Abstract
Despite its role in disease there is still no definitive method to assess oesophago-gastric junction competence (OGJ). Traditionally the OGJ has been assessed using manometry with lower oesophageal sphincter pressure as the indicator. More recently this has been shown not to be a very reliable marker of sphincter function and competence against reflux. Disorders such as gastro-oesophageal reflux disease and to a lesser extend achalasia still effects a significant number of patients. This review looks at using a new technique known as impedance planimetry to profile the geometry and pressure in the OGJ during distension of a bag. The data gathered can be reconstructed into a dynamic representation of OGJ action. This has been shown to provide a useful representation of the OGJ and to show changes to the competence of the OGJ in terms of compliance and distensibility as a result of endoluminal therapy.
Keywords: Oesophagogastric junction, Lower oesophageal sphincter, Competence, Distensibility, Functional imaging
INTRODUCTION
The oesophago-gastric junction (OGJ) is located between the oesophagus and the stomach. Its dynamic action is dependant upon the intrinsic and extrinsic effects of its own anatomical structure and its position with respect to the surrounding organs in this region of the body. Despite its relationship to diseased states of the oesophagus, no objective measurement technique has been developed to quantify the normal or abnormal functioning of the OGJ. It is generally accepted that sphincters divide the gut into functional segments. They are characterized by a resting tension, generally known as muscle tone which is greater than in the two adjacent segments. It is common nowadays to give the term sphincter a functional meaning rather than its original anatomical meaning[1]. The sphincter at the lower end of the oesophagus, the lower oesophageal sphincter (LOS), forms part of the OGJ structure. At the OGJ the oesophagus opens into the stomach at the cardia. It is important that backflow of stomach secretions into the oesophagus is controlled at the OGJ. The OGJ guards against gastric acid moving up into the oesophagus, opening only transiently to allow passage of the swallowed food into the stomach. Swallowing is a demanding reflex activity[2]. The diaphragmatic hiatus, through which the oesophagus passes just at the OGJ, has a role in this valvular mechanism[3].
Several other structures are important in maintaining a barrier in the OGJ against the back flow of food and the stomach acids into the oesophagus, known as the anti-reflux barrier. The intrinsic muscles of the distal oesophagus and the sling fibres of the proximal stomach make up the internal mechanism of the LOS[4]. The muscles of the diaphragm that connect to the OGJ make up the crural diaphragm and this constitutes the external LOS mechanism. The tissue that connects the distal oesophagus to the crural diaphragm is known as the phreno-oesophageal ligament[5].
The OGJ can be distinguished from the body of the oesophagus by its behaviour pattern characteristics. There is an increase in the tone of the circular muscle in this region. The sphincter relaxes in response to a swallow and this usually precedes the arrival of a contraction wave travelling down the oesophagus. This phase of relaxation is followed by a short-lasting elevation of pressure above resting values[6]. In recent years it has been identified that the sphincter sometimes relaxes even when a swallow does not occur. These relaxations are know as transient lower oesophageal sphincter relaxations (tLOSRs)[7]. Other factors contribute to the role of the OGJ in controlling the flow of solids, liquids and gases between the oesophagus and the stomach. Pressures in the abdominal and thoracic cavities are involved as well as the exact intra-abdominal location of the junction. Time and posture are also important factors.
THE ROLE OF THE OGJ IN DISEASE
Disorders of the OGJ in general relate to two diseased states. Conditions where the disease may be related to an OGJ not closing normally are generally referred to as gastro-oesophageal reflux disease (GORD) and conditions where the OGJ fails to relax or open, which is referred to as achalasia.
Gastro-oesophageal reflux disease
GORD symptoms are very common in the general population affecting up to 44% of the US population at least once a month and 20% of the population once a week[8,9]. It has been shown that patients suffering from GORD have a lower health related quality of life than patients with angina and mild heart failure[10]. There is solid evidence that incompetence or dysfunction of the OGJ is a primary determinant of GORD. Three mechanisms at the OGJ lead to an increased number of acid reflux events associated with GORD: tLOSRs, free reflux during periods of low LOS pressure or deglutitive relaxation of the LOS[7,11-13]. Much more needs to be learned about the dynamic action of the OGJ if we are to fully understand its role in GORD. Current treatment of GORD is with acid suppressing drugs such as proton pump inhibitors but for those refractory to pharmacological treatment surgery is often recommended. The surgery of choice for GORD is the laparoscopic Nissen fundoplication[14].
Achalasia
Achalasia is an oesophageal disorder of unknown cause characterized by aperistalsis of the oesophageal body and impaired relaxation of the lower oesophageal sphincter[2,15]. It is a relatively rare disease and in Western countries the annual incidence is in the range 0.5 to 8 cases per 100 000 people[16]. It can be difficult to make a diagnosis in some patients because of vague symptoms. Symptoms can emerge slowly and patients adapt. The diagnosis can be confirmed by endoscopy and manometry[17]. Developing a device that can profile the OGJ will contribute greatly to the understanding of this disorder, which often manifests as a permanently constricted or toned sphincter. Surgical performance of an oesophageal myotomy is the treatment of choice for some severe cases of achalasia[18]. Currently the laparoscopic oesophageal Heller-Nissen procedure is commonly used[19].
