Abstract
Simple liver cysts are congenital with a prevalence of 2.5%-4.25%. Imaging, whether by US, CT or MRI, is accurate in distinguishing simple cysts from other etiologies, including parasitic, neoplastic, duct-related, and traumatic cysts. Symptomatic simple liver cysts are rare, and the true frequency of symptoms is not known. Symptomatic simple liver cysts are predominantly large (> 4 cm), right-sided, and more common in women and older patients. The vast majority of simple hepatic cysts require no treatment or follow-up, though large cysts (> 4 cm) may be followed initially with serial imaging to ensure stability. Attribution of symptoms to a large simple cyst should be undertaken with caution, after alternative diagnoses have been excluded. Aspiration may be performed to test whether symptoms are due to the cyst; however, cyst recurrence should be expected. Limited experience with both laparoscopic deroofing and aspiration, followed by instillation of a sclerosing agent has demonstrated promising results for the treatment of symptomatic cysts. Here, we describe a patient with a large, symptomatic, simple liver cyst who experienced complete resolution of symptoms following cyst drainage and alcohol ablation, and we present a comprehensive review of the literature.
Keywords: Simple hepatic cyst, Alcohol sclerosis, Laparoscopic deroofing
INTRODUCTION
Liver cysts are classified as true or false, depending on the presence of an epithelial lining[1]. True cysts include congenital cysts (simple cysts and polycystic liver disease), parasitic (hydatid) cysts caused by Echinococcus granulosis and multilocularis tapeworms, neoplastic cysts (including cystadenoma, cystadenocarcinoma, cystic sarcoma, squamous cell carcinoma, and metastatic ovarian, pancreatic, colon, renal and neuroendocrine cancers), and biliary duct-related cysts (Caroli’s disease, bile duct duplication, and peribiliary cysts)[1]. False cysts may be caused by spontaneous intrahepatic hemorrhage, post-traumatic hematoma, or intrahepatic biloma[1].
The pathogenesis of liver cysts is not clear. Simple liver cysts are congenital[2]. They are lined by cuboidal epithelium and originate from the abnormal development of intrahepatic ducts in utero. They are generally stable in size over time, but may slowly enlarge and occasionally become symptomatic due to mass effect, rupture, hemorrhage, or infection[3]. However, an enlarging cyst should prompt consideration of diagnosis other than simple cysts. Although simple cysts are generally solitary, more than one cyst may be present (“several solitary”), even in the absence of polycystic liver disease, as is the case with the patient described below.
We report herein a case of a patient with a large, symptomatic, simple hepatic cyst with resolution of symptoms immediately after therapy. In addition, we present a comprehensive literature review of diagnosis and treatment options of symptomatic hepatic cysts.
CASE REPORT
A 59-year-old African-American woman was referred to the Division of Gastroenterology at the University of Pennsylvania for evaluation of abdominal pain for 2 years. The patient described frequent, intermittent epigastric pain and bloating not associated with meals or bowel movements. Typically, pain would last for one-half hour before spontaneously resolving. She had twice presented to the emergency room with abdominal pain and was discharged without definitive diagnosis. She also complained of early satiety and occasional nausea without weight loss.
The patient was taking daily fiber supplements and had regular, daily bowel movements. Reflux symptoms were well controlled with esomeprazole. Hypertension was treated with metoprolol and amlodipine. Other chronic medications were progesterone and estrogen. She denied non-steroidal anti-inflammatory drug usage. Physical examination was unremarkable.
Laboratory evaluation showed normal liver-associated enzymes, metabolic panel, complete blood count, and urinalysis. Screening colonoscopy, performed two years prior to presentation, revealed internal hemorrhoids and melanosis. Upon presentation, she underwent double contrast upper gastrointestinal examination, which suggested antral gastritis and a hiatal hernia. On subsequent upper gastrointestinal endoscopy, the mucosa appeared normal, and a medium-sized hiatal hernia was present. Treatment with a proton pump inhibitor, fiber, and hyoscyamine failed to improve symptoms by six months.
The patient underwent abdominal CT scan with intravenous and oral contrast, which showed a large (7.7 cm) hepatic cyst in the lateral segment of the left lobe, as well as several other smaller cysts (Figure 1). Gallstones without gallbladder wall thickening or pericholecystic fluid were also visualized. On ultrasonography, tenderness was elicited specifically over the site of the cyst, which measured 10.3 cm in its longest dimension.
Figure 1.
