Abstract
AIM: The use of low-dose aspirin to prevent cardiovascular disease events is well established. However, the incidence and predictors of upper gastrointestinal bleeding (UGIB) with its use are unknown. We studied prospectively the incidence and outcome of peptic ulceration in low-dose aspirin users.
METHODS: A total of 991 patients with coronary artery disease (CAD) on low-dose aspirin were prospectively followed-up for two years for the occurrence and clinical features of first hospitalized episode of UGIB.
RESULTS: UGIB had a bimodal presentation with 45% occurring within four months of aspirin initiation and had an overall prevalence of 1.5% per year. There was no UGIB-related death. Hypertension (OR = 4.6, 95%CI 1.5 - 14.7, P = 0.009), history of peptic ulceration (OR = 3.1, 95%CI 1.1 - 9.0, P = 0.039), tertiary education (OR = 3.08, 95%CI 1.1 - 9.0, P = 0.039) and higher lean body mass (P = 0.016) were independent factors associated with UGIB. Use of nitrate did not reduce UGIB.
CONCLUSION: The incidence of UGIB in patients with CAD on long-term low-dose aspirin is low, but is accompanied with significant morbidity. With prolonged use of aspirin, UGIB continues to be a problem for those with risk factors and especially in patients with a history of peptic ulcers, in which UGIB tends to occur early after aspirin therapy.
Keywords: Coronary artery disease, Aspirin, Gastrointestinal bleeding
INTRODUCTION
Anti-platelet therapy with low-dose aspirin (75-325 mg) reduces the risk of vascular events in patients with established coronary artery disease (CAD)[1-3]. However, the use of aspirin, even at a low dose for secondary prevention of cardiovascular events, is associated with a small but significant increase in the risk of upper gastrointestinal bleeding (UGIB). In addition, previous studies have also reported several risk factors, such as the use of non-steroidal anti-inflammatory drugs (NSAIDs) and old age, implicated in the development of UGIB in patients taking aspirin[4-8]. However, the incidence of aspirin-related UGIB in patients with CAD for the secondary prevention of cardiovascular events and the time frame of UGIB occurrence are uncertain. In addition, effects of several emerging therapies, such as the use of dual antiplatelet agents, nitrate or ulcer-protecting drugs for the development of UGIB, have not been systematically evaluated.
In a cohort of patients with established CAD recruited into a Cardiac Rehabilitation Programme, we prospectively monitored the time of occurrence of UGIB in patients taking low-dose aspirin for the secondary prevention of cardiovascular events over two years. We also sought to determine the possible predictive factors associated with UGIB.
MATERIALS AND METHODS
Study population
In this prospective study, we recruited all patients with established ischemic heart disease (myocardial infarction, angina pectoris, angiographic-proven coronary artery disease) referred to a Cardiac Rehabilitation Programme in Tung Wah Hospital (an University-affiliated hospital) between September 1997 and April 2002. All the patients were started on low-dose aspirin (75-300 mg) according to referring physicians’ discretion. Apart from collecting data on demographics, other parameters related to cardiac rehabilitation like education level, functional class, left ventricular function, exercise capacity, body mass index, body fat content, body lean mass content, medications used were also recorded. A database was specially designed to collect the required information prospectively. Patients were followed-up once every 3-4 mo and the occurrence of UGIB was noted. Detailed information about each UGIB event was obtained from interpretation and review of hospital records.
Upper gastrointestinal bleeding
The occurrence of UGIB was defined as the clinical presentation of hematemesis or passage of melena or unexplained drop in hemoglobin of ≥ 20 g/L. Upper gastrointestinal endoscopy with therapeutic intervention(s) to obtain haemostasis (using epinephrine injection or heater probe application, when needed) was performed in all these patients presented with UGIB. Helicobacter pylori (H pylori) status was determined using a 24-h rapid urease test on antral biopsy specimens and histological detection of Helicobacter-like organisms using the haematoxylin and eosin stain, and Giemsa stain on biopsies taken from the gastric antrum and/or body. The rapid urease test at 24 h has been validated in our hospital with a sensitivity and specificity of 99% and 100%, respectively[9]. In addition, the use of histological examination of these biopsy specimens, the H pylori status was correctly evaluated even in patients with active UGIB, with the understanding that rapid urease test would be rendered inaccurate at those situations.
