Abstract
Gastrointestinal (GI) sensory-motor abnormalities are common in patients with diabetes mellitus and may involve any part of the GI tract. Abnormalities are frequently sub-clinical, and fortunately only rarely do severe and life-threatening problems occur. The pathogenesis of abnormal upper GI sensory-motor function in diabetes is incompletely understood and is most likely multi-factorial of origin. Diabetic autonomic neuropathy as well as acute suboptimal control of diabetes has been shown to impair GI motor and sensory function. Morphological and biomechanical remodeling of the GI wall develops during the duration of diabetes, and may contribute to motor and sensory dysfunction. In this review sensory and motility disorders of the upper GI tract in diabetes is discussed; and the morphological changes and biomechanical remodeling related to the sensory-motor dysfunction is also addressed.
Keywords: Diabetes, Esophagus, Stomach, Intestine, Motility, Pain, Diabetic neuropathy, Hyperglycemia, Remodeling
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease requiring lifelong medical attention in order to limit the development of potentially devastating late complications and to manage them if they occur. In the USA, the per capita cost of healthcare in 2002 was $13 243 for patients with diabetes and $2560 for patients without diabetes[1]. Gastrointestinal (GI) disorders are common in diabetic patients[2,3]. As many as 75% of patients attending DM clinics report significant GI symptoms[2]. The entire GI tract from the esophagus to the anorectal region may be affected. Common complaints include dysphasia, early satiety, reflux, constipation, abdominal pain, nausea, vomiting and diarrhea. The symptoms may be severe and substantially decrease quality of life. The pathogenesis of the GI abnormalities is complex of nature, multi-factorial (motor dysfunction, autonomic neuropathy, glycemic control, psychological factors, etc.) and is not well understood[4]. A number of abnormal conditions have been described in different segments of the GI tract in patients with diabetic autonomic neuropathy (DAN): esophagus (dysmotility), stomach (dysmotility, delayed emptying) and small and large bowel (dysmotility, delayed transit, bacterial overgrowth and diarrhea)[4]. Only a few studies have addressed the visceral sensory function in DM[5-7] and have demonstrated abnormalities in perception thresholds, vagal tonus and evoked brain potentials in patients with DAN. This indicates that DM related neuronal changes may be located both in the peripheral and in the central nervous system (CNS). As mentioned the entire GI tract may be involved in DM, but our review describes the upper GI tract only.
Many studies have demonstrated prominent morphological changes of the small intestine and esophagus in DM[8-11]. Lately, several studies have described biomechanical remodeling as well as morphological remodeling in experimental diabetic rats[12-18]. Recently, Frøkjær et al[19] have shown that both the neuronal function of the contractile system as well as the structural apparatus of the GI tract may be affected in patients with longstanding DM and DAN. Therefore we suggest that along with DAN and glycemic control, the structural and biomechanical changes may play important roles in the symptomatology of GI abnormalities in long-standing DM. The future management of diabetic patients with GI symptoms may develop accordingly.
SYMPTOMS FROM THE UPPER GI TRACT IN DIABETES MELLITUS
Motor and sensory abnormalities in DM may affect the entire GI tract or part hereof, and the perceived symptoms may originate from one or several parts of the gut, such as the esophagus, stomach and small intestine[20,21]. The prevalence of upper GI symptoms is high in both insulin dependent DM (IDDM) and non-insulin dependent DM (NIDDM)[22-28]. The symptoms relating to the esophagus, stomach and small intestine are as follows: (1) Esophagus: Heartburn, dysphagia and chest pain[37]; (2) Stomach: weight loss and abdominal pain[38]; (3) Small intestine: Diarrhea, discomfort, pain and pseudo-obstruction. Most symptoms are non-specific of nature and may relate to other GI disorders not necessarily related to DM. Therefore, when dealing with GI symptoms, some specific issues need to be addressed. Chest pain may relate to reflux or esophageal motor disorders, but ischemic heart disease and other causes of non-cardiac chest pain are also possible causes and must be excluded. Dysphagia, the most characteristic symptom of impaired esophageal transit, may be caused by motility disorders of the esophagus. It is very important, however, to emphasize that dysphagia is more often caused by mechanical obstruction such as tumors and peptic stenosis than by motility disorders. A number of other incidental conditions must be excluded when diabetic gastroparesis is suspected: gastric outlet obstruction caused by tumors and ulcer disease; metabolic abnormalities such as diabetic ketoacidosis or uremia and side effects of pharmacotherapy. A gastroscopy and biochemical screening followed by physical examination are obligatory requisites to reach the correct diagnosis. The symptoms of diabetic gastroparesis tend to increase in intensity and frequency over the duration of diabetes in patients affected. Most often symptoms are vague and nonspecific such as early satiety, slight abdominal discomfort and perhaps bloating, and fortunately more rarely do nausea and vomiting develop. When nausea and vomiting becomes continuous hospitalization is needed to control glucose homeostasis, electrolytes and fluid substitution is needed. This is a potential life threatening situation. In the long run diabetic gastroparesis may be accompanied by poor diabetic control, weight loss and diminished quality of life partly due to frequent hospital contacts. The symptoms may last for days to months, and do often occur in cycles with symptom free intervals[29,30]. Diabetic patients frequently report abdominal pain and this may be the only symptom of diabetic gastroparesis; however, abdominal pain can also be seen in diabetic ketoacidosis and severe metabolic acidosis[31]. These conditions are followed by other symptoms as well and therefore distinguishable from gastroparesis. Diabetic patients with thoracic polyradiculopathy, a rare condition, may also suffer from abdominal pain[32]. Diarrhea in DM patients may be induced by a number of factors. These may include food composition, abnormal intestinal motility, small intestinal bacterial overgrowth, excessive loss of bile acids, pancreatic insufficiency and more[33]. Abnormal small intestinal motility (rapid or delayed transit) is a frequent condition in the diabetic patients as described below. Rapid transit may induce an increase in intra-luminal contents that reach the caecum, whereas delayed transit may cause bacterial overgrowth, both potentially resulting in diabetic diarrhea. Bacterial overgrowth has been reported in up to 40% of diabetic patients with diarrhea[34,35]. Celiac disease is overrepresented in IDDM and a cause of severe diarrhea to be excluded when dealing with diabetic diarrhea[36]. In its most fulminate form, diabetic diarrhea is a devastating and horrible condition for the person affected, sometimes resulting in catastrophical nightly soiling in bed and an uncontrollable condition during daytime. Most often, fortunately, this condition is self limiting and symptoms are more manageable.
SENSORY DYSFUNCTION
Although both the afferent and efferent nerves are affected in DM, the data related to the sensory dysfunction of the GI tract are sparse compared with those relating to the motor dysfunction of the upper GI tract. Elevation of perception thresholds to esophageal electrical stimulation has been observed in patients with DAN and different severity of GI symptoms[7]. Increased vagal tonus and abnormal evoked brain potentials to mechanical and electrical stimulation of the esophagus has also been shown[5,6]. Rayner et al performed isovolumetric and isobaric distensions of the proximal stomach in ten randomly selected patients with IDDM[39]. They demonstrated that the perception of gastric distension during euglycemia was increased compared with healthy controls. To study mechanisms behind postprandial symptoms in patients with diabetes, the gastric accommodation of the meal was assessed by abdominal ultrasound[40]. In DM patients, a large proximal stomach was associated with perception of fullness and a large antrum was associated with perception of pain after a meal. More recently, Frøkjær et al[41] used a multimodal stimulation device (Figure 1) to investigate the visceral sensitivity to mechanical, thermal and electrical stimulation in the esophagus and duodenum in IDDM patients with DAN and GI symptoms. This study demonstrated that the patients had decreased sensitivity to the stimulations of the esophagus and duodenum. This indicates that the affection of the sensory nerves is widespread in the GI tract. As the multimodal approach is thought to stimulate the mucosa, submucosa and muscle layers differentially, the disease seems to be generalized to nerves in all layers of the gut.
Figure 1.
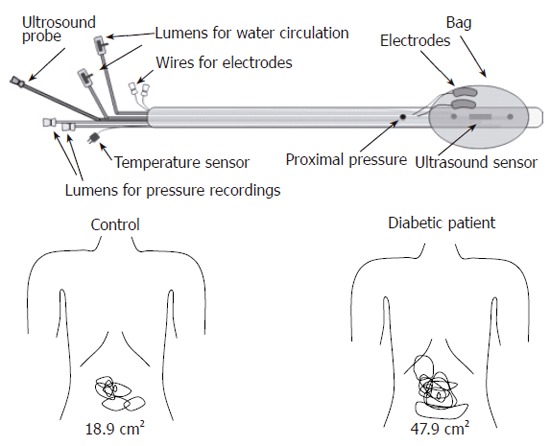
Top: The probe design allowing mechanical, heat and electrical stimulation of both the oesophagus and duodenum. The centre of the bag contains the sensor of the ultrasound probe imaging the oesophagus and duodenum during mechanical distension. The thermal stimuli were given by a pump system, which re-circulates water at 60 °C through two channels in the probe. The electrodes allowed electrical stimulation delivered directly to the mucosal surface. Bottom: The somatic referred pain areas to mechanical distension of the duodenum for the controls (left) and diabetic patients (right). The areas were larger for the diabetic patients.