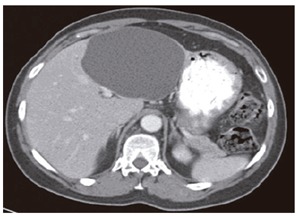
Enhanced abdominal CT scan showing large, simple hepatic cyst.
After several months of expectant management, the patient was referred to interventional radiology for drainage of the large hepatic cyst. Under ultrasonographic guidance, an 8-gauge French catheter was placed into the cyst, serous non-bilious fluid was aspirated, and the catheter was placed to gravity drainage. One week later, after drainage had ceased, the patient underwent ethanol sclerosis. At 4 mo follow-up, the patient was completely symptom-free. Follow-up ultrasound showed complete cyst resolution.
DISCUSSION
Previous studies, based on autopsy and surgical series, estimated a very low prevalence of simple non-parasitic hepatic cysts (0.14%-0.17%)[4]. More recently, among patients referred for abdominal ultrasonography, the prevalence of simple hepatic cysts has been reported 2.5%-4.65%[5,6]. Liver cysts have been recognized increasingly as the routine use of imaging studies becomes more widespread. Hepatic cysts may be more common in women[5] and in patients older than 40 years[6]. Symptoms, though quite rare, may be related to the space-occupying effect of large cysts[7] and may be more common in right-sided cysts[8]. Symptoms may include abdominal discomfort, chronic right upper quadrant or epigastric abdominal pain, early satiety, dyspnea, increasing abdominal girth, nausea, and vomiting[7,9]. Sanfelippo et al[4] reported that among 15 symptomatic patients with solitary non-parastic liver cysts, abdominal mass was present in 54%, hepatomegaly in 40%, abdominal pain in 33% and jaundice in 9% patients.
Although the natural history of simple hepatic cysts is not well known, complications appear to be quite rare (Table 1). Obstructive jaundice caused by solitary non-parasitic liver cyst is rare[11-15], and such cysts are usually large and located centrally in the liver, causing compression of the hepatic hilum[13]. However, sometimes even small hepatic cysts (3 cm in diameter) may cause common bile duct stenosis and intrahepatic biliary dilatation[15].
Table 1.
Rare complications of simple liver cysts
| Obstructive jaundice |
| Infection |
| Intracystic haemorrhage |
| Spontaneous rupture |
| Inferior vena cava obstruction |
| Neoplastic transformation |
| Primary squamous cell carcinoma |
| Cystadenocarcinoma |
| Adenosquamous carcinoma |
| Adenocarcinoma |
| Hepatocellular carinoma |
| Cholangiocarcinoma |
Liver cysts may also cause obstruction of the inferior vena cava, which may lead to massive edema of the legs[16,17] and scrotum[16]. Infections of simple hepatic cysts with Klebsiella pneumoniae[18] and Escherichia coli[19], presenting with acute onset of right upper quadrant abdominal pain, diarrhea, and fever have also been reported. Other documented complications include intracystic haemorrhage[19-23] and spontaneous rupture[23]. Neoplasms arising from solitary non-parasitic liver cysts, including primary squamous cell carcinoma[24-26], cystadenocarcinoma, adenosquamous carcinoma, adenocarcinoma, hepatocellular carinoma, and cholangiocarcinoma[27-29], have been reported, but appear to be very rare. A possible association with Peutz-Jeghers syndrome has also been suggested[30].
Imaging modalities (ultrasound, CT, and MRI) are highly accurate for diagnosing simple cysts (Table 2). Large differences in echogenicity between hepatic parenchyma and cyst fluid allow for easy recognition of simple cysts by ultrasound[31]. The ultrasonographic appearance of simple cyst is characterized by well-defined, echo-free lesions with good through transmission and an imperceptible wall[3,32]. The presence of acoustic enhancement results from relative lack of absorption and reflection of sound by cyst fluid, as compared with hepatic parenchyma[32]. On CT scan, simple cysts appear as well-demarcated, water-density sacs, which do not demonstrate peripheral enhancement after intravenous contrast [3]. The presence of septations suggests that a cyst is not simple. Occasionally, large simple cysts may have “septations” due to hemorrhage. MRI shows simple cysts as hypointense lesions on T1-weighted and hyperintense on T2-weighted images[32,33]. Simple cysts differ from cavernous hemangiomas in that they are more hypointense on T1-weighted and of equal hyperintensity on T2-weighted images[33]. Radiologic characteristics which would argue against a cyst being simple include a thick wall, peripheral enhancement on CT or MRI, heterogeneity within the cyst, and an increase in size over time. Hepatic cysts should be differentiated from hepatic abscesses, hematomas, necrotic metastases, an intrahepatic gallbladder, biliary cystadenoma, and echinococcal (hydatid) cysts[32].