BIOMECHANICS OF THE OGJ
Biomechanics are the laws of mechanics applied to biology[20]. There is an increasing recognition of the importance of tissue biomechanical properties for function, e.g. organ geometry, resting conditions and elasticity is determinants of flow in the organ and sensory responses in the neural circuits. When dealing with biological tissues, solid mechanical parameters such as the relation between forces (membrane stress resultant, tension) and deformation (often expressed as a strain) are important to evaluate. The mechanical properties of the OGJ are important determinants of its distensibility and competence. In vitro studies have shown potential in measuring biomechanical parameters in the OGJ[21-25]. Very few methods exist for assessment of mechanical properties in the GI tract in vivo. One method is impedance planimetry from where the cross-sectional area (CSA) and pressure data can be gathered and the wall tension and strain in the organ can be estimated[26]. Other methods rely on the combination of bag distension and manometry with high resolution imaging techniques such as 3D-ultrasound of the gastric antrum[27] or MRI of the rectum[28]. Manometry is of course a simple biomechanical measurement. Its use will be mentioned again later.
METHODS OF EVALUATING OGJ FUNCTION
In 1964 Mann et al[6] suggested that the zone separating the lower end of the oesophagus from the stomach can be recognized in two different ways, by its distinguishing anatomic features and its unique patterns of behaviour. A huge amount of knowledge about the anatomy of the OGJ has been assembled over the last 40 years, but much of our limited understanding of the behaviour has been related to manometric studies using single or multiple pressure recordings or a sleeve placed in the OGJ. While manometry provides useful information on motility within the lumens of the digestive tract, it may be more restrictive in what it can tell us about sphincteric regions such as the OGJ and their dynamics. Harris and Pope found that sphincters do not necessarily need to squeeze or contract tightly to be competent and therefore resistance to distension by measurement of radial force should be the prime determinant of sphincteric strength[29]. Measurement of luminal pressure, however, is not a direct measure of muscle tone and tension. It is merely a proxy of changes in force generated by the surrounding tissues[27,30].
No clinically acceptable quantitative method exists to directly measure OGJ mechanical function. Intraluminal pressure recordings is the only objective measurement technique for determining the competence of the LOS, but it is usually the more indirect method of measuring acid reflux in the oesophagus which is the key determinant of failure of the OGJ to inhibit flow. Thus, pH-metry rather than manometry is the recommended test for GORD[31].
Usually in medicine dynamic diagnostic imaging methods can be used to visualize and evaluate anatomical structures similar to the OGJ. Currently none of these methods give sufficient dynamic detail to contribute to the routine diagnosis and treatment of diseases of the OGJ. Recent developments in material sciences and technology have allowed the OGJ’s resistance to distension to be studied using parameters such as distensibility, opening patterns and dimensions and flow rate[32-34]. It is becoming apparent that these measurements may be useful in determining the success of therapeutic interventions such as surgery[35]. However what is needed is a technique with high spatial and temporal resolution for determination of sphincter geometry and mechanical factors.
Impedance planimetry
Principle of Impedance Planimetry: Impedance planimetry (IP) is a technique established by Gregersen and coworkers for performing bag distensions in the GI tract (Figure 1). It has been used extensively to provide a single measure of CSA together with bag pressure in animal and human studies using electrical measurements inside the liquid filled bag[36]. Hence the term impedance planimetry. If we fill a polyurethane bag with saline and pass a constant current through it, the CSA is inversely proportional to the voltage across the sensing electrodes. If all other parameters are kept constant then the impedance becomes related to the CSA of the bag between the sensing electrodes.
Figure 1.
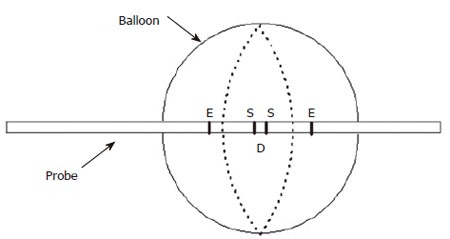
Sketch of a conventional impedance planimetry probe with a single set of excitation (E) and sensing (S) electrodes for the measurement of cross-sectional area in the middle of the bag. D is the distance between the sensing electrodes.
Vα(1/CSA),
Many studies have shown the usefulness and reliability of IP in the oesophagus of healthy volunteers and patients[37-54]. Rather than analyzing distension data based on pressure-volume relationships as is the case with the “Barostat”, IP enables data on the CSA of the bag during distension to be gathered. CSA shows a better relationship to the diametrical changes in the alimentary lumen than volume[27]. However, by far most studies have been done using a single CSA recording inside the bag rather than obtaining a series of CSAs. Due to previous limitations, it has only been used in a few studies in sphincter regions[55,56]. These limitations related to the difficulties placing the measurement electrodes at the point of most interest and the fact that distending a bag in a high-pressure zone tends to displace the bag during the measurements.
