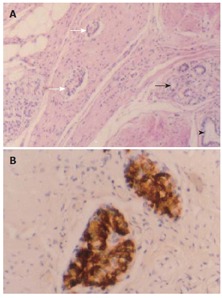
Lesion 3, heterotopic pancreatic tissue in gastric wall, which was composed of well developed pancreatic acini (darker arrow), ducts (arrowhead), and islets (lighter arrow) intermingled with the hyperplastic smooth muscle tissue, giving rise to the pattern of an adenomyoma (A) (HE × 20); Small-cell nodules of 0.1-0.4 mm in diameter scattered within the muscularis propria and serosa of the gastric wall and strongly positive for insulin (B) (S-P method, conterstained with hematoxylin).
Standard lymph node dissection was conducted and no metastasis was found in all of the twelve dissected lymph nodes.
The patient remains well 17 mo after surgery.
DISCUSSION
Gastric endocrine neoplasms are classified into carcinoid (well differentiated endocrine neoplasm), small cell carcinoma (poorly differentiated endocrine neoplasm) and tumor-like lesions (including hyperplasia and dysplasia of endocrine cells)[4]. Most endocrine tumors of the stomach are well differentiated, nonfunctioning enterochromaffin-like (ECL) cell carcinoids arising from lamina propria in the corpus or fundus[5]. Three distinct types have been recognized. Type I carcinoids are associated with autoimmune chronic atrophic gastritis. Type II carcinoids are linked to multiple endocrine neoplasia type 1(MEN-I)) and Zollinger-Ellison syndrome (ZES). Type III carcinoids are sporadic, without hypergastrinemia or autoimmune atrophic gastritis[6]. Types I and II carcinoids develop through a sequence of “hyperplasia-dysplasia-neoplasia”, and ECL-cell hyperplasia and dysplasia are identified as the precancerous lesions of ECL-cell carcinoid[7]. Lesions up to 0.5 cm in diameter or infiltrating submucosa are recognized as carcinoids[8]. In this case, mild to moderate atrophic gastritis was noted, coexisted with intestinal metaplasia and a high level of serum gastrin. A carcinoid of 0.7 cm in diameter was identified. All of the lesions listed above were in line with Type I carcinoid associated with endocrine cell hyperplasia.
In this case, superficial SRC (Lesion 2) was identified in corpus mucosa of the lesser curvature, which was failed to be detected by gastroscopy and biopsy before surgery. An unusual type of signet-ring-like carcinoid[9-11] was reported in literature, but the following three points in this case ruled out the possibility. First, cells in Lesions 1 and 2 were different from each other in morphology. Second, they occurred in two different sites, without anatomical continuity. Third, their histochemical and immunohistochemical phenotypes were different. Lesion 1 was negative for mucin, while positive for neuroendocrine markers (Table 1). Lesion 2, however, showed positive staining of mucin, while negative for neuroendocrine markers. Therefore, the carcinoid and SRC in this case were two independent lesions. Up to date, 5 cases of gastric carcinoid coexisting with adenocarcinoma have been reported[12-16], and only one of them with SRC[16].