Table 2.
Radiologic features of simple hepatic cysts
| Features supporting diagnosis of a simple cyst | Features not supporting diagnosis of a simple cyst |
| Anechoic lesion | Echoic lesion |
| Thin wall | Thick wall |
| Absence of septations | Presence of septations |
| No peripheral enhancement on CT/MRI | Peripheral enhancement on CT/MRI |
| Homogeneous appearance | Heterogeneity within the cyst |
| Hydatid sand | |
| Presence of daughter cysts | |
| Heavy wall calcifications |
Radiologic imaging can accurately identify hydatid cysts, with an accuracy of 96% in one series[34]. Presence of hydatid sand, internal sepatations, daughter cysts, and heavy wall calcifications argue for Echinococcus granulosis infection, instead of a simple cyst. Epidemiologic features and serology in combination with radiologic imaging generally lead to the correct diagnosis non-invasively.
Distinction between cystic and solid hepatic lesions can be made accurately by ultrasonography, though CT or MR imaging may be more sensitive for the detection of focal hepatic masses[35]. The presence of any peripheral enhancement or thick-walled component suggests the possibility of hepatic abscess or neoplasm[32]. Small hepatic lesions with diameter < 1 cm are difficult to classify[36]. Such lesions should be differentiated from benign cysts, hepatic metastases with central necrosis, and microabscesses[36]. In order to differentiate small cysts from small metastases, follow-up imaging may be considered in selected cases[36].
The vast majority of simple hepatic cysts require no treatment. Large cysts (diameter of 4 cm or more) can be followed for stability with repeated imaging[37]. If the cyst remains unchanged for 2 years, further monitoring may be discontinued[37]. Symptomatic or enlarging cysts require consideration of alternative diagnoses, including cystadenoma, cystadenocarcinoma, and hepatic metastases. It should be stressed that attribution of symptoms to simple cysts should be undertaken with caution after excluding alternative diagnoses. Epigastric or right upper quadrant abdominal pain provoked by eating may indicate biliary colic, if gallstones are present. A successful trial of acid suppression therapy points to gastroesophageal reflux disease. Selected patients may undergo upper gastrointestinal endoscopy to diagnose erosive esophagitis or peptic ulcer disease. Esophageal pH monitoring can confirm the diagnosis of gastroesophageal reflux. If symptoms fluctuate in concert with changes in stool frequency or form, a diagnosis of irritable bowel syndrome should be suspected. Finally, a diagnosis of non-ulcer dyspepsia may be entertained in patients with unremarkable upper gastrointestinal endoscopy who have continued prominent upper abdominal pain, possibly in association with nausea and vomiting (Table 3). If the preceding diagnoses can be confidently excluded, then treatment of a large, symptomatic hepatic cyst may be undertaken. Treatment options include needle aspiration with or without injection of sclerosing solution, internal drainage with cystojejunostomy, wide deroofing, and different degrees of liver resection[37].
Table 3.
Alternative explanations for symptoms in patients with simple hepatic cysts
| Diagnosis |
| Biliary colic |
| Gastroesophageal reflux |
| Peptic ulcer |
| Non-ulcer dyspepsia |
| Irritable bowel syndrome |
| Chronic pancreatitis |
| Abdominal wall pain syndrome |
Percutaneous US- or CT-guided needle aspiration of hepatic cysts is associated with high recurrence rates (78%-100%)[38,39]. Several small case series have demonstrated efficacy for the performance of US- or CT-guided needle aspiration of hepatic cysts combined with alcohol injection[40-44]. Because US-guided aspiration with ethanol sclerosis is generally safe, effective, and relatively non-invasive, it may be a first-line treatment for selected symptomatic congenital hepatic cysts, especially in patients with high surgical risk or polycystic liver disease[15]. The 95%, 96% and 99% alcohol solutions are equally safe and effective[15,42,45]. Enough alcohol should be instilled to replace 25% of the aspirated cyst fluid volume[46]. For larger cysts (> 400 mL), multiple alcohol injections in the same sitting have been proposed[46]. Alcohol fixes the cells lining the cyst cavity, disabling their ability to secrete fluid and promote cyst enlargement[46]. Recurrence may occur if alcohol does not come in contact with all cells lining the cyst cavity[46] and may be more common in uncooperative patients[47]. Combinations of percutaneous aspiration with other sclerosing substances, such as iophendylate (pantopaque)[48], tetracycline chloride[49-52], doxycycline[52], minocycline chloride[53-56], and hypertonic saline solution[8] have also achieved good outcomes.