Multiple measurements: A recent pilot study in the porcine rectum demonstrates how an IP probe placed in the rectum with 5 sensing electrode pairs with 2mm between pairs and 2cms between sets can detect contractile events at different levels along the longitudinal axis of the gut (Figure 2)[39,57]. This multi-electrode technique demonstrated how a balloon with a conducting liquid and a constant current source set up across two excitation electrodes can measure multiple CSAs by measuring the voltage across multiple electrode pairs within the electric field.
Figure 2.
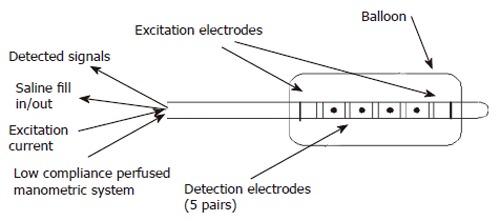
Diagram showing multi-electrode probe by Andersen et al for measuring 5 CSAs in the porcine rectum.
There are a number of potential sources of error in multiple measurement IP systems. Electrodes placed too close together will interfere with each other. The number of wires required to make the electrical connections goes up and therefore the potential for interference, liquid leaking into the probe and bad electrical connections is much.
Manometry
Manometry has been used for more than 50 years to monitor motility in the gastrointestinal tract. Since 1977 reliable methods have been available to obtain accurate measurements of force of closure of the lumens of the intestine when contracting. These system often use reliable perfused hydraulic-capillary systems where pressure at a sensor outside the body can be used to accurately measure pressure at a point along a catheter via a low flow rate perfused channel[58]. This system has been used extensively to measure force via a pressure measurement in lumens and in liquid filled balloons. Errors in manometric readings can be caused by air bubbles in the capillary lines, occlusion of the measurement site and the compliance of the recording system but in general if well set up they produce accurate readings. In more recent years catheters with solid state transducers are becoming more popular for pressure reading and these overcome some of the measurement problems.
Using manometry to determine the competence of sphincteric or valvular regions in the lumens of the digestive tract is however dubious. Dent recognized the problem of positioning the measurement point 30 years ago and proposed a solution[59] and others tried to follow suit[60]. But pressure is a measurement of force over area and this may not be a proper measure of the function of a valvular region. As suggested by Pope and Harris we cannot assume that just because a sphincter is closed that it has a contract force or in the terminology of manometry that if it has a high lower oesophageal pressure it is deemed to be competent.
Calculation of wall tension
Law of laplace: For a membraneous cylinder with transmural pressure P and radius r the circumferential tension T can be given by :
Pr = T
Use of Laplace’s law is based on the assumption that the geometry of the segment is circular and cylindrical and that the wall thickness is very small.
Measurements using multi-electrode Impedance planime-try in the oesophagogastric junction
We have demonstrated that a multi-electrode probe with a cylindrical bag mounted and filled with saline can be placed in the OGJ and that data on a number of CSAs in this area can be measured[49]. Figure 3 shows results of 3 CSA measurements 8 mm apart measured from distension of a bag in the OGJ of a healthy volunteer. The plot shows how the most distal CSA (D) measurement increases more rapidly than the middle CSA (M) as a constant volume of saline is filled in to the bag. For the most proximal CSA (P) the converse is true. This suggests that distally the OGJ is more compliant than proximally. This work also demonstrated for the first time how wall tension in the OGJ can be estimated from simple distension measurements. This preliminary version of the multi-electrode technique demonstrated that a probe can be placed in the OGJ and that data can be successfully collected. However, there were limitations such as the fact that there were only three measurements made. A narrowing region such a sphincter can not be profiled very well with three CSA measurements unless the exact position of the sphincter is located and maintained. Another limitation is the outer diameter of the catheter on which the bag is mounted. In this case it was 4.5 mm. If the sphincter is to be distended from as close to the closed position as possible then a smaller catheter needs to be used.
Figure 3.
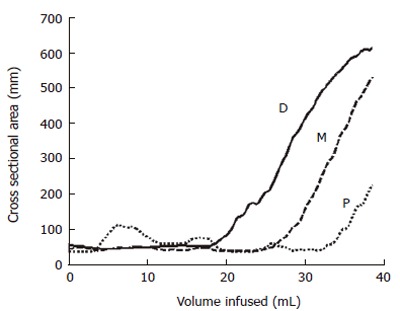
Plot showing the changes in the CSAs at the proximal (P), middle (M) and distal (D) electrode pairs as the balloon is distended at a constant flow rate indicated by the increase in volume.
Figure 4 shows how a probe capable of measuring 8 CSAs could be used to profile a sphincter region. It is quite obvious that a larger number of sensing pairs could be more useful in providing a geometric profile. Consequently a more elaborate probe for measurement of up to 8 CSAs in the region of the oesophago-gastric junction was developed. This probe can measure CSAs at 4 mm intervals through the OGJ thus spanning a measurement range of 28 mm.
Figure 4.