Statistical analysis
Univariate analyses were performed, using Student’s t test and Mann-Whitney U test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables to assess the risk factors associated with UGIB. A multiple logistic regression model was designed to determine the factors (age, gender, educational level, occupation, hypertension, diabetes mellitus, renal impairment, history of peptic ulcer diseases, lean body mass, body fat, obesity, New York Heart Association (NYHA) functional class, left ventricular function, use of dual anti-platelet agents/NSAID/ulcer protective drug) associated with UGIB. To find the best model, a backward elimination stepwise procedure was carried out such that the factor would be eliminated from the analysis if the corresponding P value was greater than 0.15. A two-tailed P value of 0.05 or less was considered statistically significant.
RESULTS
Characteristics of patients and incidence of upper gastrointestinal bleeding (Table 1)
Table 1.
Baseline characteristics of 991 patients with coronary artery disease started on low-dose aspirin (mean ± SD, n, %)
| No UGIB | UGIB | P values | |
| (n = 962) | (n = 29) | ||
| Age (mean ± SD) | 64 ± 11 | 67 ± 11 | 0.663 |
| Sex (M/F) | 693/268 | 23/6 | 0.683 |
| Hypertension (%) | 519 (54) | 22 (76) | 0.008 |
| Diabetes mellitus (%) | 342 (35.5) | 10 (34.5) | 0.906 |
| Renal impairment (Cr > 200 umol/L) (%) | 42 (4.4) | 5 (17.2) | 0.001 |
| Serum creatinine | 120 ± 93 | 158 ± 103 | 0.002 |
| History of MI (%) | 536 (56) | 16 (55) | 0.095 |
| History of PU (%) | 74 (7.7) | 9 (31.0) | 0.006 |
| LVEF < 40% (%) | 388 (40.3) | 11 (37.9) | 0.034 |
| NYHA (III-IV) (%) | 82 (8.5) | 6 (20.7) | 0.048 |
| % Body fat | 34 ± 7 | 30 ± 7 | 0.036 |
| % Lean body mass | 66 ± 8 | 70 ± 7 | 0.049 |
| Education level (tertiary) (%) | 107 (11.1) | 5 (17.2) | 0.05 |
| Nitrate use (%) | 540 (56.1) | 21 (72.4) | 0.09 |
Cr: creatinine; MI: myocardial infarction; PU: peptic ulcer; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association functional class.
Between September 1997 and April 2002, a total of 991 patients with established ischemic heart disease were enrolled into the study and followed-up prospectively for two years. Of these, 716 (72.3%) were male (mean age, 64.8 ± 11 years); 552 (55.7%) had history of myocardial infarction (MI); 541 (54.8%) were hypertensive; 352 (35.5%) had diabetic mellitus; and 546 (54.7%) patients were smokers. Nitrate use was found in 561 (57.1%) patients. One hundred and twelve patients (11.3%) had received tertiary education. Twenty-nine patients developed UGIB over a follow-up period of two years, giving the incidence of 1.5% per year. For those who had a bleeding event, 72% did not have new-onset ulcer pain; 52% required blood transfusion (mean, 3.3 units; range, 1-8 units); 62% with a drop in hemoglobin of greater than 3 g/dL; 24% of these patients required more than one therapeutic upper gastrointestinal endoscopies for haemostasis. Peptic ulceration was the main cause of UGIB in our series (gastric ulcer, 38%; duodenum ulcer, 52%); aspirin-related hemorrhagic gastritis did not occur in our cohort. H pylori positivity status was present in 50% of patients presented with UGIB.