MOTILITY DISORDERS
Long ago, it was known that abnormal motility of the GI tract occurred during the development of DM[42]. So far many studies have demonstrated that DM patients have slow transit and abnormal motility. The most frequent motility disorders of upper GI tract are shown in Table 1.
Table 1.
Motility disorders of upper GI tract in DM
| Organ | Motility disorder |
| Esophagus | Amplitude[88,89] and number[46,90] of peristaltic contractions↑ |
| Number of spontaneous and non-propagated contractions↑[45] | |
| Amplitude of lower esophageal sphincter pressure↓[88] | |
| Multi-peaked contractions[78,79] | |
| Stomach | Antral IMMC↓[87] |
| Post-prandial antral activity and the number of antral contractions↓[91] | |
| Pyloric dysmotility[92] | |
| Small intestine | Frequency and amplitude of the antropyloroduodenal contractions↑↓[93] |
| Duration of MMC cycle↑[85] | |
| Early recurrence of the MMC and clusters of contractile activity[94] |
Upper GI transit disorders in DM
Esophagus: Impaired esophageal transit has been reported both in IDDM and NIDDM patients[43-47]. The esophageal transit appears to be delayed in about 50% of patients with long-standing DM[48]. The retarded esophageal transit in the DM usually reflects either peristaltic failure or focal low-amplitude pressure waves[49].
Stomach: Several animal studies reported a slowing gastric emptying in IDDM and NIDDM rats[50-55], whereas other studies on IDDM and NIDDM animals demonstrated that gastric emptying increased[56-59]. Using radionuclide measuring techniques it has been demonstrated that gastric empting of solid, or liquid meals was abnormally slow in 30%-50% of patients with long-standing IDDM and NIDDM[60,61]. The gastroparesis in DM has been known clinically for more than 50 years[29]. It is not surprising that the gastric emptying delay in DM is related to both slow transit with increased retention of food in the proximal and distal stomach[62,63], and abnormal motility of the gastric wall[64].
Small intestine: Delayed and rapid transit in the small intestine was observed in animal diabetic models[65,66,67,68]. El-Salhy reported that the GI transit was rapid in non-obese diabetic mice[67] and was slower in obese diabetic mice[68]. Anjaneyulu and Ramarao[69] reported an increase in intestinal transit and a decrease in intestinal tone due to increased cholinergic and decreased beta-adrenergic receptor activities in DM rats. Slow small intestinal transit in DM patients have been documented using breath hydrogen appearance time after the ingestion of lactulose[70,71], by using radiopaque markers[72,73] and by use of metal-detector test[74]. On the other hand, Keshavarzian and Iber[75] investigated intestinal transit in IDDM patients after the ingestion of both liquid and solid meals and showed abnormal fast intestinal transit in their sample of diabetic patients. Nguyen et al[76] used intraluminal multiple impedance measurements to identify the postprandial duodenal chyme transport in patients with long standing IDDM. They demonstrated that the patients had disturbed propulsive chyme transport through the duodenum and the duodenal chyme clearance activity was decreased.
The transit disorders can occur in any region of the gut and in every stage of diabetes[74], and can affect each other. Since transit of food through the esophagus is relative fast, the gastric emptying rate is the major determinant of the food delivery to the small intestine. The relationship between esophageal transit and the rate of gastric empting appears to be poor[43] and the gastroparesis is often associated with the intestinal transit delay in DM[74,77].
Abnormal patterns of upper GI motility in DM
Esophagus: Esophageal manometric abnormalities occur in over 50% of patients with DM (see Table 1)[37]. The reduced amplitude of lower esophageal sphincter pressure is in accordance with the increased prevalence of gastroesophageal reflux disease in DM. More recently the evoked esophageal contractile activity to standardized bag distension was assessed using a specialized ultrasound-based probe by Frøkjær et al[19]. A balloon-like bag was positioned 10 cm above the lower esophageal sphincter and inflated. It was demonstrated, both at the bag and 6 cm proximally, that the distension induced hyperreactivity and impaired the coordination of the contractions in the diabetic patients.
Stomach: Motility disorders of the fundus and pylorus have been demonstrated in the diabetic animals[80-83]. In the human studies, it is recognized that disordered gastric contractile activity as assessed by manometry and gastric emptying occurs frequently in DM[84,85]. The motility disorders may include three aspects: Inter-digestive migrating motor complex (IMMC), amplitude and frequency of contractions, and pyloric dysfunction (Table 1).
Small intestine: Camilleri and Malagelada reported that small intestinal motility was abnormal in about 80% patients of long-standing DM with delayed gastric emptying[86]. Both postprandial and fasting small intestinal dysmotility in the DM was reported (Table 1). Dooley et al[87] studied fasting GI motility by manometry for a mean of 210 min in a group of 12 NIDDM patients with diarrhea and DAN. The patients showed grossly disordered motility. The migrating motor complex (MMC) disorders reflect the prolongation of phase II without change in the duration of phase I and III. The results of studies on postprandial motility at the level of the small intestine are inconsistent. However, abnormal motility patterns were observed in the diabetic subjects[85].
PATHOPHYSIOLOGY OF GI MOTILITY AND SENSORY DYSFUNCTION IN DM
Hyperglycemia
Disordered GI function in DM has been attributed to irreversible DAN but it is now clear that acute high blood glucose concentration per se have a major reversible influence on upper-GI tract motility and sensory function. An animal study demonstrated that the correction of hyperglycemia to euglycemic levels restores the delayed transit[65]. Several studies in both healthy subjects and DM patients have shown that the GI motor function is impaired during acute hyperglycemia (Table 2)[84,95-97]. Marked acute hyperglycemia affects the motility in every region of the GI tract[98]. This may indicate that cholinergic activity is affected during hyperglycemia. Hyperglycemia may also affect the perception of sensations arising from the GI tract (Table 2). However, much of the data have been observational, and there is relatively little information relating to potential mechanisms by which these effects are mediated. Because both stimulatory and inhibitory effects occur during the hyperglycemia, the effects of glucose are likely to be mediated by neural or humoral mechanisms, rather than a direct effect on the smooth muscle of the GI tract. The secretion of pancreatic polypeptide, which is under vagal cholinergic control, is diminished during acute hyperglycemia in healthy control subjects[99]. In healthy volunteers, plasma concentrations of motilin are less during hyperglycemia when compared with euglycemia[100]. Considering the effects of systemic changes in blood glucose concentrations, animal studies have revealed the presence of glucose-responsive neurons in the central nervous system, which may modify vagal efferent activity[101]. Neurons responsive to glucose have recently been identified in the rat small intestine[102], but their response to systemic rather than luminal glucose is unclear. Much work is required to elucidate the neural, humoral, and cellular mechanisms by which systemic glucose levels affects GI motility and sensation.
Table 2.
Acute hyperglycemia and upper GI disorders
| Motility disorder | Sensory disorder |
| Peristaltic wave duration[98]↑ | Amplitude of the cortical evoked |
| Peristaltic velocity in the distal | brain potentials to moderate |
| part of the esophagus↓[103] | esophageal distension↑[98] |
| Pressure of the low esophageal | Perception during proximal |
| sphincter↓[103] | gastric distension in healthy subjects |
| Gastric emptying of both solids | both in the fasted state and during |
| and nutrient liquids↓[104] | intra-duodenal lipid infusion↑[95] |
| Motility index and propagation | Influencing the postprandial |
| of duodenal and jejunal waves↓[84] | fullness in IDDM patients↑[105] |
| Cycle length of inter-digestive | |
| motor activity in the fasted state↓[56] | |
| Small intestinal transit↓[71] | |
| Proximal duodenal pressure waves↓[104] |
Peripheral and central neuronal changes
DAN is seen as a major factor in the pathogenesis of disordered GI motor and sensory functions in DM[106,107]. Although GI manifestations related to DAN are diverse, many GI complications of DM seem to be related to DAN[19,48,72,108-110].