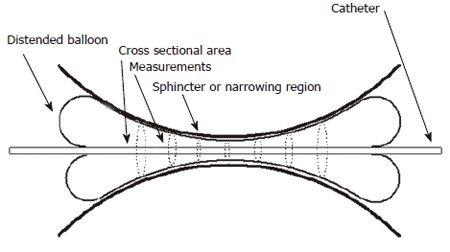
An example of how a probe which can measure 8 CSAs could profile the geometry of the narrowing region of a bodily sphincter.
Figure 5 shows the data from the 8 CSAs during a bag distension at a fixed flow rate of 40 mL/min to a volume of 60 mL. The information in this format can be difficult to decipher. A probe to make these measurements has been validated and test in a healthy control subject[57]. Since the OGJ is such a dynamic organ it is its functional activity which can be more of interest. Hence it is possible to generate an illustration of the geometric changes in the OGJ.
Figure 5.
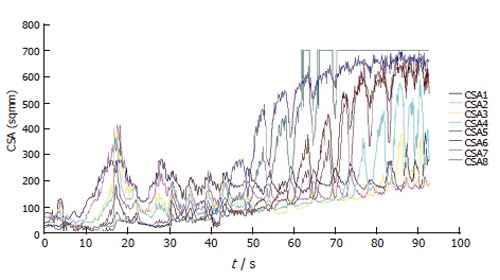
Plot showing changes in the 8 measured CSAs during bag distension at 40 mL/min. CSA1 is the most distal and CSA8 is the most proximal.
If we introduce the idea of demonstrating the CSA changes dynamically as reconstructed data we introduce a new concept known as the functional lumen imaging probe (FLIP). The concept suggests that by gathering data on the dynamic changes in the geometry of the OGJ during normal physiological changes we can represent these changes by recreating the geometry in a three-dimensional image. The FLIP technique can be used to monitor geometric changes in the behaviour of the OGJ during bag distension as can be seen in Figure 6. Here the dynamic data is reconstructed demonstrating the effect of the balloon distension on the amount of OGJ opening.
Figure 6.
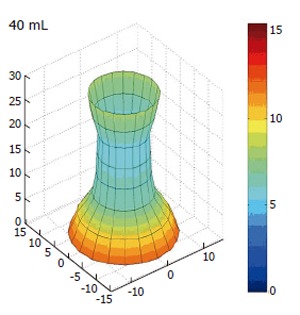
Three dimensional reconstruction of the OGJ geometry in a human volunteer after distension in to a volume of 40 mL. All dimensions are in millimeters. Colour change is indicating increased radius.
FLIP can quantify changes in compliance of the OGJ after endoscopic suturing at the OGJ in a pig model[61]. Figure 7 indicates how the geometric reconstruction can be used to indicate changes in the profile of the OGJ after endoscopic plication. The pressure is higher, the CSA is smaller and subsequently the wall tension has increased after the plications are inserted. These measurements indicate that the OGJ becomes less compliant after plication. No direct measure of the effects of these therapies on the OGJ has been demonstrated before. Recent studies have expressed concerns about the efficacy and durability of these procedures[62-65]. FLIP may in fact reveal new information on the mechanism of action behind these new reflux therapies. In effect it suggests that endoscopic suturing augments the compliance of the OGJ to make it less compliant. Perhaps patients could now be selected better for these procedures if their compliance can be confirmed to be abnormal in the first place and maybe the success of the procedure could be determined directly at the time of the procedure so for example more stitches can be added if necessary.
Figure 7.
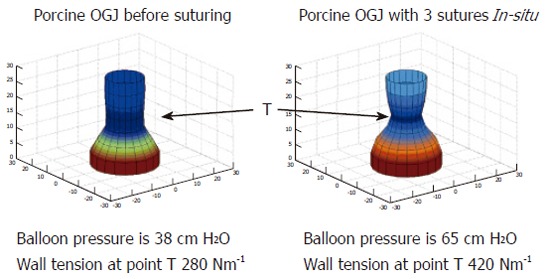
Geometric profile of the porcine OGJ before and after the placement of 3 sutures in the OGJ. T indicates the point where wall tension was measured.
Since GORD patients are such a large group perhaps this is the most important area for FLIP to be developed. The probe can distinguish between patients for reflux surgery and normal controls. These are of course the extreme group of patients. Further studies are of course necessary on larger cohorts of patients and controls.
We have recorded with the FLIP the compliance of the OGJ intra-operatively during oesophageal myotomy for achalasia (as yet unpublished findings by the authors). FLIP offers a more sensitive and possibly a better tool than manometry and may be used for evaluating effects of surgery during the procedure as opposed to using manometry more as a blunt instrument before and after as used by Arain et al[66]. FLIP could provide a valuable new tool for the oesophageal surgeon.
Summary
Work already carried out shows for the first time that CSA and pressure measurements in the OGJ can be used to measure distensibility by an entirely new technique. It demonstrates how the already established technique of impedance planimetry can be used to measure geometry in vivo in the human oesophagus. It also shows that there is validity of these new measurements, their appropriateness for use in sphincteric regions in the body and their comparable accuracy has been shown with ultrasound. It has been indicated how the measurement could be used practically. Safety and efficacy has been demonstrated. The work also demonstrates how this new measure could be used as a functional imaging technique. Preliminary studies have shown potential applications of the probe. It could prove to be a very valuable tool in endoscopic therapies. It can distinguish between normal and a stitched OGJs in live pigs.
