Abstract
Retroperitoneal fibrosis is an uncommon disorder characterized by the formation of a dense plaque of fibrous tissue in the retroperitoneum, and its etiology remains unknown. Autoimmune pancreatitis is a rare type of chronic pancreatitis characterized by fibrosis with abundant infiltration of IgG4-positive plasma cells and lymphocytes and obliterative phlebitis in the pancreas. We present a case of autoimmune pancreatitis that developed 10 mo after the occurrence of retroperitoneal fibrosis. Histological findings of the resected retroperitoneal mass were marked periureteral fibrosis with abundant infiltration of IgG4-positive plasma cells and lymphocytes and obliterative phlebitis. These findings suggest a common pathophysiological mechanism for retroperitoneal fibrosis and autoimmune pancreatitis in this case. Some cases of retroperitoneal fibrosis might be a retroperitoneal lesion of IgG4-related sclerosing disease.
Keywords: Autoimmune pancreatitis, Retroperitoneal fibrosis, IgG4
INTRODUCTION
Retroperitoneal fibrosis is an uncommon disorder characterized by the formation of a dense plaque of fibrous tissue in the retroperitoneum. Although retroperitoneal fibrosis leads to ureteral encasement and consequently hydronephrosis in most cases, it may also involve other structures in the retroperitoneum as well as in multiple unattached sites. Retroperitoneal fibrosis may be part of a systemic fibrosing disease[1,2]. Although the exact etiology of retroperitoneal fibrosis remains unknown, some cases respond well to steroid therapy[2,3].
Autoimmune pancreatitis is a recently noted clinical entity and a rare group of pancreatitis in which autoimmunity is suspected as a pathogenic mechanism. It is characterized by enlargement of the pancreas, irregular narrowing of the main pancreatic duct, elevated serum IgG4 concentrations, favorable response to steroid therapy and lymphoplasmacytic infiltration with fibrosis in the pancreas[4,5]. Recent studies have revealed occasional coexistence of autoimmune pancreatitis with other systemic exocrinopathy, such as sclerosing cholangitis[6] or sclerosing sialadenitis[7]. We have previously reported 2 other cases of autoimmune pancreatitis associated with retroperitoneal fibrosis[8]. Here, we report a case of autoimmune pancreatitis that occurred 10 mo after retroperitoneal fibrosis. We also focus on the common histopathological features with abundant IgG4-positive plasma cell infiltration in both the pancreas and retroperitoneal mass.
CASE REPORT
A 75-year-old male was admitted to another hospital with a complaint of back pain. Abdominal computed tomography (CT) showed left hydronephrosis (Figure 1A) caused by a retroperitoneal mass with encasement of the ureter (Figure 1B), which was highly suggestive of a ureteral tumor. Resection of the left lower ureter and ureterostomy were performed. Histological examination of the retroperitoneal mass revealed marked periureteral fibrosis with dense perivascular infiltration of lymphocytes and plasma cells (Figure 2A) and obliterative phlebitis (Figure 2B). These findings were compatible with retroperitoneal fibrosis. Ten months later, jaundice with abdominal pain appeared, and he was referred to our hospital for further examination.
Figure 1.
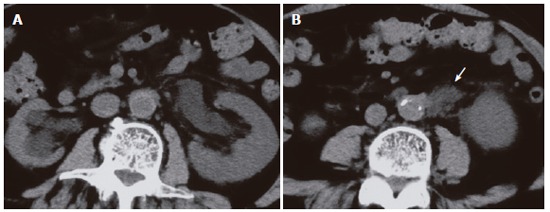
CT performed performed in another hospital showing left hydronephrosis (A) caused by a retroperitoneal mass (arrow) (B).
Figure 2.
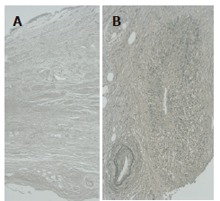
Histological findings of the retroperitoneal mass showing marked periureteral fibrosis with dense perivascular infiltration of lymphocytes and plasma cells (A, H&E) and obliterative phlebitis (B, Elastica Van Gienson stain).