Histological findings: The best-characterized signs of damage to the autonomic nervous system during DM are morphological animal studies[111-117]. The number of myelinated axons in the vagosympathetic trunk is decreased in diabetic rats[118]. In the GI tract, many changes of nerves and ganglia were observed i.e. (1) dystrophic axonopathy[119]; (2) degeneration of mesenteric nerves and ganglia[111]; (3) number of vasoactive intestinal peptide (VIP)-IR neurons in myenteric ganglia[112]; (4) relative volume density in myenteric plexus[120]; (5) number of adrenergic and serotonergic neurons[111]. In addition, the surrounding tissue is also often disturbed. There is a thickening of the endothelium[121,122], which may sensitize axons to damage from increased pressure or decreased oxygen and glucose availability. It is thus possible that axonal damage may be secondary to disorders in tissue surrounding the neurons. Nitric oxide (NO) is a key neurotransmitter in the regulation of GI motor function[123,124]. In rodents with streptozotocin (STZ)-induced diabetes, NO synthase expression in the gastric myenteric neurons is diminished[125] and associated with delayed gastric emptying. The results of morphologic studies of the vagal nerves in diabetic patients are less consistent. One study demonstrated a decrease in unmyelinated axons in the abdominal vagus, another study found no abnormalities[126,127].
Clinical findings: One study has shown that abnormal esophageal motility is more frequent in the diabetic patients with evidence of DAN than in those without[37]. The presence of abnormal gastroesophageal reflux in diabetic patients has also been associated with the existence of DAN[128]. Increased cholinergic tone may relate to multi-peaked waves in the diabetic esophagus in these patients[78]. Gastric emptying largely depends on vagus nerve function, which can be severely disrupted in DM[60]. Studies using cardiac autonomic nerve function tests to assess the involvement of the autonomic nerve system in diabetic patients have indicated that the prevalence and severity of dysmotility of the small intestine is substantially greater in DM patients with DAN compared to patients with normal autonomic function[129]. Diarrhea is evident in 20% of diabetic patients, particularly those with known DAN[130,131]. Altogether, abnormal upper GI motility in DM has been associated to DAN. However, the question arises as to whether neuropathic changes in the intramural GI plexuses, the extrinsic neuronal pathways or in the central nervous system are primary or secondary. In a recent study, Frøkjær et al found hyperreactivity and impaired coordination to bag distension of the esophagus in IDDM patients, which indicates that a neuronal dysfunction is responsible for the hyperreactivity[19]. This dysfunction can theoretically be addressed to the mechano-sensitive afferent fibers, inter-neurons or efferent fibers located in the esophageal wall. However, impaired extra-intestinal pathways can potentially also affect the motor response. In another study, Frøkjær et al demonstrated that IDDM patients had decreased sensitivity to the stimulations of the esophagus and duodenum[41]. This was accompanied by an increase in somatic referred pain areas to the gut stimulation (Figure 1). As the referred pain is a result of convergence between visceral and somatic afferents on central neurons, the findings are indirect evidence for central hyperexcitability and neuroplastic changes. Altogether, this indicate that the neuronal changes can be located both peripherally (receptors, nerve fibers, ganglions) and in the CNS. Sensory nerve dysfunction in the gut may explain why diabetic patients with severe GI motility disorders often do not suffer from the GI symptoms expected from the abnormal motility and motor function. On the another hand a large subgroup of patients with longstanding DM suffers from severe GI symptoms[2,26,27]. This overall hyperalgesia/hypersensitivity is in contrast to the peripheral autonomic afferent neuropathy that most likely impairs the perception from the GI tract. The mismatch between hypo-and hypersensitivity can likely be explained by an impaired balance between peripheral and central neuronal changes. Hence, the impaired function of the peripheral afferent nerves is likely counterbalanced (or even overruled) by increased central (spinal and/or brain) neuronal excitability and this balance may determine the symptoms in individual patients.
It is likely that different pathophysiological mechanisms contribute to the peripheral and central neuronal changes in DM, including metabolic alterations, microvascular changes, and inflammatory changes[132]. Hypoxia, hyperglycemia, and increased oxidative stress in DM contribute directly and indirectly to Schwann cell dysfunction[133]. This will result in impaired paranodal barrier function, damaged myelin, reduced antioxidative capacity, and decreased neurotrophic support for axons. Furthermore, the direct effects of prolonged hyperglycemia through the glycation on nervous tissue are also important in the development of DAN[134-136].
Morphological and biomechanical remodeling
Although the hyperglycemia and neuropathy seem to be the main mechanisms to the motor-sensory dysfunction in the upper GI tract in DM, the question remains whether the disordered motor and sensory functions of the GI tract are only due to the neuronal changes and dysfunction or if primary diabetes-induced structural and biomechanical changes in the GI tract also play a role Data on the biomechanical properties are crucial for the understanding of the motor function of the GI tract because, (1) peristaltic motion that propels the food through the GI tract is a result of interaction of the passive and active tissue forces and the hydrodynamic forces in the food bolus and (2) remodeling of the mechanical properties reflects the changes in the tissue structure that determine a specific motor dysfunction. Therefore, the morphological and biomechanical remodeling of the GI wall may also be an important factor in the pathogenesis to the GI motor-sensory dysfunction in the diabetic patients.
Esophageal remodeling: Many studies have shown that DM causes morphological changes and biomechanical remodeling in the esophagus. Yang et al[13] in the in vitro study on the STZ-induced diabetic rat esophagus found that the wall thickness and cross-sectional wall area increased after the induction of diabetes. Twenty-eight days after the diabetic esophagus became stiffer both in shear and in the longitudinal direction. The esophageal remodeling was also found in NIDDM rat esophagus[18]. In the diabetic rats the opening angle and residual strain distribution in the outer surface of the wall decreased, the collagen fraction in the mucosa-submucosa layer and the passive circumferential stiffness of the esophageal wall increased (Figure 2). Furthermore, the esophageal weight per length, wall thickness and cross-sectional wall area increased in the NIDDM rats. One previous human study reported that among six cases of diffuse muscular hypertrophy of the esophagus, four cases were associated with DM[137]. Recently, Frøkjær et al[19] in an human study found an increase in esophageal wall thickness and altered deformation to the distension with reduced longitudinal shortening and the radial stretch in IDDM patients (Figure 3).
Figure 2.
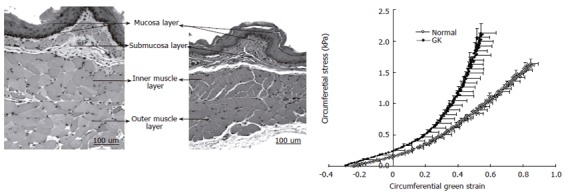
Microphotos of esophageal layer thickness in GK rats (left) and normal rats (middle). The muscle layer was biggest in the GK esophagus. Correspondingly the circumferential stress-strain curves of diabetic and non-diabetic esophageal wall showed in the right. The curve in the GK rats was shifted to the left, indicating that the esophageal wall stiffness increased.
Figure 3.
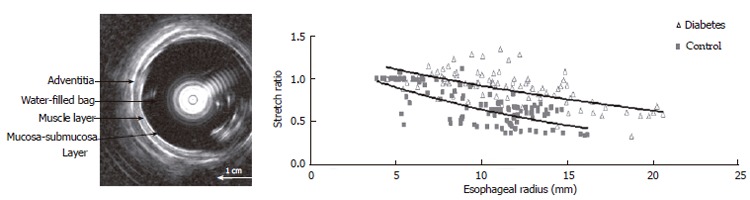
Left: Cross-sectional ultrasound image of the distended distal esophagus allows identification of the esophageal layers, i.e. mucosa-submucosa, muscle and adventitia layers. The white round shadow in the center is caused by the intraluminal ultrasound probe. Right: The distension-induced change in longitudinal stretch ratio is illustrated as function of the esophageal radius. The curves were obtained during smooth muscle relaxation with butylscopolamine. Exponential trend lines of the patients and controls are shown. The shortening during distension was clearly reduced in the diabetic patients.
Stomach remodeling: A morphological study in DM rats demonstrated that the gastric mucosa thickness increased in DM rats compared with controls[138]. Remodeling of the interstitial cells network of Cajal in the stomach were found both in animals and humans with DM[139-141]. A histopathological study of the human stomach in DM patients with severe gastroparesis showed prominent collagenization and smooth muscle atrophy of the muscle layer[142]. Regarding the biomechanical remodeling of the stomach only one report by Liao et al is available[17]. The rat stomach was distended in vitro. Gastric compliance, the surface tension, and circumferential and longitudinal deformation-pressure curves were calculated based on three-dimensional ultrasound reconstructions of non-diabetic and diabetic stomach models. In experimental DM, gastric compliance was lowered both in the non-glandular stomach (proximal part) and the whole stomach (Figure 4). Furthermore, the circumferential stiffness in the non-glandular part increased. The structural changes of the stomach due to DM may together with the sensory and motor nerve dysfunction contribute to the delayed gastric emptying and the symptoms in diabetic patients.
Figure 4.