As a resistance to distension test this better describes the sphincteric action of the OGJ than tonic contraction. Even advanced manometry uses such as volume vector analysis, the Dent Sleeve or the Sphinctometer all rely on the principal that there is a force of contraction always present when the sphincter is closed[59,60,67-70]. With FLIP this assumption is not made and it presents more of a challenge test to the sphincter. The technique also represents a more direct technique to evaluate the OGJ using opening patterns with manometry and intraluminal impedance[71].
Some other techniques for measurement of resistance to distension by other investigators are promising and confirm that the principals describe here have great potential for merit. However these other approaches are limited in a number of ways. The technique described by Pandolfino, although it is similar in its measurement design to FLIP, it requires fluoroscopic imaging[32,33,35]. It also depends on the pressure being controlled. Pressure control is difficult to achieve by increasing and reducing volume through a narrow catheter. Using pressure-volume data from the barostat to quantify distensibility does not a good basis in the application of the physical sciences. The bag on a barostat can expand in all directions we cannot be sure that an increase in the volume is reflected by a change in the geometry of the OGJ. This suggest that using this technique is of limited use[34]. Our findings in patients with achalasia confirmed this.
Perspectives
FLIP represents the first validated data on a probe capable of measuring OGJ compliance in a simple and time-saving procedure. It represents the opportunity to define the exact role of the OGJ in dysmotility and disease. As a new technique rather than representing a completed work it opens up a new door and perspectives on the junction whose function has been the subject of debate for many years. Future work and improvements in electronics and material sciences will mean that the resolution of the FLIP can be increased dramatically. Design materials have been carefully chosen to minimize signal error. The lower oesophageal sphincter or the oesophagogastric junction are often cited as being the prime causes of reflux disease, yet despite this very few research groups are investigating new ways to evaluate this dynamic structure in the gastrointestinal tract[32,34].
Much work has been done in extending manometric measurements enhancing pH measurement techniques to further evaluate the effects of acid in the oesophagus[72] and developing new techniques for measuring bolus transit both down to the stomach and back up into the oesophagus using intra-luminal impedance[71]. However, none of these techniques have overcome the fundamental question of how the OGJ function can be quantified.
FLIP may provide the answer to this question. In a way trialing the probe in the OGJ was probably the most challenging and perhaps experiments in another sphincteric region would have been more straight-forward where the dynamic activity would be slower. FLIP may have a role in determining biomechanically the root cause of diseases. It can be used for evaluating therapy for example endoscopic and surgical therapy for reflux. It may be possible to use it intra-operatively during Nissen fundoplication, for example where the tension of the wrap is extremely important. It may also be possible to explore other sphincters in the body with FLIP so that the relationship between their biomechanical properties and their relationship to disease can be further defined.
Footnotes
S- Editor Pan BR E- Editor Ma WH
References
- 1.Liebermann-Meffert D. What anatomic structures are undoubtedly responsible for gastroesophageal competence. In: Guili R, Galmiche J, Jamieson G, Scarpignato C, eds , et al., editors. The esophagogastric junction. London: John Libbey Eurotext; 1998. pp. 3–6. [Google Scholar]
- 2.Goyal RK, Prasad M, Chang HY. Functional anatomy and physiology of swallowing and esophageal motility. In: The Esophagus, et al., editors. Castell DO, Richter JE eds. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1–36. [Google Scholar]
- 3.Boyle JT, Altschuler SM, Nixon TE, Tuchman DN, Pack AI, Cohen S. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88:723–730. doi: 10.1016/0016-5085(85)90143-x. [DOI] [PubMed] [Google Scholar]
- 4.Liebermann-Meffert D, Allgöwer M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- 5.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–932. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 6.Mann CV, Greenwood RK, Ellis FH Jr. The Esophagogastric Junction. Surg Gynecol Obstet. 1964;118:853–862. [PubMed] [Google Scholar]
- 7.Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 8.Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 9.The Gallup Organisation. A Gallup Survey on Heartburn Across America Princeton. NJ: 1988. [Google Scholar]