Several recent series have demonstrated good results for laparoscopic deroofing procedures. Widest possible excision of the cystic wall and concomitant argon beam coagulation or electrocoagulation may improve the durability of results[57-61]. Recurrence has ranged from 0% to 20% with morbidity in 0% to 25%[57-60,62]. In one series, laparoscopic fenestration of simple hepatic cysts was found to be technically feasible in 90% with symptomatic relief in 95% during 38.5 mo follow-up[61]. Careful selection of patients who have not previously undergone surgical treatment and have large, symptomatic, superficial, and anterior cysts may improve outcomes[63]. US- or CT-guided cyst aspiration can be performed prior to laparoscopic fenestration in selected cases to assess whether symptoms are truly referable to the cyst[63]. Reported complications associated with laparoscopic deroofing include wound infection, bile leak, chest infection, subphrenic hematoma, and prolonged post-procedure drainage[37]. In selected cases, an open surgical procedure (fenestration, excision, or resection) may be preferred, despite longer recovery times and larger surgical scar, because of cyst location, surgeon expertise, or the presence of complicating factors[8,39,61,63,64].
Laparoscopic deroofing and cyst aspiration, followed by sclerosis, are both reasonable approaches for the majority of symptomatic simple liver cysts (Table 4)[64]. Whereas sclerosis may be less invasive and associated with lower rates of complications, laparoscopy is effective and provides the opportunity to directly examine the cyst interior to rule out etiologies other than a simple hepatic cyst[64,65].
Table 4.
Comparison of treatment options for symptomatic simple liver cysts
| Treatment options | Advantages | Disadvantages |
| Observation alone | - Because most cysts are asymptomatic, intervention is unlikely to be helpful and may be harmful | - Only effective cyst treatment can prove whether symptoms are related to the cyst |
| US-guided aspiration | - Simple procedure | - High recurrence rate |
| - May be used as a diagnostic test to assess whether symptoms are related to the cyst | ||
| US-guided aspiration with sclerotherapy | - Relatively non-invasive | - Less effective for uncooperative patients |
| - Complications are rare | - Can not be performed if cyst communicates with biliary tree | |
| - Effective | ||
| - Possible in poor surgical candidates | ||
| Laparoscopic unroofing | - Technically feasible and effective in > 80% cases | - More invasive |
| - Improved results with extensive fenestration and argon beam coagulation or electrocoagulation | - Morbidity in up to 25% | |
| - Low recurrence rate (0%-20%) | - Less effective for cysts which are superior, posterior, or deep within hepatic parenchyma | |
| - Visualization of cyst interior (exclude other diagnoses) | - Less effective if prior surgery has been attempted | |
| Laparotomy (resection, fenestration, or excision) | - Effective | - Most invasive |
| - Allows treatment of laparoscopically inaccessible cysts | - Larger scars | |
| - Useful for cysts with complications | - Longer hospital stays compare to laparoscopy | |
| - May perform cystojejunostomy at time of laparotomy for cysts with biliary communication | - Significant post-surgical morbidity |
It is important to rule out biliary communication before sclerosing a cyst, as irreversible sclerosing cholangitis has been reported as a complication[7,16,46,52,66]. The presence of cystobiliary communication may be established by endoscopic retrograde cholangiography or aspiration of bile-stained cystic fluid and is often treated by open cystojejunostomy[39], although the cystobiliary communication may be closed laparoscopically[67].
In summary, we present the case of a large, symptomatic simple hepatic cyst. We established that the cyst was the cause of symptoms only after systematically excluding alternative diagnoses through testing and empiric therapy. The usual standard of care for patients with large, simple hepatic cysts is observation, but our case demonstrates that large cysts can occasionally be responsible for symptoms. In selected cases, symptoms may respond to cyst treatment. Our treatment approach was ultrasound-guided aspiration and ethanol sclerotherapy. We believe that aspiration alone is associated with unacceptably high rates of recurrence. In our patient, excellent symptomatic and radiologic responses were achieved. In the case of cyst recurrence laparoscopic deroofing may be considered, though complications may occur in up to 25% of cases.