The incidence of gastric ectopic pancreas ranges from 0.6% to 13.7%, frequently in the antrum and prepyloric region on the greater curvature or posterior wall. It often locates in submucosa, lamina muscularis or subserosa. Histologically, heterotopic pancreas can be divided into 4 types: type 1, composed of all cell types, namely complete heterotopia; type 2, composed of ductal components only, the canalicular heterotopia; type 3, composed of acinar cells only, the exocrine heterotopia; and type 4, composed of islet cells only; the endocrine heterotopia[17]. In this case, the heterotopic pancreas belongs to the first type. Most of the patients with gastric ectopic pancreas are asymptomatic. When mucosa is involved, however, patients may complain of an upper abdominal pain, bleeding, and obstruction may occur. Malignant transformation has been encountered, albeit rarely, giving rise to an adenocarcinoma[18-25] or a neuroendocrine neoplasm[26]. Caution should be paid to establishing a diagnosis of a carcinoma from heterotopic pancreas. For this, 3 points were considered necessary. First, the carcinoma should be found within or close to the heterotopic pancreas. Second, the transitional area between pancreatic structures and carcinoma should be observed and a metastatic carcinoma or dissemination of carcinoma from the adjacent gastrointestinal tract must be excluded. In addition, the non-neoplastic heterotopic pancreatic tissue should contain at least fully developed acinar or ductal structures[3]. It is evident that SRC in this case is independent from Lesion 3. Besides, a functional pancreatic endocrine tumor is composed of neoplastic cells secreting the same hormone (insulin, glucagon or somatostatin), differing from the endocrine cell hyperplasia whose constituent cells produce multiple hormones. In this case, Lesion 3 belonged to the complete heterotopic pancreas, with endocrine cell proliferation, which scattered preferentially among bundles of smooth muscle fibers or along vascular structures, mimicking intramural infiltration or metastasis of the carcinoid. It is important to know whether the endocrine tissue is hyperplastic or neoplastic, in consideration of the presence of a carcinoid (Lesion 1) at the corpus of the stomach and their structural and morphologic similarities. The following data revealed that endocrine component in Lesion 3 is non-neoplastic. The endocrine tissue was accompanied by the exocrine components in most parts of the lesion. Functionally, the endocrine tissue was composed of cells separately producing insulin, glucagon or somatostatin, reflecting its polyclonal nature, the phenotypes being similar to that of islets and different from Lesion 1 (Table 1).
In summary, we presented a case with coexistence of carcinoid, SRC and heterotopic pancreas in stomach. Differential diagnosis of the three abnormalities was of great importance for prognosis evaluation of the patient.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Xin-Hua Xue and Ms. Xiu-Yun Liu for their excellent technical assistance.
References
- 1.Modlin IM, Kidd M, Lye KD. Biology and management of gastric carcinoid tumours: a review. Eur J Surg. 2002;168:669–683. doi: 10.1080/11024150201680022. [DOI] [PubMed] [Google Scholar]
- 2.Yang XF, Yang L, Mao XY, Wu DY, Zhang SM, Xin Y. Pathobiological behavior and molecular mechanism of signet ring cell carcinoma and mucinous adenocarcinoma of the stomach: a comparative study. World J Gastroenterol. 2004;10:750–754. doi: 10.3748/wjg.v10.i5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lack EE. Congenital and developmental abnormalities of the pancreas. In: Pathology of the pancreas, gallbladder , extrahepatic biliary, and ampullary region., editors. 1st ed. New York: Oxford University Press Inc; 2003. p. 44–62. [Google Scholar]
- 4.Hamilton SR, Aaltonen LA(eds) Endocrine tumours of the stomach. In: World Health Organization Classification of Tumours., editor. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. p. 57. [Google Scholar]