- 10.Dimenäs E. Methodological aspects of evaluation of Quality of Life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl. 1993;199:18–21. [PubMed] [Google Scholar]
- 11.Dent J, Dodds WJ, Friedman RH, Sekiguchi T, Hogan WJ, Arndorfer RC, Petrie DJ. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–267. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547–1552. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- 13.Barham CP, Gotley DC, Mills A, Alderson D. Precipitating causes of acid reflux episodes in ambulant patients with gastro-oesophageal reflux disease. Gut. 1995;36:505–510. doi: 10.1136/gut.36.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozmen V, Oran ES, Gorgun E, Asoglu O, Igci A, Kecer M, Dizdaroglu F. Histologic and clinical outcome after laparoscopic Nissen fundoplication for gastroesophageal reflux disease and Barrett's esophagus. Surg Endosc. 2006;20:226–229. doi: 10.1007/s00464-005-0434-9. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson AD, Scott SM, Yazaki E, Fusai G, Walker SM, Kadirkamanathan SS, Evans DF. Compliance measurement of lower esophageal sphincter and esophageal body in achalasia and gastroesophageal reflux disease. Dig Dis Sci. 2001;46:1937–1942. doi: 10.1023/a:1010639232137. [DOI] [PubMed] [Google Scholar]
- 16.St Peter SD, Swain JM. Achalasia: a comprehensive review. Surg Laparosc Endosc Percutan Tech. 2003;13:227–240. doi: 10.1097/00129689-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Richter J. Achalasia. In: Castell D, Richter J, eds , et al., editors. The Esophagus. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 221–261. [Google Scholar]
- 18.Ramacciato G, D'Angelo FA, Aurello P, Del Gaudio M, Varotti G, Mercantini P, Bellagamba R, Ercolani G. Laparoscopic Heller myotomy with or without partial fundoplication: a matter of debate. World J Gastroenterol. 2005;11:1558–1561. doi: 10.3748/wjg.v11.i10.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patti MG, Fisichella PM, Perretta S, Galvani C, Gorodner MV, Robinson T, Way LW. Impact of minimally invasive surgery on the treatment of esophageal achalasia: a decade of change. J Am Coll Surg. 2003;196:698–703; discussion 703-705. doi: 10.1016/S1072-7515(02)01837-9. [DOI] [PubMed] [Google Scholar]
- 20.Fung YC. Biomechanical aspects of growth and tissue engineering. In: Biomechanics Motion, Flow , Stress amd Growth, et al., editors. New York: Springer-Verlag; 1990. pp. 499–500. [Google Scholar]
- 21.Gregersen H, Giversen IM, Rasmussen LM, Tøttrup A. Biomechanical wall properties and collagen content in the partially obstructed opossum esophagus. Gastroenterology. 1992;103:1547–1551. doi: 10.1016/0016-5085(92)91176-5. [DOI] [PubMed] [Google Scholar]
- 22.Madsen G, Tøttrup A, Andersson KE, Forman A. Mechanical effects of some prostanoids on isolated human oesophageal submucosal veins. Pharmacol Toxicol. 1992;71:330–334. doi: 10.1111/j.1600-0773.1992.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 23.Biancani P, Barwick K, Selling J, McCallum R. Effects of acute experimental esophagitis on mechanical properties of the lower esophageal sphincter. Gastroenterology. 1984;87:8–16. [PubMed] [Google Scholar]
- 24.Uc A, Murray JA, Conklin JL. Effects of calcitonin gene-related peptide on opossum esophageal smooth muscle. Gastroenterology. 1997;113:514–520. doi: 10.1053/gast.1997.v113.pm9247471. [DOI] [PubMed] [Google Scholar]
- 25.Leichus LS, Thomas RM, Murray JA, Conklin JL. Effects of oxygen radicals and radical scavenging on opossum lower esophageal sphincter. Dig Dis Sci. 1997;42:592–596. doi: 10.1023/a:1018859428869. [DOI] [PubMed] [Google Scholar]
- 26.Gregersen H, Barlow J, Thompson D. Development of a computer-controlled tensiometer for real-time measurements of tension in tubular organs. Neurogastroenterol Motil. 1999;11:109–118. doi: 10.1046/j.1365-2982.1999.00144.x. [DOI] [PubMed] [Google Scholar]
- 27.Gregersen H, Gilja OH, Hausken T, Heimdal A, Gao C, Matre K, Ødegaard S, Berstad A. Mechanical properties in the human gastric antrum using B-mode ultrasonography and antral distension. Am J Physiol Gastrointest Liver Physiol. 2002;283:G368–G375. doi: 10.1152/ajpgi.00144.2001. [DOI] [PubMed] [Google Scholar]
- 28.Frokjaer JB, McMahon BP, Liao DH, Drewes AM, Gregersen H. Modelling of the 3D geometry in the human rectum based on magnetic resonance imaging (MRI) Gastroenterology. 2004;126:A202–A203. [Google Scholar]
- 29.Harris LD, Pope CE 2nd. “Squeeze” Vs. Resistance: An Evaluation of the Mechanism of Sphincter Competence. J Clin Invest. 1964;43:2272–2278. doi: 10.1172/JCI105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregersen H, Christensen J. Gastrointestinal tone. Neurogastroenterol Motil. 2000;12:501–508. doi: 10.1046/j.1365-2982.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 31.Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Pandolfino JE, Shi G, Curry J, Joehl RJ, Brasseur JG, Kahrilas PJ. Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1052–G1058. doi: 10.1152/ajpgi.00279.2001. [DOI] [PubMed] [Google Scholar]