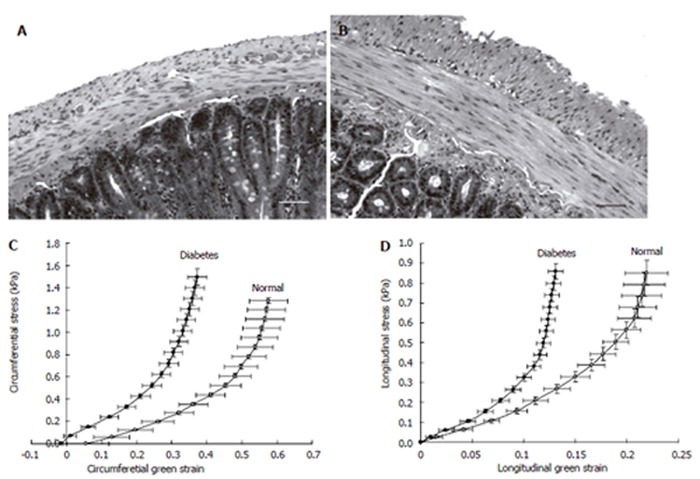
The micro-photographs show the normal (A) and 4 wk STZ-induced diabetic (B) duodenal histological sections. It clearly demonstrates that the muscle and submucosa layers in the diabetic duodenum became much thicker than in the normal duodenum. The bar is 100 μm. Correspondingly, the circumferential (C) and longitudinal (D) stress-strain relation curves of the duodenum in the STZ-induced diabetic rats shifted to the left indicating the wall became stiffer.
Small intestinal remodeling: Many human[2,11,143,144] and animal [2,3,10,145-148] studies have shown that DM causes morphological alterations in the small intestine (Table 3). However, only few data exist relating to biomechanical remodeling of the small intestine in DM[12]. Recently we did a series of studies on the morphological and biomechanical remodeling of small intestine in the STZ-induced diabetic rats[14-16]. The major findings in our studies are summarized in Table 3 and briefly showed that the stiffness of the diabetic intestinal wall increased in a time-dependent manner (Figure 5). The viscoelastic behavior of intestinal wall also changed during the development of diabetes (Table 3). The stress-strain distribution and viscoelastic behavior mainly reflects the elastic properties of intestine. The changes in elastic properties reflect the structural remodeling of the intestinal wall during the diabetic development.
Table 3.
Morphological and biomechanical changes of small intestine in DM
| Morphological change | Biomechanical change |
| Intestinal weight, length, and weight per unit length↑ | Opening angle and residual strain in duodenum↓ |
| Surface area of mucosa↑ | Opening angle and residual |
| Number of goblet cells per villus↑ | strain in jejunum and ileum↑ Circumferential stiffness of |
| Smooth muscle mass↑ | the intestinal wall↑ |
| Different layer thickness↑ | Longitudinal stiffness of the |
| Proliferating cell nuclear antigen (PCNA) ↑ | intestinal wall↑ Stress relaxation of small |
| interstitial cells of Cajal volume↓ | intestine↓ |
Figure 5.
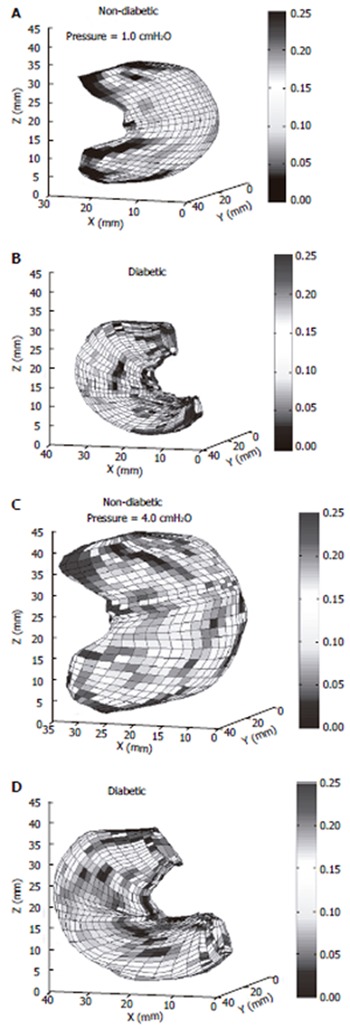
The circumferential curvature at the distension pressure of 1.0 (A,B) and 4.0 (C,D) cmH2O in the non-diabetic (A,C) and the diabetic stomach (B,D). The circumferential deformation of the stomach in the non-diabetic rats was significantly bigger than that in the diabetic Goto-Kakizaki (GK) rats, indicating the stiffness of the diabetic gastric wall increased in the diabetic GK rats.
Advanced glycation end products (AGEs) produced during the development of DM likely relate to the morphological changes and biomechanical remodeling of the GI tract in DM. AGEs are generated by the sequential non-enzymatic glycation of protein amino groups and by oxidation reaction[149]. The accumulation of AGEs in tissues will alter the structure and function of matrix proteins[150]. Studies on DM and ageing show that AGEs are causing cross-linking of collagen molecules responsible for basement membrane thickening and loss of matrix elasticity[151-153]. AGEs may contribute to the diabetic GI morphological and biomechanical remodeling by at least two major mechanisms. The first is receptor-independent alteration of the extracellular matrix architecture by non-enzymatic glycation and the formation of protein cross-links. The second mechanism is receptor-dependent and consists of modulation of cellular functions through ligation of specific cell surface receptors[154-156]. Sanchez SS and coworkers have demonstrated that the expression of small intestinal extracellular matrix proteins have changed in STZ-induced diabetic rats[152]. Therefore, the nonenzymatic glycation of the GI tissue induced by long-term hyperglycemia seems to be an important mechanism behind the GI wall remodeling in DM.
Implication of GI remodeling on mechanosensory assessments: Mechanosensation is of fundamental importance for the GI function. The mechanosensitive nerve endings exist extensively in the GI tract where they serve a critical role in homeostasis. Several mechanoreceptor-like structures have been identified such as intraganglionic laminar nerve endings (IGLE) and intramuscular arrays (IMA) in the GI tract[157-164]. The mechanosensitive afferents in the intrinsic and extrinsic pathways were described as low-, wide-dynamic- or high-threshold tension-receptors[165]. Therefore, the GI tract structure as well as the tension, stress and strain distribution in the wall is important for the GI sensory and motor function. The GI wall structure or deformation changes in the DM will alter the relative positions of the mechanosensitive afferents (zero setting of the mechanosensitive afferents)[166]. The biomechanical remodeling in the DM such as alterations of residual strain and stress distribution and increase the wall stiffness will alter the tension and stress distribution of the mechanosensitive afferents. As results, the perception and motility of the GI tract will change as well. Hence, the morphological changes and biomechanical remodeling of GI tract in the DM is likely to affect the function of mechanosensitive afferents in the GI wall and further affect the motor and sensory function. However, so far the data are sparse about the association between the morphological and biomechanical remodeling of GI tract and the motor-sensory dysfunction in the DM[19,41]. The multimodal stimulations have proven accurate and reliable in the assessment of visceral sensation in several studies[167-169]. The geometry data of GI tract can be obtained by impedance and cross-sectional imaging such as ultrasound[170-172]. Combined with pressure recordings, biomechanical parameters such as tension, stress and strain can be obtained and correlated to the symptoms and mechanosensory data in the DM subjects[41]. Therefore, combined studies the GI motor-sensory dysfunction and morphological and biomechanical remodeling in the diabetic GI tract will improve our understanding about the pathophysiology of GI disorders in the DM patients. In the future, animal studies are needed to investigate the passive and active biomechanical and mechanosensory properties of the GI tract related to DM. The experiments should simulate the physiological conditions both in vivo and in vitro in normal and diabetic animals. The identification of mechanosensitive afferents and enteric nerve responses to the different stimuli will obviously be beneficial to understand the mechanisms of GI motor-sensory dysfunction in the DM.
In conclusion, GI symptoms are frequent in the diabetic patients and are associated with sensory-motor abnormalities, such as impaired perception and motility of the GI tract. The pathogenesis of abnormal GI sensory-motor function in DM is clearly multi-factorial. DAN seems to be the major factor in the pathogenesis of disordered GI motor and sensory functions in DM. Hyperglycemia has also been shown to impair GI motor and sensory function. Furthermore, the morphological changes and biomechanical remodeling of the GI wall may compromise the GI motor function and affect the function of the mechanosensitive afferents in the GI wall. Studies of the relation between the GI motor-sensory dysfunction, morphological changes and biomechanical remodeling in the diabetic GI tract may shed of more light to understand the mechanism of GI motor-sensory dysfunction in the diabetic patients. This knowledge may prove to be valuable in the development of new treatment strategies, which can also be evaluated with the developed methods. New treatments that may be based on the present knowledge and methods are: (1) neurostimulation of afferent visceral nerves, i.e. gastric electric stimulation, (2) agents which can break down already formed glycation end product protein-protein crosslinks, and (3) modulation of the central nervous system excitability by drugs.