Footnotes
S- Editor Guo SY L- Editor Kumar M E- Editor Bi L
References
- 1.Taylor BR, Langer B. Current surgical management of hepatic cyst disease. Adv Surg. 1997;31:127–148. [PubMed] [Google Scholar]
- 2.Cowles RA, Mulholland MW. Solitary hepatic cysts. J Am Coll Surg. 2000;191:311–321. doi: 10.1016/s1072-7515(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 3.Nisenbaum HL, Rowling SE. Ultrasound of focal hepatic lesions. Semin Roentgenol. 1995;30:324–346. doi: 10.1016/s0037-198x(05)80021-5. [DOI] [PubMed] [Google Scholar]
- 4.Sanfelippo PM, Beahrs OH, Weiland LH. Cystic disease of the liver. Ann Surg. 1974;179:922–925. doi: 10.1097/00000658-197406000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989;62:335–337. doi: 10.1259/0007-1285-62-736-335. [DOI] [PubMed] [Google Scholar]
- 6.Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound. 1993;21:115–118. doi: 10.1002/jcu.1870210207. [DOI] [PubMed] [Google Scholar]
- 7.Lai EC, Wong J. Symptomatic nonparasitic cysts of the liver. World J Surg. 1990;14:452–456. doi: 10.1007/BF01658666. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez H, Gagner M, Rossi RL, Jenkins RL, Lewis WD, Munson JL, Braasch JW. Surgical management of nonparasitic cystic liver disease. Am J Surg. 1991;161:113–118; discussion 113-118;. doi: 10.1016/0002-9610(91)90370-s. [DOI] [PubMed] [Google Scholar]
- 9.Karavias DD, Tsamandas AC, Payatakes AH, Solomou E, Salakou S, Felekouras ES, Tepetes KN. Simple (non-parasitic) liver cysts: clinical presentation and outcome. Hepatogastroenterology. 2000;47:1439–1443. [PubMed] [Google Scholar]
- 10.Azar GM, Kutin N, Kahn E. Unusual hepatic tumor with features of mesenchymal hamartoma and congenital solitary nonparasitic cyst. Pediatr Dev Pathol. 2003;6:265–269. doi: 10.1007/s10024-003-7073-8. [DOI] [PubMed] [Google Scholar]
- 11.Spivey JR, Garrido JA, Reddy KR, Jeffers LJ, Schiff ER. ERCP documentation of obstructive jaundice caused by a solitary, centrally located, benign hepatic cyst. Gastrointest Endosc. 1990;36:521–523. doi: 10.1016/s0016-5107(90)71133-9. [DOI] [PubMed] [Google Scholar]
- 12.Terada N, Shimizu T, Imai Y, Kobayashi T, Terashima M, Furukawa K, Kumazawa S, Kiyosawa K. Benign, non-parasitic hepatic cyst causing obstructive jaundice. Intern Med. 1993;32:857–860. doi: 10.2169/internalmedicine.32.857. [DOI] [PubMed] [Google Scholar]
- 13.Kanai T, Kenmochi T, Takabayashi T, Hangai N, Kawano Y, Suwa T, Yonekawa H, Miyazawa N. Obstructive jaundice caused by a huge liver cyst riding on the hilum: report of a case. Surg Today. 1999;29:791–794. doi: 10.1007/BF02482330. [DOI] [PubMed] [Google Scholar]
- 14.Cappell MS. Obstructive jaundice from benign, nonparasitic hepatic cysts: identification of risk factors and percutaneous aspiration for diagnosis and treatment. Am J Gastroenterol. 1988;83:93–96. [PubMed] [Google Scholar]
- 15.Inaba T, Nagashima I, Ogawa F, Tomioka M, Okinaga K. Diffuse intrahepatic bile duct dilation caused by a very small hepatic cyst. J Hepatobiliary Pancreat Surg. 2003;10:106–108. doi: 10.1007/s10534-002-0816-6. [DOI] [PubMed] [Google Scholar]
- 16.Frisell J, Röjdmark S, Arvidsson H, Lundh G. Compression of the inferior caval vein--a rare complication of a large non-parasitic liver cyst. Acta Med Scand. 1979;205:541–542. doi: 10.1111/j.0954-6820.1979.tb06098.x. [DOI] [PubMed] [Google Scholar]
- 17.Kairaluoma MI, Leinonen A, Ståhlberg M, Päivänsalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg. 1989;210:208–215. doi: 10.1097/00000658-198908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Onda M, Tajiri T, Mamada Y, Taniai N, Mineta S, Hirakata A, Futami R, Arima Y, Inoue M, et al. Infected hepatic cyst. Hepatogastroenterology. 2003;50:507–509. [PubMed] [Google Scholar]