Laboratory studies on admission were as follows: total serum bilirubin 10.4 mg/dL (normal range: 0.2-1.1 mg/dL), alkaline phosphatase 1 418 IU/L (115-359 IU/L), γ-glutamyl trasnpeptidase 558 IU/L (9-70 IU/L), glutamic-oxaloacetic transaminase 277 IU/L (12-32 IU/L), amylase 79 IU/L (45-155 IU/L), and elastase-1 1313 ng/mL (72-432 ng/mL). Tumor marker levels were within normal range. Although serum IgG level was 1380 mg/dL (870-1 700 mg/dL), serum IgG4 levels was elevated to 240 mg/dL (< 70 mg/dL). Antinuclear antibody and rheumatoid factor were negative. CT showed the lower common bile duct obstructed with focally swollen pancreatic head (Figure 3A) and soft tissue surrounding the aorta (Figure 3B). Magnetic resonance cholangiopancreatography (MRCP) showed the stricture of the lower common bile duct with dilatation of the upper common bile duct, but the pancreatic duct was not visualized. Percutaneous transhepatic biliary drainage was performed, and the common bile duct was completely obstructed in the lower portion (Figure 4A). Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated segmental narrowing of the main pancreatic duct in the pancreatic head (Figure 4B). Biopsy specimen taken from the major duodenal papilla during duodenoscopy revealed severe lymphoplasmacytic infiltration. Immunohistochemical studies using anti-IgG4 antibody (Binding Site, Birmingham, UK) with avidin-biotin-peroxidase complex (ABC) revealed abundant infiltration of IgG4-positive plasma cells in tissue specimens of both resected retroperitoneal mass (Figure 5A) and biopsied major duodenal papilla (Figure 5B). We diagnosed autoimmune pancreatitis and treated him with 30 mg predonisolone daily for 2 wk. The dose was tapered by 2.5-5 mg every 1-2 wk until a daily maintenance dose of 5 mg. After steroid therapy, findings on cholangiopancreatography and CT improved (Figure 4C), and the biliary drainage tube could be withdrawn. Serum IgG4 lowered to 83 mg/dL.
Figure 3.
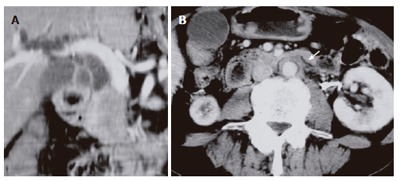
CT performed in our hospital showing the lower common bile duct obstructed with focally swollen pancreatic head (A) and soft tissue surrounding the aorta (arrow) (B).
Figure 4.
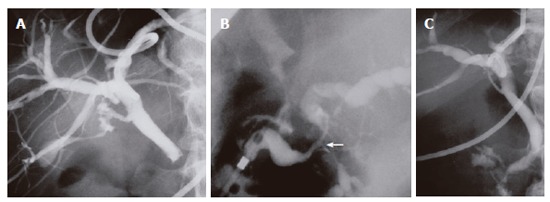
Percutaneous transhepatic cholangiography showing complete obstruction of the lower common bile duct (A) and resolution of the bile duct after steroid therapy (C). Endoscopic retrograde cholangiopancreatography demonstrating segmental narrowing of the main pancreatic duct in the pancreatic head (arrow) (B).
Figure 5.
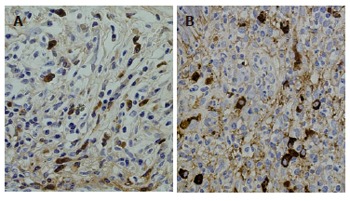
IgG4 immunostaining showing abundant infiltration of IgG4-positive plasma cells in tissue specimens of resected retroperitoneal mass (A) and biopsied major duodenal papilla (B).