Footnotes
Supported by the Danish Diabetes Association, the Research Council of North Jutland County, the Toyota Foundation and the SparNord Foundation
S- Editor Pan BR E- Editor Bai SH
References
- 1.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 2.Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: Part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803–815. [PubMed] [Google Scholar]
- 3.Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am. 1998;27:861–874, vi-vii. doi: 10.1016/s0889-8553(05)70035-2. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Samsom M. Gastrointestinal function in diabetes mellitus. Chichester: John Wiley & Sons, Ltd; 2004. pp. 1–337. [Google Scholar]
- 5.Kamath MV, Tougas G, Fitzpatrick D, Fallen EL, Watteel R, Shine G, Upton AR. Assessment of the visceral afferent and autonomic pathways in response to esophageal stimulation in control subjects and in patients with diabetes. Clin Invest Med. 1998;21:100–113. [PubMed] [Google Scholar]
- 6.Kamath MV, May A, Hollerbach S, Fitzpatrick D, Bulat R, Bajwa A, Tougas G, Fallen EL, Shine G, Upton AR. Effects of esophageal stimulation in patients with functional disorders of the gastrointestinal tract. Crit Rev Biomed Eng. 2000;28:87–93. doi: 10.1615/critrevbiomedeng.v28.i12.150. [DOI] [PubMed] [Google Scholar]
- 7.Rathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care. 1991;14:1086–1089. doi: 10.2337/diacare.14.11.1086. [DOI] [PubMed] [Google Scholar]
- 8.Mayhew TM, Carson FL, Sharma AK. Small intestinal morphology in experimental diabetic rats: a stereological study on the effects of an aldose reductase inhibitor (ponalrestat) given with or without conventional insulin therapy. Diabetologia. 1989;32:649–654. doi: 10.1007/BF00274251. [DOI] [PubMed] [Google Scholar]
- 9.Tahara T, Yamamoto T. Morphological changes of the villous microvascular architecture and intestinal growth in rats with streptozotocin-induced diabetes. Virchows Arch A Pathol Anat Histopathol. 1988;413:151–158. doi: 10.1007/BF00749677. [DOI] [PubMed] [Google Scholar]
- 10.Zoubi SA, Mayhew TM, Sparrow RA. The small intestine in experimental diabetes: cellular adaptation in crypts and villi at different longitudinal sites. Virchows Arch. 1995;426:501–507. doi: 10.1007/BF00193174. [DOI] [PubMed] [Google Scholar]
- 11.Charlton M, Ahlman B, Nair KS. The effect of insulin on human small intestinal mucosal protein synthesis. Gastroenterology. 2000;118:299–306. doi: 10.1016/s0016-5085(00)70212-5. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A. Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci. 2001;46:960–967. doi: 10.1023/a:1010737323153. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhao J, Zeng Y, Gregersen H. Biomechanical properties of the rat oesophagus in experimental type-1 diabetes. Neurogastroenterol Motil. 2004;16:195–203. doi: 10.1111/j.1365-2982.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Sha H, Zhou S, Tong X, Zhuang FY, Gregersen H. Remodelling of zero-stress state of small intestine in streptozotocin-induced diabetic rats. Effect of gliclazide. Dig Liver Dis. 2002;34:707–716. doi: 10.1016/s1590-8658(02)80022-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Yang J, Gregersen H. Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia. 2003;46:1688–1697. doi: 10.1007/s00125-003-1233-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Liao D, Yang J, Gregersen H. Viscoelastic behavior of small intestine in streptozotocin-induced diabetic rats. Dig Dis Sci. 2003;48:2271–2277. doi: 10.1023/b:ddas.0000007862.50690.85. [DOI] [PubMed] [Google Scholar]
- 17.Liao D, Zhao J, Gregersen H. Three-dimensional geometry analysis of the stomach in type II diabetic GK rats. Diabetes Res Clin Pract. 2006;71:1–13. doi: 10.1016/j.diabres.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Liao D, Gregersen H. Biomechanical and histomorphometric esophageal remodeling in type 2 diabetic GK rats. J Diabetes Complications. 2007;21:34–40. doi: 10.1016/j.jdiacomp.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Frokjaer JB, Andersen SD, Ejskjaer N, Funch-Jensen P, Drewes AM, Gregersen H. Impaired contractility and remodeling of the upper gastrointestinal tract in diabetes mellitus type-1. World J Gastroenterol. 2007;13:4881–4890. doi: 10.3748/wjg.v13.i36.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996;25:361–378. doi: 10.1016/s0889-8529(05)70328-5. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz M, Fraser R. Disordered gastric motor function in diabetes mellitus. Diabetologia. 1994;37:543–551. doi: 10.1007/BF00403371. [DOI] [PubMed] [Google Scholar]
- 22.Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 23.Talley NJ, Howell S, Jones MP, Horowitz M. Predictors of turnover of lower gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2002;97:3087–3094. doi: 10.1111/j.1572-0241.2002.07104.x. [DOI] [PubMed] [Google Scholar]
- 24.Schvarcz E, Palmér M, Ingberg CM, Aman J, Berne C. Increased prevalence of upper gastrointestinal symptoms in long-term type 1 diabetes mellitus. Diabet Med. 1996;13:478–481. doi: 10.1002/(SICI)1096-9136(199605)13:5<478::AID-DIA104>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Maleki D, Locke GR 3rd, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ 3rd. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–2816. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 26.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670–674. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 27.Spångéus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol. 1999;34:1196–1202. doi: 10.1080/003655299750024706. [DOI] [PubMed] [Google Scholar]
- 28.Ricci JA, Siddique R, Stewart WF, Sandler RS, Sloan S, Farup CE. Upper gastrointestinal symptoms in a U.S. national sample of adults with diabetes. Scand J Gastroenterol. 2000;35:152–159. doi: 10.1080/003655200750024317. [DOI] [PubMed] [Google Scholar]
- 29.Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63:1339–1358. doi: 10.2165/00003495-200363130-00002. [DOI] [PubMed] [Google Scholar]
- 30.Rayner CK, Horowitz M. New management approaches for gastroparesis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:454–462; quiz 493. doi: 10.1038/ncpgasthep0283. [DOI] [PubMed] [Google Scholar]
- 31.Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care. 2002;17:63–67. doi: 10.1053/jcrc.2002.33030. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth GF. Diabetic thoracic polyradiculopathy: ten patients with abdominal pain. Am J Gastroenterol. 1997;92:502–505. [PubMed] [Google Scholar]
- 33.Samsom M, Verhagen MAMT. Intestinal function. In: Horowitz M, Samsom M, eds , editors. Gastrointestinal function in diabetes mellitus. Chichester: John Wiley & Sons, Ltd; 2004. pp. 177–218. [Google Scholar]
- 34.Virally-Monod M, Tielmans D, Kevorkian JP, Bouhnik Y, Flourie B, Porokhov B, Ajzenberg C, Warnet A, Guillausseau PJ. Chronic diarrhoea and diabetes mellitus: prevalence of small intestinal bacterial overgrowth. Diabetes Metab. 1998;24:530–536. [PubMed] [Google Scholar]
- 35.Zietz B, Lock G, Straub RH, Braun B, Schölmerich J, Palitzsch KD. Small-bowel bacterial overgrowth in diabetic subjects is associated with cardiovascular autonomic neuropathy. Diabetes Care. 2000;23:1200–1201. doi: 10.2337/diacare.23.8.1200. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RP. Coeliac disease. Aust Fam Physician. 2005;34:239–242. [PubMed] [Google Scholar]
- 37.Smout AJPM. Oesophageal function. In: Horowitz M, Samsom M, eds , editors. Gastrointestinal function in diabetes mellitus. Chichester: John Wiley & Sons, Ltd; 2004. pp. 97–116. [Google Scholar]
- 38.Kong MF, Horowitz M. Diabetic gastroparesis. Diabet Med. 2005;22 Suppl 4:13–18. doi: 10.1111/j.1464-5491.2005.1761e.x. [DOI] [PubMed] [Google Scholar]
- 39.Rayner CK, Verhagen MA, Hebbard GS, DiMatteo AC, Doran SM, Horowitz M. Proximal gastric compliance and perception of distension in type 1 diabetes mellitus: effects of hyperglycemia. Am J Gastroenterol. 2000;95:1175–1183. doi: 10.1111/j.1572-0241.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- 40.Undeland KA, Hausken T, Gilja OH, Aanderud S, Berstad A. Gastric meal accommodation and symptoms in diabetes. A placebo-controlled study of glyceryl trinitrate. Eur J Gastroenterol Hepatol. 1998;10:677–681. [PubMed] [Google Scholar]