- 19.Yoshida H, Tajiri T, Mamada Y, Taniai N, Kawano Y, Mizuguchi Y, Shimizu T, Takahashi T, Uchida E, Watanabe M, et al. Infected solitary hepatic cyst. J Nippon Med Sch. 2003;70:515–518. doi: 10.1272/jnms.70.515. [DOI] [PubMed] [Google Scholar]
- 20.Hanazaki K, Wakabayashi M, Mori H, Sodeyama H, Yoshizawa K, Yokoyama S, Sode Y, Kawamura N, Miyazaki T. Hemorrhage into a simple liver cyst: diagnostic implications of a recent case. J Gastroenterol. 1997;32:848–851. doi: 10.1007/BF02936967. [DOI] [PubMed] [Google Scholar]
- 21.Kitajima Y, Okayama Y, Hirai M, Hayashi K, Imai H, Okamoto T, Aoki S, Akita S, Gotoh K, Ohara H, et al. Intracystic hemorrhage of a simple liver cyst mimicking a biliary cystadenocarcinoma. J Gastroenterol. 2003;38:190–193. doi: 10.1007/s005350300032. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H, Onda M, Tajiri T, Mamada Y, Taniai N, Uchida E, Arima Y, Akimaru K, Yamashita K. Intracystic hemorrhage of a simple hepatic cyst. Hepatogastroenterology. 2002;49:1095–1097. [PubMed] [Google Scholar]
- 23.Yamaguchi M, Kuzume M, Matsumoto T, Matsumiya A, Nakano H, Kumada K. Spontaneous rupture of a nonparasitic liver cyst complicated by intracystic hemorrhage. J Gastroenterol. 1999;34:645–648. doi: 10.1007/s005350050388. [DOI] [PubMed] [Google Scholar]
- 24.Pliskin A, Cualing H, Stenger RJ. Primary squamous cell carcinoma originating in congenital cysts of the liver. Report of a case and review of the literature. Arch Pathol Lab Med. 1992;116:105–107. [PubMed] [Google Scholar]
- 25.Monteagudo M, Vidal G, Moreno M, Bella R, Díaz MJ, Colomer O, Santesmasses A. Squamous cell carcinoma and infection in a solitary hepatic cyst. Eur J Gastroenterol Hepatol. 1998;10:1051–1053. doi: 10.1097/00042737-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Banbury J, Conlon KC, Ghossein R, Brennan MF. Primary squamous cell carcinoma within a solitary nonparasitic hepatic cyst. J Surg Oncol. 1994;57:210–212. doi: 10.1002/jso.2930570316. [DOI] [PubMed] [Google Scholar]
- 27.Jones WL, Mountain JC, Warren KW. Symptomatic non-parasitic cysts of the liver. Br J Surg. 1974;61:118–123. doi: 10.1002/bjs.1800610211. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka T, Tsunoda T, Harada N, Tsuchiya R, Kajiwara Y, Tokunaga S, Matsuo T, Ikeda T. Adenosquamous carcinoma of the liver. Am J Gastroenterol. 1987;82:1203–1206. [PubMed] [Google Scholar]
- 29.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–434. doi: 10.1097/00000658-198810000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thrasher S, Adelman S, Chang CH. Hepatic cyst associated with Peutz-Jeghers syndrome. Arch Pathol Lab Med. 1990;114:1278–1280. [PubMed] [Google Scholar]
- 31.Marn CS, Bree RL, Silver TM. Ultrasonography of liver. Technique and focal and diffuse disease. Radiol Clin North Am. 1991;29:1151–1170. [PubMed] [Google Scholar]
- 32.Mergo PJ, Ros PR. Benign lesions of the liver. Radiol Clin North Am. 1998;36:319–331. doi: 10.1016/s0033-8389(05)70025-7. [DOI] [PubMed] [Google Scholar]
- 33.Kanzer GK, Weinreb JC. Magnetic resonance imaging of diseases of the liver and biliary system. Radiol Clin North Am. 1991;29:1259–1284. [PubMed] [Google Scholar]
- 34.Munzer D. New perspectives in the diagnosis of Echinococcus disease. J Clin Gastroenterol. 1991;13:415–423. doi: 10.1097/00004836-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz JH, Ellison EC. Focal liver lesions. Evaluation of simple and complex cysts. Postgrad Med. 1994;95:149–152. [PubMed] [Google Scholar]
- 36.Foley WD, Jochem RJ. Computed tomography. Focal and diffuse liver disease. Radiol Clin North Am. 1991;29:1213–1233. [PubMed] [Google Scholar]