- 5.Modlin IM, Lye KD, Kidd M. Carcinoid tumors of the stomach. Surg Oncol. 2003;12:153–172. doi: 10.1016/s0960-7404(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 7.Solcia E, Fiocca R, Villani L, Luinetti O, Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S1–S7. [PubMed] [Google Scholar]
- 8.Bordi C, Annibale B, Azzoni C, Marignani M, Ferraro G, Antonelli G, D'Adda T, D'Ambra G, Delle Fave G. Endocrine cell growths in atrophic body gastritis. Critical evaluation of a histological classification. J Pathol. 1997;182:339–346. doi: 10.1002/(SICI)1096-9896(199707)182:3<339::AID-PATH854>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435–444. [PubMed] [Google Scholar]
- 10.Sugihara A, Nakasho K, Yamada N, Nakagomi N, Tsujimura T, Terada N, Tsuji M. Neuroendocrine differentiation of periodic-acid Schiff and Alcian blue-negative signet-ring cell-like cells and tubular adenocarcinoma cells within a gastric cancer. Scand J Gastroenterol. 2004;39:795–800. doi: 10.1080/00365520410005775. [DOI] [PubMed] [Google Scholar]
- 11.Morii S, Oka K, Hakozaki H, Nihei T, Mori N. CEA-producing mucin-negative gastric signet-ring cell carcinoma with neuroendocrine markers: a case report. J Clin Gastroenterol. 1999;29:82–85. doi: 10.1097/00004836-199907000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Olinici CD, Crişan D, Racu I. Synchronous gastric adenocarcinoma and carcinoid. Rom J Gastroenterol. 2004;13:135–137. [PubMed] [Google Scholar]
- 13.De Marco L, Carlinfante G, Botticelli L, Di Maira PV, Putrino I, Cavazza A. [Mixed neoplasia of the stomach: description of a case of tubular adenoma combined with carcinoid] Pathologica. 2003;95:214–216. [PubMed] [Google Scholar]
- 14.Adhikari D, Conte C, Eskreis D, Urmacher C, Ellen K. Combined adenocarcinoma and carcinoid tumor in atrophic gastritis. Ann Clin Lab Sci. 2002;32:422–427. [PubMed] [Google Scholar]
- 15.Zai MJ, Zhang MZ, Zhao ZQ, Wu LJ, Huang YP. Mixed carcinoid and adenocarcinoma of gastrointestinal tracts: three cases of reports. Zhonghua Zhongliu Zazhi. 2003;25:447. [Google Scholar]
- 16.Emerson L, Layfield LJ, Rohr LR, Dayton MT. A case showing collision of gastric carcinoid tumor and signet-ring cell carcinoma treated by endoscopic mucosal resection. Endosc Forum Dig Dis. 1995;11:219–223. [Google Scholar]
- 17.Hammock L, Jorda M. Gastric endocrine pancreatic heterotopia. Arch Pathol Lab Med. 2002;126:464–467. doi: 10.5858/2002-126-0464-GEPH. [DOI] [PubMed] [Google Scholar]
- 18.Ura H, Denno R, Hirata K, Saeki A, Hirata K, Natori H. Carcinoma arising from ectopic pancreas in the stomach: endosonographic detection of malignant change. J Clin Ultrasound. 1998;26:265–268. doi: 10.1002/(sici)1097-0096(199806)26:5<265::aid-jcu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Song DE, Kwon Y, Kim KR, Oh ST, Kim JS. Adenocarcinoma arising in gastric heterotopic pancreas: a case report. J Korean Med Sci. 2004;19:145–148. doi: 10.3346/jkms.2004.19.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Wasserman PG. Acinar cell carcinoma arising in the stomach: a case report with literature review. Hum Pathol. 2004;35:263–265. doi: 10.1016/j.humpath.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 2004;87:53–57. doi: 10.1002/jso.20087. [DOI] [PubMed] [Google Scholar]
- 22.Osanai M, Miyokawa N, Tamaki T, Yonekawa M, Kawamura A, Sawada N. Adenocarcinoma arising in gastric heterotopic pancreas: clinicopathological and immunohistochemical study with genetic analysis of a case. Pathol Int. 2001;51:549–554. doi: 10.1046/j.1440-1827.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 23.Herold G, Kraft K. Adenocarcinoma arising from ectopic gastric pancreas: two case reports with a review of the literature. Z Gastroenterol. 1995;33:260–264. [PubMed] [Google Scholar]
- 24.Hickman DM, Frey CF, Carson JW. Adenocarcinoma arising in gastric heterotopic pancreas. West J Med. 1981;135:57–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Tanimura A, Yamamoto H, Shibata H, Sano E. Carcinoma in heterotopic gastric pancreas. Acta Pathol Jpn. 1979;29:251–257. doi: 10.1111/j.1440-1827.1979.tb03179.x. [DOI] [PubMed] [Google Scholar]
- 26.Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57:314–317. doi: 10.1136/jcp.2003.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]