- 41.Frøkjaer JB, Andersen SD, Ejskaer N, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Gut sensations in diabetic autonomic neuropathy. Pain. 2007;131:320–329. doi: 10.1016/j.pain.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Rothstein RD. Gastrointestinal motility disorders in diabetes mellitus. Am J Gastroenterol. 1990;85:782–785. [PubMed] [Google Scholar]
- 43.Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJ. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18:229–234. doi: 10.1007/BF00186645. [DOI] [PubMed] [Google Scholar]
- 44.Jørgensen F, Boesen F, Andersen EB, Hesse B. Oesophageal transit in patients with autonomic dysfunction. The effect of treatment with fludrocortisone. Clin Physiol. 1991;11:83–92. doi: 10.1111/j.1475-097x.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Keshavarzian A, Iber FL, Nasrallah S. Radionuclide esophageal emptying and manometric studies in diabetes mellitus. Am J Gastroenterol. 1987;82:625–631. [PubMed] [Google Scholar]
- 46.Stewart IM, Hosking DJ, Preston BJ, Atkinson M. Oesophageal motor changes in diabetes mellitus. Thorax. 1976;31:278–283. doi: 10.1136/thx.31.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai SC, Kao CH, Pan DY, ChangLai SP, Wang SJ. Effects of oral erythromycin on esophageal motility in patients with noninsulin-dependent diabetes mellitus. Gaoxiong Yixue Kexue Zazhi. 1995;11:430–435. [PubMed] [Google Scholar]
- 48.Annese V, Bassotti G, Caruso N, De Cosmo S, Gabbrielli A, Modoni S, Frusciante V, Andriulli A. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol. 1999;29:171–177. doi: 10.1097/00004836-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Holloway RH, Tippett MD, Horowitz M, Maddox AF, Moten J, Russo A. Relationship between esophageal motility and transit in patients with type I diabetes mellitus. Am J Gastroenterol. 1999;94:3150–3157. doi: 10.1111/j.1572-0241.1999.01508.x. [DOI] [PubMed] [Google Scholar]
- 50.Chang FY, Doong ML, Chen TS, Lee SD, Wang PS. Altered intestinal transit is independent of gastroparesis in the early diabetic rats. Chin J Physiol. 1997;40:31–35. [PubMed] [Google Scholar]
- 51.Yamano M, Kamato T, Nagakura Y, Miyata K. Effects of gastroprokinetic agents on gastroparesis in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:145–150. doi: 10.1007/pl00005022. [DOI] [PubMed] [Google Scholar]
- 52.Mehta N, Veliath S, Thombre DP. Effect of experimental diabetes and vagotomy on gastric emptying in rats. Indian J Physiol Pharmacol. 2002;46:441–448. [PubMed] [Google Scholar]
- 53.Ogata M, Uchimura T, Iizuka Y, Murata R, Suzuki S, Toyota T, Hikichi N. Effect of non-insulin dependent diabetes on cyclosporin A disposition in Goto-Kakizaki (GK) rats. Biol Pharm Bull. 1997;20:1026–1029. doi: 10.1248/bpb.20.1026. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Qiao X, Micci MA, Pasricha PJ, Chen JD. Improvement of gastric motility with gastric electrical stimulation in STZ-induced diabetic rats. Digestion. 2004;70:159–166. doi: 10.1159/000081516. [DOI] [PubMed] [Google Scholar]
- 55.Le Blanc-Louvry I, Guerre F, Songné B, Ducrotté P. Gastric stimulation: influence of electrical parameters on gastric emptying in control and diabetic rats. BMC Surg. 2002;2:5. doi: 10.1186/1471-2482-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogata M, Iizuka Y, Murata R, Hikichi N. Effect of streptozotocin-induced diabetes on cyclosporin A disposition in rats. Biol Pharm Bull. 1996;19:1586–1590. doi: 10.1248/bpb.19.1586. [DOI] [PubMed] [Google Scholar]
- 57.Young AA, Gedulin B, Vine W, Percy A, Rink TJ. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia. 1995;38:642–648. doi: 10.1007/BF00401833. [DOI] [PubMed] [Google Scholar]
- 58.Nowak TV, Roza AM, Weisbruch JP, Brosnan MR. Accelerated gastric emptying in diabetic rodents: effect of insulin treatment and pancreas transplantation. J Lab Clin Med. 1994;123:110–116. [PubMed] [Google Scholar]
- 59.Granneman JG, Stricker EM. Food intake and gastric emptying in rats with streptozotocin-induced diabetes. Am J Physiol. 1984;247:R1054–R1061. doi: 10.1152/ajpregu.1984.247.6.R1054. [DOI] [PubMed] [Google Scholar]
- 60.Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16–S22. [PubMed] [Google Scholar]
- 61.Horowitz M, Su YC, Rayner CK, Jones KL. Gastroparesis: prevalence, clinical significance and treatment. Can J Gastroenterol. 2001;15:805–813. doi: 10.1155/2001/628102. [DOI] [PubMed] [Google Scholar]
- 62.Jones KL, Horowitz M, Wishart MJ, Maddox AF, Harding PE, Chatterton BE. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med. 1995;36:2220–2228. [PubMed] [Google Scholar]
- 63.Urbain JL, Vekemans MC, Bouillon R, Van Cauteren J, Bex M, Mayeur SM, Van den Maegdenbergh V, Bataille G, Charkes ND, Malmud LS. Characterization of gastric antral motility disturbances in diabetes using a scintigraphic technique. J Nucl Med. 1993;34:576–581. [PubMed] [Google Scholar]
- 64.Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43:491–496. doi: 10.1023/a:1018894520557. [DOI] [PubMed] [Google Scholar]
- 65.Chang FY, Lee SD, Yeh GH, Wang PS. Hyperglycaemia is responsible for the inhibited gastrointestinal transit in the early diabetic rat. Acta Physiol Scand. 1995;155:457–462. doi: 10.1111/j.1748-1716.1995.tb09995.x. [DOI] [PubMed] [Google Scholar]
- 66.Kumar MS, Prashanth KV. alpha-Lipoic acid ameliorates altered colonic contractility and intestinal transit in STZ-diabetic rats. Indian J Exp Biol. 2004;42:279–282. [PubMed] [Google Scholar]
- 67.El-Salhy M. Gastrointestinal transit in nonobese diabetic mouse: an animal model of human diabetes type 1. J Diabetes Complications. 2001;15:277–284. doi: 10.1016/s1056-8727(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 68.El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci. 2002;107:101–110. doi: 10.3109/2000-1967-133. [DOI] [PubMed] [Google Scholar]
- 69.Anjaneyulu M, Ramarao P. Studies on gastrointestinal tract functional changes in diabetic animals. Methods Find Exp Clin Pharmacol. 2002;24:71–75. doi: 10.1358/mf.2002.24.2.677129. [DOI] [PubMed] [Google Scholar]
- 70.Scarpello JH, Greaves M, Sladen GE. Small intestinal transit in diabetics. Br Med J. 1976;2:1225–1226. doi: 10.1136/bmj.2.6046.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Boer SY, Masclee AA, lam WF, Schipper J, Jansen JB, Lamers CB. Hyperglycemia modulates gallbladder motility and small intestinal transit time in man. Dig Dis Sci. 1993;38:2228–2235. doi: 10.1007/BF01299901. [DOI] [PubMed] [Google Scholar]
- 72.Iida M, Ikeda M, Kishimoto M, Tsujino T, Kaneto H, Matsuhisa M, Kajimoto Y, Watarai T, Yamasaki Y, Hori M. Evaluation of gut motility in type II diabetes by the radiopaque marker method. J Gastroenterol Hepatol. 2000;15:381–385. doi: 10.1046/j.1440-1746.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 73.Kawagishi T, Nishizawa Y, Okuno Y, Sekiya K, Morii H. Segmental gut transit in diabetes mellitus: effect of cisapride. Diabetes Res Clin Pract. 1992;17:137–144. doi: 10.1016/0168-8227(92)90159-o. [DOI] [PubMed] [Google Scholar]
- 74.Folwaczny C, Hundegger K, Volger C, Sorodoc J, Kühn M, Tatsch K, Landgraf R, Karbach U. Measurement of transit disorders in different gastrointestinal segments of patients with diabetes mellitus in relation to duration and severity of the disease by use of the metal-detector test. Z Gastroenterol. 1995;33:517–526. [PubMed] [Google Scholar]
- 75.Keshavarzian A, Iber FL, Dangleis MD, Cornish R. Intestinal-transit and lactose intolerance in chronic alcoholics. Am J Clin Nutr. 1986;44:70–76. doi: 10.1093/ajcn/44.1.70. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen HN, Silny J, Wüller S, Marschall HU, Rau G, Matern S. Abnormal postprandial duodenal chyme transport in patients with long standing insulin dependent diabetes mellitus. Gut. 1997;41:624–631. doi: 10.1136/gut.41.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iber FL, Parveen S, Vandrunen M, Sood KB, Reza F, Serlovsky R, Reddy S. Relation of symptoms to impaired stomach, small bowel, and colon motility in long-standing diabetes. Dig Dis Sci. 1993;38:45–50. doi: 10.1007/BF01296772. [DOI] [PubMed] [Google Scholar]
- 78.Loo FD, Dodds WJ, Soergel KH, Arndorfer RC, Helm JF, Hogan WJ. Multipeaked esophageal peristaltic pressure waves in patients with diabetic neuropathy. Gastroenterology. 1985;88:485–491. doi: 10.1016/0016-5085(85)90511-6. [DOI] [PubMed] [Google Scholar]