- 37.Regev A, Reddy KR. Benign solid and cystic tumors of the liver. In: The requisities in gastroenterology., editor. Volume 3: Hepatobiliary tract and pancreas. Reddy KR, Long WB, eds. Edinburgh: Mosby; 2004. pp. 189–214. [Google Scholar]
- 38.Saini S, Mueller PR, Ferrucci JT Jr, Simeone JF, Wittenberg J, Butch RJ. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. AJR Am J Roentgenol. 1983;141:559–560. doi: 10.2214/ajr.141.3.559. [DOI] [PubMed] [Google Scholar]
- 39.Koperna T, Vogl S, Satzinger U, Schulz F. Nonparasitic cysts of the liver: results and options of surgical treatment. World J Surg. 1997;21:850–854; discussion 850-854;. doi: 10.1007/s002689900316. [DOI] [PubMed] [Google Scholar]
- 40.Montorsi M, Torzilli G, Fumagalli U, Bona S, Rostai R, De Simone M, Rovati V, Mosca F, Filice C. Percutaneous alcohol sclerotherapy of simple hepatic cysts. Results from a multicentre survey in Italy. HPB Surg. 1994;8:89–94. doi: 10.1155/1994/10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikkakoski T, Mäkelä JT, Leinonen S, Päivänsalo M, Merikanto J, Karttunen A, Siniluoto T, Kairaluoma MI. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: technique and outcome. J Vasc Interv Radiol. 1996;7:235–239. doi: 10.1016/s1051-0443(96)70767-4. [DOI] [PubMed] [Google Scholar]
- 42.Larssen TB, Viste A, Jensen DK, Søndenaa K, Røkke O, Horn A. Single-session alcohol sclerotherapy in benign symptomatic hepatic cysts. Acta Radiol. 1997;38:993–997. doi: 10.1080/02841859709172116. [DOI] [PubMed] [Google Scholar]
- 43.Okano A, Hajiro K, Takakuwa H, Nishio A. Alcohol sclerotherapy of hepatic cysts: its effect in relation to ethanol concentration. Hepatol Res. 2000;17:179–184. doi: 10.1016/s1386-6346(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 44.Furuta T, Yoshida Y, Saku M, Honda H, Muranaka T, Oshiumi Y, Kanematsu T, Sugimachi K. Treatment of symptomatic non-parasitic liver cysts--surgical treatment versus alcohol injection therapy. HPB Surg. 1990;2:269–277; discussion 277-279. doi: 10.1155/1990/60495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCullough KM. Alcohol sclerotherapy of simple parenchymal liver cysts. Australas Radiol. 1993;37:177–181. doi: 10.1111/j.1440-1673.1993.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 46.Bean WJ, Rodan BA. Hepatic cysts: treatment with alcohol. AJR Am J Roentgenol. 1985;144:237–241. doi: 10.2214/ajr.144.2.237. [DOI] [PubMed] [Google Scholar]
- 47.Horsmans Y, Laka A, Gigot JF, Geubel AP. Serum and cystic fluid CA 19-9 determinations as a diagnostic help in liver cysts of uncertain nature. Liver. 1996;16:255–257. doi: 10.1111/j.1600-0676.1996.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein HM, Carlyle DR, Nelson RS. Treatment of symptomatic hepatic cyst by percutaneous instillation of Pantopaque. AJR Am J Roentgenol. 1976;127:850–853. doi: 10.2214/ajr.127.5.850. [DOI] [PubMed] [Google Scholar]
- 49.Lopes HM, Portela FA, e Silva Pontes JM, Leitão MJ, Ribeiro JC, Freitas DS. Treatment of benign hepatic cysts by instillation of tetracycline hydrochloride. Hepatogastroenterology. 1998;45:496–499. [PubMed] [Google Scholar]
- 50.McFarlane ME, Venugopal R, McDonald A, Ewing R, Newnham MS, Johnson L. Management of hepatic cysts by percutaneous drainage and instillation of tetracycline hydrochloride. West Indian Med J. 2001;50:230–233. [PubMed] [Google Scholar]
- 51.Davies CW, McIntyre AS. Treatment of a symptomatic hepatic cyst by tetracycline hydrochloride instillation sclerosis. Eur J Gastroenterol Hepatol. 1996;8:173–175. doi: 10.1097/00042737-199602000-00015. [DOI] [PubMed] [Google Scholar]
- 52.vanSonnenberg E, Wroblicka JT, D'Agostino HB, Mathieson JR, Casola G, O'Laoide R, Cooperberg PL. Symptomatic hepatic cysts: percutaneous drainage and sclerosis. Radiology. 1994;190:387–392. doi: 10.1148/radiology.190.2.8284385. [DOI] [PubMed] [Google Scholar]