Of those who had UGIB, 21% of all the events occurred in the first month and 45% occurred in the first 4 mo after initiation of low-dose aspirin (early bleeders) (Figure 1). The ‘late bleeders’ (55%) developed UGIB at a mean of 20.8 ± 5.3 mo after initiation of therapy, with 88% (14/16) of them bled at least once after one year of aspirin therapy. In addition, about a third of those who presented with UGIB (9/29) had a history of peptic ulcer/GIB/gastrectomy, in which 56% (5/9) of them were early bleeders. This indicates a 1:8 chance of having UGIB in patients with history of peptic ulceration while started on low-dose aspirin, which have a tendency to have early bleeding event; 97% of patients with UGIB required hospitalization. Death or myocardial infarction did not occur during the UGIB episodes and no patient required surgery for treatment of the UGIB. Nitrate use was recorded in 62% of the bleeders; 21% of those who had an UGIB were on ulcer-protecting drugs during presentation, but only 10% of them reported concurrent use of NSAID.
Figure 1.
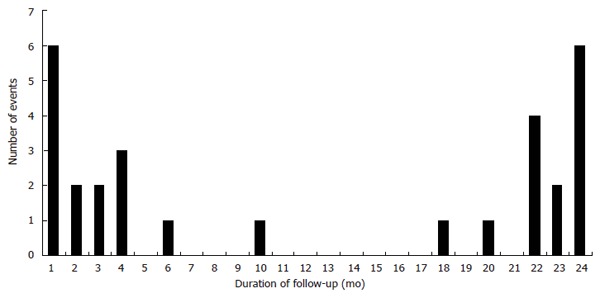
Bimodal presentation of upper gastrointestinal bleeding events in a two-year follow-up period.
Predictors of UGIB
Univariate analyses showed that the presence of hypertension (OR = 3.1, 95%CI 1.3 - 7.7, P = 0.008), renal impairment (OR = 4.8, 95%CI 1.7 - 13.1, P = 0.001), history of peptic ulcer (OR = 4.6, 95%CI 2.0 - 10.7, P = 0.002), patients with impaired left ventricle function (EF < 40%) (P = 0.034) or NYHA functional class III-IV (OR = 3.0, 95%CI 1.2 - 7.6, P = 0.048), patients with high lean body mass (70% ± 7% vs 66% ± 8%, P = 0.049) or low body fat content (30% ± 7% vs 34% ± 7%, P = 0.036) and patients with tertiary education (OR = 3.1, 95%CI 1.1 - 9.0, P = 0.05) were predictors of the development of UGIB. Use of anti-ulcer therapy (in small number of patients) was not associated in prevention of UGIB. The use of nitrate did not reduce the risk of UGIB (P = 0.09). However, multivariate analyses using a logistic regression model demonstrated that only patients with hypertension (OR = 4.63, 95%CI 1.5 - 14.7, P = 0.009), history of peptic ulcer (OR = 3.1, 95%CI 1.1 - 9.0, P = 0.039), tertiary education (OR = 3.1, 95%CI 1.1 - 9.0, P = 0.039) or with high lean body mass (707% ± 7% vs 667% ± 8%, P = 0.0157) had a higher probability of UGIB while taking low-dose aspirin for secondary prevention of cardiovascular events (Table 2).
Table 2.