- 79.Borgström PS, Olsson R, Sundkvist G, Ekberg O. Pharyngeal and oesophageal function in patients with diabetes mellitus and swallowing complaints. Br J Radiol. 1988;61:817–821. doi: 10.1259/0007-1285-61-729-817. [DOI] [PubMed] [Google Scholar]
- 80.Belai A, Lefebvre RA, Burnstock G. Motor activity and neurotransmitter release in the gastric fundus of streptozotocin-diabetic rats. Eur J Pharmacol. 1991;194:225–234. doi: 10.1016/0014-2999(91)90109-4. [DOI] [PubMed] [Google Scholar]
- 81.James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612–G619. doi: 10.1152/ajpgi.00431.2003. [DOI] [PubMed] [Google Scholar]
- 82.Korolkiewicz R, Rekowski P, Szyk A, Kato S, Yasuhiro T, Kubomi M, Tashima K, Takeuchi K. Effects of diabetes mellitus on the contractile activity of carbachol and galanin in isolated gastric fundus strips of rats. Pharmacology. 1998;57:65–78. doi: 10.1159/000028227. [DOI] [PubMed] [Google Scholar]
- 83.Sakai Y, Inazu M, Shamoto A, Zhu B, Homma I. Contractile hyperreactivity and alteration of PKC activity in gastric fundus smooth muscle of diabetic rats. Pharmacol Biochem Behav. 1994;49:669–674. doi: 10.1016/0091-3057(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 84.Björnsson ES, Urbanavicius V, Eliasson B, Attvall S, Smith U, Abrahamsson H. Effects of hyperglycemia on interdigestive gastrointestinal motility in humans. Scand J Gastroenterol. 1994;29:1096–1104. doi: 10.3109/00365529409094894. [DOI] [PubMed] [Google Scholar]
- 85.Samsom M, Jebbink RJ, Akkermans LM, van Berge-Henegouwen GP, Smout AJ. Abnormalities of antroduodenal motility in type I diabetes. Diabetes Care. 1996;19:21–27. doi: 10.2337/diacare.19.1.21. [DOI] [PubMed] [Google Scholar]
- 86.Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420–427. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 87.Dooley CP, el Newihi HM, Zeidler A, Valenzuela JE. Abnormalities of the migrating motor complex in diabetics with autonomic neuropathy and diarrhea. Scand J Gastroenterol. 1988;23:217–223. doi: 10.3109/00365528809103971. [DOI] [PubMed] [Google Scholar]
- 88.Murray FE, Lombard MG, Ashe J, Lynch D, Drury MI, O'Moore B, Lennon J, Crowe J. Esophageal function in diabetes mellitus with special reference to acid studies and relationship to peripheral neuropathy. Am J Gastroenterol. 1987;82:840–843. [PubMed] [Google Scholar]
- 89.Clouse RE, Lustman PJ, Reidel WL. Correlation of esophageal motility abnormalities with neuropsychiatric status in diabetics. Gastroenterology. 1986;90:1146–1154. doi: 10.1016/0016-5085(86)90379-3. [DOI] [PubMed] [Google Scholar]
- 90.Hollis JB, Castell DO, Braddom RL. Esophageal function in diabetes mellitus and its relation to peripheral neuropathy. Gastroenterology. 1977;73:1098–1102. [PubMed] [Google Scholar]
- 91.Ishiguchi T, Tada H, Nakagawa K, Yamamura T, Takahashi T. Hyperglycemia impairs antro-pyloric coordination and delays gastric emptying in conscious rats. Auton Neurosci. 2002;95:112–120. doi: 10.1016/s1566-0702(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 92.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 93.Fraser R, Fried M, Beglinger C. [Assessment of gastric emptying] Schweiz Med Wochenschr Suppl. 1993;54:15–21. [PubMed] [Google Scholar]
- 94.Jebbink HJ, Bravenboer B, Akkermans LM, vanBerge-Henegouwen GP, Smout AJ. Relationships between dyspeptic symptoms and gastrointestinal motility in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:948–954. doi: 10.1007/BF02374478. [DOI] [PubMed] [Google Scholar]
- 95.Hebbard GS, Sun WM, Dent J, Horowitz M. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. 1996;8:211–217. doi: 10.1097/00042737-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Hebbard GS, Samsom M, Sun WM, Dent J, Horowitz M. Hyperglycemia affects proximal gastric motor and sensory function during small intestinal triglyceride infusion. Am J Physiol. 1996;271:G814–G819. doi: 10.1152/ajpgi.1996.271.5.G814. [DOI] [PubMed] [Google Scholar]
- 97.Hebbard GS, Samson M, Andrews JM, Carman D, Tansell B, Sun WM, Dent J, Horowitz M. Hyperglycemia affects gastric electrical rhythm and nausea during intraduodenal triglyceride infusion. Dig Dis Sci. 1997;42:568–575. doi: 10.1023/a:1018851227051. [DOI] [PubMed] [Google Scholar]
- 98.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 99.Lam WF, Masclee AA, Souverijn JH, Lamers CB. Effect of acute hyperglycemia on basal, secretin and secretin + cholecystokinin stimulated exocrine pancreatic secretion in humans. Life Sci. 1999;64:617–626. doi: 10.1016/s0024-3205(98)00604-3. [DOI] [PubMed] [Google Scholar]
- 100.Barnett JL, Owyang C. Serum glucose concentration as a modulator of interdigestive gastric motility. Gastroenterology. 1988;94:739–744. doi: 10.1016/0016-5085(88)90248-x. [DOI] [PubMed] [Google Scholar]
- 101.Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- 102.Liu M, Seino S, Kirchgessner AL. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Boer SY, Masclee AA, Lamers CB. Effect of hyperglycemia on gastrointestinal and gallbladder motility. Scand J Gastroenterol Suppl. 1992;194:13–18. doi: 10.3109/00365529209096020. [DOI] [PubMed] [Google Scholar]
- 104.Samsom M, Akkermans LM, Jebbink RJ, van Isselt H, vanBerge-Henegouwen GP, Smout AJ. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40:641–646. doi: 10.1136/gut.40.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones KL, Horowitz M, Berry M, Wishart JM, Guha S. Blood glucose concentration influences postprandial fullness in IDDM. Diabetes Care. 1997;20:1141–1146. doi: 10.2337/diacare.20.7.1141. [DOI] [PubMed] [Google Scholar]
- 106.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 107.Samsom M, Smout AJ. Abnormal gastric and small intestinal motor function in diabetes mellitus. Dig Dis. 1997;15:263–274. doi: 10.1159/000171603. [DOI] [PubMed] [Google Scholar]
- 108.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 109.Mearin F, De Ribot X, Balboa A, Antolín M, Varas MJ, Malagelada JR. Duodenogastric bile reflux and gastrointestinal motility in pathogenesis of functional dyspepsia. Role of cholecystectomy. Dig Dis Sci. 1995;40:1703–1709. doi: 10.1007/BF02212691. [DOI] [PubMed] [Google Scholar]
- 110.Mearin F, Malagelada JR. Gastroparesis and dyspepsia in patients with diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:717–723. [PubMed] [Google Scholar]
- 111.Belai A, Burnstock G. Changes in adrenergic and peptidergic nerves in the submucous plexus of streptozocin-diabetic rat ileum. Gastroenterology. 1990;98:1427–1436. doi: 10.1016/0016-5085(90)91072-e. [DOI] [PubMed] [Google Scholar]
- 112.Belai A, Facer P, Bishop A, Polak JM, Burnstock G. Effect of streptozotocin-diabetes on the level of VIP mRNA in myenteric neurones. Neuroreport. 1993;4:291–294. doi: 10.1097/00001756-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 113.Belai A, Burnstock G. Acrylamide-induced neuropathic changes in rat enteric nerves: similarities with effects of streptozotocin-diabetes. J Auton Nerv Syst. 1996;58:56–62. doi: 10.1016/0165-1838(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 114.Di Giulio AM, Lesma E, Gorio A. Diabetic neuropathy in the rat: 1. Alcar augments the reduced levels and axoplasmic transport of substance P. J Neurosci Res. 1995;40:414–419. doi: 10.1002/jnr.490400317. [DOI] [PubMed] [Google Scholar]
- 115.Yagihashi S, Wada R, Kamijo M, Nagai K. Peripheral neuropathy in the WBN/Kob rat with chronic pancreatitis and spontaneous diabetes. Lab Invest. 1993;68:296–307. [PubMed] [Google Scholar]
- 116.Yagihashi S, Kamijo M, Baba M, Yagihashi N, Nagai K. Effect of aminoguanidine on functional and structural abnormalities in peripheral nerve of STZ-induced diabetic rats. Diabetes. 1992;41:47–52. doi: 10.2337/diab.41.1.47. [DOI] [PubMed] [Google Scholar]
- 117.Yagihashi S, Zhang WX, Sima AA. Neuroaxonal dystrophy in distal symmetric sensory polyneuropathy of the diabetic BB-rat. J Diabet Complications. 1989;3:202–210. doi: 10.1016/0891-6632(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 118.Yagihashi S, Sima AA. Diabetic autonomic neuropathy. The distribution of structural changes in sympathetic nerves of the BB rat. Am J Pathol. 1985;121:138–147. [PMC free article] [PubMed] [Google Scholar]
- 119.Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Zhang W, Sima AA. Experimental rat models of types 1 and 2 diabetes differ in sympathetic neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:450–460. doi: 10.1093/jnen/63.5.450. [DOI] [PubMed] [Google Scholar]