- 33.Pandolfino JE, Shi G, Trueworthy B, Kahrilas PJ. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology. 2003;125:1018–1024. doi: 10.1016/s0016-5085(03)01210-1. [DOI] [PubMed] [Google Scholar]
- 34.Shaker R, Bardan E, Gu C, Massey BT, Sanders T, Kern MK, Hoffmann RG, Hogan WJ. Effect of lower esophageal sphincter tone and crural diaphragm contraction on distensibility of the gastroesophageal junction in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G815–G821. doi: 10.1152/ajpgi.00120.2004. [DOI] [PubMed] [Google Scholar]
- 35.Pandolfino JE, Curry J, Shi G, Joehl RJ, Brasseur JG, Kahrilas PJ. Restoration of normal distensive characteristics of the esophagogastric junction after fundoplication. Ann Surg. 2005;242:43–48. doi: 10.1097/01.sla.0000167868.44211.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregersen H. Biomechanics of the Gastrointestinal Tract. London: Springer-Verlag; 2003. pp. 80–91. [Google Scholar]
- 37.Rao SS, Hayek B, Summers RW. Impedance planimetry: an integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. Am J Gastroenterol. 1995;90:431–438. [PubMed] [Google Scholar]
- 38.Pedersen J, Reddy H, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Cold and heat pain assessment of the human oesophagus after experimental sensitisation with acid. Pain. 2004;110:393–399. doi: 10.1016/j.pain.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Andersen IS, Gregersen H, Buntzen S, Djurhuus JC, Laurberg S. New probe for the measurement of dynamic changes in the rectum. Neurogastroenterol Motil. 2004;16:99–105. doi: 10.1046/j.1365-2982.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 40.Assentoft JE, Gregersen H, O'Brien WD Jr. Determination of biomechanical properties in guinea pig esophagus by means of high frequency ultrasound and impedance planimetry. Dig Dis Sci. 2000;45:1260–1266. doi: 10.1023/a:1005579214416. [DOI] [PubMed] [Google Scholar]
- 41.Barlow JD, Gregersen H, Thompson DG. Identification of the biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2002;282:G683–G689. doi: 10.1152/ajpgi.00134.2001. [DOI] [PubMed] [Google Scholar]
- 42.Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol. 2005;11:4367–4374. doi: 10.3748/wjg.v11.i28.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregersen H, Djurhuus JC. Impedance planimetry: a new approach to biomechanical intestinal wall properties. Dig Dis. 1991;9:332–340. doi: 10.1159/000171320. [DOI] [PubMed] [Google Scholar]
- 44.Gregersen H, Orvar K, Christensen J. Biomechanical properties of duodenal wall and duodenal tone during phase I and phase II of the MMC. Am J Physiol. 1992;263:G795–G801. doi: 10.1152/ajpgi.1992.263.5.G795. [DOI] [PubMed] [Google Scholar]
- 45.Gregersen H, Christensen LL. Pressure-cross-sectional area relations and elasticity in the rabbit oesophagus in vivo. Digestion. 1996;57:174–179. doi: 10.1159/000201333. [DOI] [PubMed] [Google Scholar]
- 46.Gregersen H, Vinter-Jensen L, Juhl CO, Dajani EZ. Impedance planimetric characterization of the distal oesophagus in the Goettingen minipig. J Biomech. 1996;29:63–68. doi: 10.1016/0021-9290(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 47.Jørgensen CS, Dall FH, Storkholm J, Jensen SL, Gregersen H. Elastic properties of the isolated perfused porcine duodenum. Dig Dis. 1991;9:401–407. doi: 10.1159/000171330. [DOI] [PubMed] [Google Scholar]
- 48.Juhl CO, Vinter-Jensen L, Djurhuus JC, Gregersen H, Dajani EZ. Biomechanical properties of the oesophagus damaged by endoscopic sclerotherapy. An impedance planimetric study in minipigs. Scand J Gastroenterol. 1994;29:867–873. doi: 10.3109/00365529409094855. [DOI] [PubMed] [Google Scholar]
- 49.McMahon BP, Frøkjaer JB, Drewes AM, Gregersen H. A new measurement of oesophago-gastric junction competence. Neurogastroenterol Motil. 2004;16:543–546. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 50.Petersen JA, Djurhuus C, Koff J, Vinter-Jensen L, Gregersen H. Endoscopic sclerotherapy in porcine esophagus changes luminal cross-sectional area and wall distensibility dose- and time-dependently. Dig Dis Sci. 1998;43:521–528. doi: 10.1023/a:1018802922374. [DOI] [PubMed] [Google Scholar]
- 51.Rao SS, Gregersen H, Hayek B, Summers RW, Christensen J. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950–958. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 52.Rao SS, Hayek B, Mudipalli R, Gregersen H. Does esophageal function vary at the striated and smooth muscle segments in functional chest pain. Am J Gastroenterol. 2002;97:2201–2207. doi: 10.1111/j.1572-0241.2002.05973.x. [DOI] [PubMed] [Google Scholar]