Multivariate analysis of predictors of upper gastrointestinal bleeding in patients with coronary artery disease on low-dose aspirin (mean ± SD)
| 95%CI | Odds ratio | P values | |
| Education level (tertiary) | 1.05 - 8.98 | 3.08 | 0.039 |
| Hypertension | 1.46 - 14.74 | 4.63 | 0.009 |
| History of peptic ulcer | 1.05 - 9.00 | 3.08 | 0.039 |
| % Lean body mass (body fat content) | 0.0157 |
DISCUSSION
To the best of our knowledge, this is the first prospective study for the first UGIB in patients started with low-dose aspirin for secondary prevention of cardiovascular events. UGIB occurred at a rate of 1.5% per year, and was due to peptic ulceration that required hospitalization and blood transfusion. Twenty-four percent of those with UGIB required more than one therapeutic endoscopy to secure haemostasis. On the other hand, new-onset ulcer syndrome was not present in majority of those patients that developed an UGIB. These data also showed that when patients started with low-dose aspirin and bled subsequently, 21% of them had the event in the first month of treatment, 45% of all bleeding events occurred in the first 4 mo of starting low-dose aspirin; whereas most of the other bleeding events took place after at least one year of therapy. This observation implies that not only the risk of UGIB associated with low-dose aspirin use does not decrease with time, rather, there exists a bimodal presentation of the event in our series with one peak occurring in the early phase of starting treatment and the second occurred beyond the first year. This is in contrary to previous studies, in which the rate of UGIB occurred in a constant fashion throughout the entire period of study[4,8]. There are several possible explanations for the difference. Unlike other studies, we prospectively recorded first episode of UGIB after aspirin initiation instead of recruiting those presenting with the event[5]. In addition, others reported the use of a higher-dose of aspirin for the prevention of cardiovascular events[6]. Furthermore, some animal studies reported the possibility of adaptation of the gastric mucosa to aspirin therapy with time, and this might explain why patients begin to have less bleeding after receiving several mo of aspirin therapy[14].
We demonstrated the predictors of UGIB events for patients taking low-dose aspirin. Better-educated people were more likely to experience UGIB (OR = 3.1, the odds of UGIB was about 3 times more among people who received tertiary education than those who received secondary education or less). One explanation is that better educated people might be more compliant to prescribed medications, and they generally have much higher pressure/stress at work, and hence have a higher incidence of UGIB[10-11]. Consistent to previous reports, patients with a past history of peptic ulcer problems or UGIB were still at high risk of developing UGIB despite the use of low-dose aspirin[4]. Furthermore, we showed that most of them bled early on initiation of aspirin and thus deserve treatment for ulcer prophylaxis. In our study, patients with hypertension had the highest odds ratio for developing UGIB, concur with the traditional association between hypertension and the development of peptic ulcer[15]. Another interesting finding was that patients with a higher lean body mass had a higher probability for having an UGIB. A recent report suggested that obese patients are more prone to the development of aspirin resistance[12], so whether non-obese patients with slender-build (and hence a higher lean body mass content) are more susceptible to aspirin effect, including its toxicity on the stomach, will need further evaluation. Unlike a previous report[4], the use of nitrate was not linked to a reduction of UGIB in our study (P = 0.09). This may be due to a purely statistical problem as a large majority (57.1%) of the CAD patients in our cohort were on nitrate, which might mask the protective effect, if any of the latter; alternatively, other confounding factors, such as hypertension, leaner subjects in our series, might negate the effect of nitrate. The presence of renal impairment or heart failure/left ventricular dysfunction was believed to be predictors of UGIB as reported in our series using an univariate analysis, but these factors dropped out after multivariate adjustment. This might be due to the small number of events recorded in the present cohort. Our patients were younger (mean, 65 years; range, 35-87 years) compared to those in the previous study. With this age range, age appears not to be a predictive factor for UGIB.
The incidence of UGIB event in patients with CAD started on low-dose aspirin was low, and if occurred, was associated with good clinical outcome though most patients required hospitalisation. Nevertheless, with better understanding of the pathogenesis of the former and, in particular, its relation to H pylori status, our group has reported a possible method for the prevention of recurrence of peptic ulcer complication from long-term low-dose aspirin use[12]. On the other hand, our study showed that certain high-risk group of patients, especially those with history of peptic ulceration who tend to have early UGIB, should be target for primary prevention of peptic ulceration development and continued surveillance needed as well for these patients while on low-dose aspirin.