- 120.Spångéus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- 121.Thomson AB, Keelan M, Thiesen A, Clandinin MT, Ropeleski M, Wild GE. Small bowel review: diseases of the small intestine. Dig Dis Sci. 2001;46:2555–2566. doi: 10.1023/a:1012782321827. [DOI] [PubMed] [Google Scholar]
- 122.Tomlinson DR, Carrington AL, Diemel LT, Ettlinger CB, Smith WJ, Fernyhough P. Limitations of the polyol hypothesis in the pathobiology of experimental diabetic neuropathy. Diabet Med. 1993;10 Suppl 2:27S–30S. doi: 10.1111/j.1464-5491.1993.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 123.Coulie B, Tack J, Sifrim D, Andrioli A, Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol. 1999;276:G373–G377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- 124.Russo A, Fraser R, Adachi K, Horowitz M, Boeckxstaens G. Evidence that nitric oxide mechanisms regulate small intestinal motility in humans. Gut. 1999;44:72–76. doi: 10.1136/gut.44.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106–2110. doi: 10.1023/a:1018830820537. [DOI] [PubMed] [Google Scholar]
- 126.Yagihashi S, Kamijo M, Ido Y, Mirrlees DJ. Effects of long-term aldose reductase inhibition on development of experimental diabetic neuropathy. Ultrastructural and morphometric studies of sural nerve in streptozocin-induced diabetic rats. Diabetes. 1990;39:690–696. doi: 10.2337/diab.39.6.690. [DOI] [PubMed] [Google Scholar]
- 127.Undeland KA, Hausken T, Aanderud S, Berstad A. Lower postprandial gastric volume response in diabetic patients with vagal neuropathy. Neurogastroenterol Motil. 1997;9:19–24. doi: 10.1046/j.1365-2982.1997.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 128.Lluch I, Ascaso JF, Mora F, Minguez M, Peña A, Hernandez A, Benages A. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999;94:919–924. doi: 10.1111/j.1572-0241.1999.987_j.x. [DOI] [PubMed] [Google Scholar]
- 129.Bharucha AE, Camilleri M, Low PA, Zinsmeister AR. Autonomic dysfunction in gastrointestinal motility disorders. Gut. 1993;34:397–401. doi: 10.1136/gut.34.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:928–30, 932, 934-944. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 131.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 132.Kelkar P. Diabetic neuropathy. Semin Neurol. 2005;25:168–173. doi: 10.1055/s-2005-871325. [DOI] [PubMed] [Google Scholar]
- 133.Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- 134.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 135.Cellek S, Qu W, Schmidt AM, Moncada S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: a new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331–339. doi: 10.1007/s00125-003-1298-y. [DOI] [PubMed] [Google Scholar]
- 136.King RH. The role of glycation in the pathogenesis of diabetic polyneuropathy. Mol Pathol. 2001;54:400–408. [PMC free article] [PubMed] [Google Scholar]
- 137.Iyer SK, Chandrasekhara KL, Sutton A. Diffuse muscular hypertrophy of esophagus. Am J Med. 1986;80:849–852. doi: 10.1016/0002-9343(86)90627-3. [DOI] [PubMed] [Google Scholar]
- 138.Watanabe T, Asanuma A, Tanaka M, Akiba T, Koga T. [Morphological study on the gastric mucosa in diabetes mellitus rats induced by streptozotocin] Exp Anim. 1995;43:693–696. doi: 10.1538/expanim1978.43.5_693. [DOI] [PubMed] [Google Scholar]
- 139.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 140.Horváth VJ, Vittal H, Ordög T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–1533. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 141.Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol. 2004;10:1227–1230. doi: 10.3748/wjg.v10.i8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–495. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 143.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 144.Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, Mezzogiomo A, Sasso FC, Cartenì M, De Rosa R, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. 2004;36:35–45. doi: 10.1016/j.dld.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 145.Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol. 2004;36:89–97. doi: 10.1016/s1357-2725(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 146.Noda T, Iwakiri R, Fujimoto K, Yoshida T, Utsumi H, Sakata H, Hisatomi A, Aw TY. Suppression of apoptosis is responsible for increased thickness of intestinal mucosa in streptozotocin-induced diabetic rats. Metabolism. 2001;50:259–264. doi: 10.1053/meta.2001.21030. [DOI] [PubMed] [Google Scholar]
- 147.Tormo MA, Martínez IM, Romero de Tejada A, Gil-Exojo I, Campillo JE. Morphological and enzymatic changes of the small intestine in an n0-STZ diabetes rat model. Exp Clin Endocrinol Diabetes. 2002;110:119–123. doi: 10.1055/s-2002-29088. [DOI] [PubMed] [Google Scholar]
- 148.Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K. Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J. 2003;50:271–279. doi: 10.1507/endocrj.50.271. [DOI] [PubMed] [Google Scholar]
- 149.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 150.Monnier VM, Sell DR, Nagaraj RH, Miyata S, Grandhee S, Odetti P, Ibrahim SA. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41 Suppl 2:36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 151.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 152.Sánchez SS, Genta SB, Aybar MJ, Honoré SM, Villecco EI, Sánchez Riera AN. Changes in the expression of small intestine extracellular matrix proteins in streptozotocin-induced diabetic rats. Cell Biol Int. 2000;24:881–888. doi: 10.1006/cbir.2000.0581. [DOI] [PubMed] [Google Scholar]
- 153.Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132–142. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 154.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 155.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 156.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- 158.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 159.Raab M, Neuhuber WL. Intraganglionic laminar endings and their relationships with neuronal and glial structures of myenteric ganglia in the esophagus of rat and mouse. Histochem Cell Biol. 2004;122:445–459. doi: 10.1007/s00418-004-0703-z. [DOI] [PubMed] [Google Scholar]
- 160.Raab M, Neuhuber WL. Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem. 2005;53:1023–1031. doi: 10.1369/jhc.4A6582.2005. [DOI] [PubMed] [Google Scholar]
- 161.Swithers SE, Baronowsky E, Powley TL. Vagal intraganglionic laminar endings and intramuscular arrays mature at different rates in pre-weanling rat stomach. Auton Neurosci. 2002;102:13–19. doi: 10.1016/s1566-0702(02)00172-8. [DOI] [PubMed] [Google Scholar]
- 162.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–G406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- 165.Gregersen H. Biomechanics of the Gastrointestinal Tract. London: Springer-Verlag; 2002. [Google Scholar]
- 166.Pedersen J, Gao C, Egekvist H, Bjerring P, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain and biomechanical responses to distention of the duodenum in patients with systemic sclerosis. Gastroenterology. 2003;124:1230–1239. doi: 10.1016/s0016-5085(03)00265-8. [DOI] [PubMed] [Google Scholar]
- 167.Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115–1130. doi: 10.1080/00365520310004399. [DOI] [PubMed] [Google Scholar]
- 168.Drewes AM, Schipper KP, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L. Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastrointest Liver Physiol. 2002;283:G95–G103. doi: 10.1152/ajpgi.00496.2001. [DOI] [PubMed] [Google Scholar]
- 169.Pedersen J, Reddy H, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Cold and heat pain assessment of the human oesophagus after experimental sensitisation with acid. Pain. 2004;110:393–399. doi: 10.1016/j.pain.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 170.Drewes AM, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H. Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol. 2003;38:27–35. [PubMed] [Google Scholar]
- 171.Frøkjaer JB, Andersen SD, Gale J, Arendt-Nielsen L, Gregersen H, Drewes AM. An experimental study of viscero-visceral hyperalgesia using an ultrasound-based multimodal sensory testing approach. Pain. 2005;119:191–200. doi: 10.1016/j.pain.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 172.Pedersen J, Drewes AM, Gregersen H. New analysis for the study of the muscle function in the human oesophagus. Neurogastroenterol Motil. 2005;17:767–772. doi: 10.1111/j.1365-2982.2005.00652.x. [DOI] [PubMed] [Google Scholar]