- 53.Hagiwara H, Kasahara A, Hayashi N, Kono M, Suzuki K, Kashio S, Fusamoto H, Kamada T. Successful treatment of a hepatic cyst by one-shot instillation of minocycline chloride. Gastroenterology. 1992;103:675–677. doi: 10.1016/0016-5085(92)90864-u. [DOI] [PubMed] [Google Scholar]
- 54.Yamada N, Shinzawa H, Ukai K, Makino N, Matsuhashi T, Wakabayashi H, Togashi H, Takahashi T. Treatment of symptomatic hepatic cysts by percutaneous instillation of minocycline hydrochloride. Dig Dis Sci. 1994;39:2503–2509. doi: 10.1007/BF02087673. [DOI] [PubMed] [Google Scholar]
- 55.Cellier C, Cuenod CA, Deslandes P, Auroux J, Landi B, Siauve N, Barbier JP, Frija G. Symptomatic hepatic cysts: treatment with single-shot injection of minocycline hydrochloride. Radiology. 1998;206:205–209. doi: 10.1148/radiology.206.1.9423674. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H, Onda M, Tajiri T, Arima Y, Mamada Y, Taniai N, Akimaru K. Long-term results of multiple minocycline hydrochloride injections for the treatment of symptomatic solitary hepatic cyst. J Gastroenterol Hepatol. 2003;18:595–598. doi: 10.1046/j.1440-1746.2003.03025.x. [DOI] [PubMed] [Google Scholar]
- 57.Zacherl J, Scheuba C, Imhof M, Jakesz R, Függer R. Long-term results after laparoscopic unroofing of solitary symptomatic congenital liver cysts. Surg Endosc. 2000;14:59–62. doi: 10.1007/s004649900012. [DOI] [PubMed] [Google Scholar]
- 58.Regev A, Reddy KR, Berho M, Sleeman D, Levi JU, Livingstone AS, Levi D, Ali U, Molina EG, Schiff ER. Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg. 2001;193:36–45. doi: 10.1016/s1072-7515(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 59.Fiamingo P, Tedeschi U, Veroux M, Cillo U, Brolese A, Da Rold A, Madia C, Zanus G, D'Amico DF. Laparoscopic treatment of simple hepatic cysts and polycystic liver disease. Surg Endosc. 2003;17:623–626. doi: 10.1007/s00464-002-9088-z. [DOI] [PubMed] [Google Scholar]
- 60.Tagaya N, Nemoto T, Kubota K. Long-term results of laparoscopic unroofing of symptomatic solitary nonparasitic hepatic cysts. Surg Laparosc Endosc Percutan Tech. 2003;13:76–79. doi: 10.1097/00129689-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Gigot JF, Metairie S, Etienne J, Horsmans Y, van Beers BE, Sempoux C, Deprez P, Materne R, Geubel A, Glineur D, et al. The surgical management of congenital liver cysts. Surg Endosc. 2001;15:357–363. doi: 10.1007/s004640090027. [DOI] [PubMed] [Google Scholar]
- 62.Diez J, Decoud J, Gutierrez L, Suhl A, Merello J. Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg. 1998;85:25–27. doi: 10.1046/j.1365-2168.1998.02870.x. [DOI] [PubMed] [Google Scholar]
- 63.Gigot JF, Legrand M, Hubens G, de Canniere L, Wibin E, Deweer F, Druart ML, Bertrand C, Devriendt H, Droissart R, et al. Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patients and surgical technique. World J Surg. 1996;20:556–561. doi: 10.1007/s002689900086. [DOI] [PubMed] [Google Scholar]
- 64.Moorthy K, Mihssin N, Houghton PW. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl. 2001;83:409–414. [PMC free article] [PubMed] [Google Scholar]
- 65.Katkhouda N, Mavor E. Laparoscopic management of benign liver disease. Surg Clin North Am. 2000;80:1203–1211. doi: 10.1016/s0039-6109(05)70220-6. [DOI] [PubMed] [Google Scholar]
- 66.Klingler PJ, Gadenstätter M, Schmid T, Bodner E, Schwelberger HG. Treatment of hepatic cysts in the era of laparoscopic surgery. Br J Surg. 1997;84:438–444. [PubMed] [Google Scholar]
- 67.Masatsugu T, Shimizu S, Noshiro H, Mizumoto K, Yamaguchi K, Chijiiwa K, Tanaka M. Liver cyst with biliary communication successfully treated with laparoscopic deroofing: a case report. JSLS. 2003;7:249–252. [PMC free article] [PubMed] [Google Scholar]