- 53.Villadsen GE. Impedance planimetry characteritics of the normal and diseased oesophagus. Surg Res Comm. 1995;17:225–242. [Google Scholar]
- 54.Villadsen GE, Storkholm J, Zachariae H, Hendel L, Bendtsen F, Gregersen H. Oesophageal pressure-cross-sectional area distributions and secondary peristalsis in relation to subclassification of systemic sclerosis. Neurogastroenterol Motil. 2001;13:199–210. doi: 10.1046/j.1365-2982.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 55.Villadsen GE, Storkholm J, Zachariae H, Hendel L, Bendtsen F, Gregersen H. Oesophageal pressure-cross-sectional area distributions and secondary peristalsis in relation to subclassification of systemic sclerosis. Neurogastroenterol Motil. 2001;13:199–210. doi: 10.1046/j.1365-2982.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 56.Sørensen SM, Gregersen H, Sørensen S, Djurhuus JC. Spontaneous anorectal pressure activity. Evidence of internal anal sphincter contractions in response to rectal pressure waves. Scand J Gastroenterol. 1989;24:115–200. doi: 10.3109/00365528909092248. [DOI] [PubMed] [Google Scholar]
- 57.McMahon BP, Frøkjaer JB, Liao D, Kunwald P, Drewes AM, Gregersen H. A new technique for evaluating sphincter function in visceral organs: application of the functional lumen imaging probe (FLIP) for the evaluation of the oesophago-gastric junction. Physiol Meas. 2005;26:823–836. doi: 10.1088/0967-3334/26/5/019. [DOI] [PubMed] [Google Scholar]
- 58.Arndorfer RC, Stef JJ, Dodds WJ, Linehan JH, Hogan WJ. Improved infusion system for intraluminal esophageal manometry. Gastroenterology. 1977;73:23–27. [PubMed] [Google Scholar]
- 59.Dent J. A new technique for continuous sphincter pressure measurement. Gastroenterology. 1976;71:263–267. [PubMed] [Google Scholar]
- 60.Gotley DC, Barham CP, Miller R, Arnold R, Alderson D. The sphinctometer: a new device for measurement of lower oesophageal sphincter function. Br J Surg. 1991;78:933–935. doi: 10.1002/bjs.1800780812. [DOI] [PubMed] [Google Scholar]
- 61.McMahon BP, Bergstrom M, Frokjaer JB, Hansson L, Drewes AM, Gregersen H, Park PO. Use of FLIP in determining changes in the geometry of the oesophago-gastric junction with endoscopic suturing in a procine model. Gut. 2005;54(Suppl VII):A104. [Google Scholar]
- 62.Schiefke I, Zabel-Langhennig A, Neumann S, Feisthammel J, Moessner J, Caca K. Long term failure of endoscopic gastroplication (EndoCinch) Gut. 2005;54:752–758. doi: 10.1136/gut.2004.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arts J, Lerut T, Rutgeerts P, Sifrim D, Janssens J, Tack J. A one-year follow-up study of endoluminal gastroplication (Endocinch) in GERD patients refractory to proton pump inhibitor therapy. Dig Dis Sci. 2005;50:351–356. doi: 10.1007/s10620-005-1610-4. [DOI] [PubMed] [Google Scholar]
- 64.Schiefke I, Soeder H, Zabel-Langhennig A, Teich N, Neumann S, Borte G, Mössner J, Caca K. Endoluminal gastroplication: what are the predictors of outcome. Scand J Gastroenterol. 2004;39:1296–1303. doi: 10.1080/00365520410003579. [DOI] [PubMed] [Google Scholar]
- 65.Mahmood Z, McMahon BP, Arfin Q, Byrne PJ, Reynolds JV, Murphy EM, Weir DG. Endocinch therapy for gastro-oesophageal reflux disease: a one year prospective follow up. Gut. 2003;52:34–39. doi: 10.1136/gut.52.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arain MA, Peters JH, Tamhankar AP, Portale G, Almogy G, DeMeester SR, Crookes PF, Hagen JA, Bremner CG, DeMeester TR. Preoperative lower esophageal sphincter pressure affects outcome of laparoscopic esophageal myotomy for achalasia. J Gastrointest Surg. 2004;8:328–334. doi: 10.1016/j.gassur.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 67.Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:476–482. doi: 10.1136/gut.44.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Straathof JW, Lüchtenborg M, Masclee AA. Comparison of two techniques for lower oesophageal sphincter manometry: sleeve and sphinctometer. Neurogastroenterol Motil. 2004;16:265–268. doi: 10.1111/j.1365-2982.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 69.Pehlivanov N, Liu J, Arora T, Yamamoto Y, Mittal RK. Lower esophageal sphincter monitoring with sphinctometer: in vitro and in vivo studies. Am J Physiol. 1999;277:G577–G584. doi: 10.1152/ajpgi.1999.277.3.G577. [DOI] [PubMed] [Google Scholar]
- 70.Trudgill NJ, Riley SA. Monitoring the lower oesophageal sphincter: sphinctometer or sleeve. Neurogastroenterol Motil. 1999;11:173–178. doi: 10.1046/j.1365-2982.1999.00151.x. [DOI] [PubMed] [Google Scholar]
- 71.Pandolfino JE, Shi G, Zhang Q, Ghosh S, Brasseur JG, Kahrilas PJ. Measuring EGJ opening patterns using high resolution intraluminal impedance. Neurogastroenterol Motil. 2005;17:200–206. doi: 10.1111/j.1365-2982.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 72.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]