The small number of patients in our cohort that were on regular NSAID (1.1%) might not be able to show its deleterious effect on patients taking low-dose aspirin. Likewise, there was insufficient number of patients taking ulcer-protecting agents 21% to show a statistically significant benefit for patients taking these medications for ulcer prevention. However, the low usage of NSAID and ulcer-protecting drugs allowed us to determine the natural history of UGIB secondary to low-dose aspirin in this patient cohort, in which future therapeutic strategy can be planned. Finally, we failed to obtain the H pylori status for all patients, Regardless of whether they had or had not had an event, from which the value of that might be much more elicited. In addition, serological assay for H pylori infection was not universally available at time of our study. Nevertheless, local study reported that 55% of the population was infected with H pylori though symptoms and clinical disease developed in only a minority of infected individuals during their lifetime[13].
In summary, the incidence of UGIB in patients with CAD on low-dose aspirin remains low at 1.5% per year. Patients with hypertension, history of peptic ulcer problem, low body fat content (high lean body mass) and those that had received tertiary education were at risk of having an UGIB event. There exists bimodal presentation of the UGIB event with the early bleeders presenting in the first few months of starting treatment and the late bleeders after at least one year of therapy. Patients who had an event were likely to be hospitalized and required blood transfusion.
ACKNOWLEDGMENTS
We thank Ms Kam-Bing Lam RN and Ms Jeanette Kwok RN for their help with the data collection; and Ms Yuki Lo for preparing the manuscript.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Bai SH
References
- 1.Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 2.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz RL, Schacky CV, Weber M, Meister W, Kotzur J, Reichardt B, Theisen K, Weber PC. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation. Lancet. 1984;1:1261–1264. doi: 10.1016/s0140-6736(84)92446-2. [DOI] [PubMed] [Google Scholar]
- 4.Serrano P, Lanas A, Arroyo MT, Ferreira IJ. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945–1953. doi: 10.1046/j.1365-2036.2002.01355.x. [DOI] [PubMed] [Google Scholar]
- 5.Lanas A, Bajador E, Serrano P, Fuentes J, Carreño S, Guardia J, Sanz M, Montoro M, Sáinz R. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834–839. doi: 10.1056/NEJM200009213431202. [DOI] [PubMed] [Google Scholar]
- 6.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 7.Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sáinz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther. 2002;16:779–786. doi: 10.1046/j.1365-2036.2002.01230.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurata JH, Abbey DE. The effect of chronic aspirin use on duodenal and gastric ulcer hospitalizations. J Clin Gastroenterol. 1990;12:260–266. doi: 10.1097/00004836-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Wong BC, Wong WM, Wang WH, Tang VS, Young J, Lai KC, Yuen ST, Leung SY, Hu WH, Chan CK, et al. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2001;15:505–511. doi: 10.1046/j.1365-2036.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 10.Feldman EJ, Sabovich KA. Stress and peptic ulcer disease. Gastroenterology. 1980;78:1087–1089. [PubMed] [Google Scholar]
- 11.Maree AO, Curtin RJ, Dooley M, Conroy RM, Crean P, Cox D, Fitzgerald DJ. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol. 2005;46:1258–1263. doi: 10.1016/j.jacc.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 13.Ching CK, Wong BC. Who should be treated for Helicobacter pylori infection. Hong Kong Med J. 1999;5:151–157. [PubMed] [Google Scholar]
- 14.St John DJ, Yeomans ND, McDermott FT, De Boer WG. Adaptation of the gastric mucosa to repeated administration of aspirin in the rat. Am J Dig Dis. 1973;18:881–885. doi: 10.1007/BF01073339. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg A. Concordant occurrence of gastric and hypertensive diseases. Gastroenterology. 1988;95:42–48. doi: 10.1016/0016-5085(88)90288-0. [DOI] [PubMed] [Google Scholar]