DISCUSSION
We have previously reported 2 other cases of autoimmune pancreatitis associated with retroperitoneal fibrosis[8]. A total of 9 cases including the present case have been reported[8-12]. All were middle-aged or elderly males. Autoimmune pancreatitis was synchronous to retroperitoneal fibrosis in 4 cases, and metachronous to retroperitoneal fibrosis in 3 cases. Retroperitoneal fibrosis was metachronous to autoimmune pancreatitis in 2 cases. Steroid therapy was effective for both pancreatic and retroperitoneal lesions.
Histological findings characteristic to autoimmune pancreatitis are dense lymphoplasmacytic infiltration with fibrosis and obliterative phlebitis in the pancreas[3,4]. We have reported that this lymphoplasmacytic infiltration with fibrosis is observed in the peripancreatic retroperitoneal tissue, biliary tract, and salivary glands, as well as in the pancreas of patients with autoimmune pancreatitis. Furthermore, an abundant infiltration of IgG4-positive plasma cells was observed in the various organs of patients with autoimmune pancreatitis, including the peripancreatic retroperitoneal tissue, biliary tract, salivary glands, gastric mucosa, lymph nodes, and pancreas, but an abundant infiltration of IgG4-positive plasma cells was not detected in the organs of patients with other diseases[13,14]. Major duodenal papilla also showed fibrosis with abundant infiltration of IgG4-positive plasma cells[15]. We proposed the existence of a novel clinicopathological entity, an IgG4-related systemic disease characterized by a preponderance for elderly male, and extensive IgG4-positive plasma cell and T lymphocyte infiltration of organs[14]. We also suggest that autoimmune pancreatitis is not simply a pancreatitis but that, in fact, it is a pancreatic lesion reflecting an IgG4-related systemic disease, and that in some cases only organs other than the pancreas might be clinically involved[14].
In the present case, the retroperitoneal mass, that occurred 10 mo prior to autoimmune pancreatitis, histologically showed marked periureteral fibrosis with abundant infiltration of IgG4-positive plasma cells and lymphocytes and obliterative phlebitis, which were similar findings to those in the pancreas and peripancreatic retroperitoneal tissues of patients with autoimmune pancreatitis. These findings suggest a common pathophysiological mechanism for retroperitoneal fibrosis and autoimmune pancreatitis in this case. Although the exact etiology of retroperitoneal fibrosis remains unknown, some cases might be a retroperitoneal lesion of IgG4-related sclerosing disease.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Bi L
References
- 1.Koep L, Zuidema GD. The clinical significance of retroperitoneal fibrosis. Surgery. 1977;81:250–257. [PubMed] [Google Scholar]
- 2.Mitchinson MJ. Some clinical aspects of idiopathic retroperitoneal fibrosis. Br J Surg. 1972;59:58–60. doi: 10.1002/bjs.1800590115. [DOI] [PubMed] [Google Scholar]
- 3.Moody TE, Vaughan ED Jr. Steroids in the treatment of retroperitoneal fibrosis. J Urol. 1979;121:109–111. doi: 10.1016/s0022-5347(17)56683-5. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, Okamoto T, Nomura T, Joh T, Itoh M. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20–25. [PubMed] [Google Scholar]
- 7.Dooreck BS, Katz P, Barkin JS. Autoimmune pancreatitis in the spectrum of autoimmune exocrinopathy associated with sialoadenitis and anosmia. Pancreas. 2004;28:105–107. doi: 10.1097/00006676-200401000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Kamisawa T, Matsukawa M, Ohkawa M. Autoimmune pancreatitis associated with retroperitoneal fibrosis. JOP. 2005;6:260–263. [PubMed] [Google Scholar]
- 9.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 10.Fukukura Y, Fujiyoshi F, Nakamura F, Hamada H, Nakajo M. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR Am J Roentgenol. 2003;181:993–995. doi: 10.2214/ajr.181.4.1810993. [DOI] [PubMed] [Google Scholar]
- 11.Uchida K, Okazaki K, Asada M, Yazumi S, Ohana M, Chiba T, Inoue T. Case of chronic pancreatitis involving an autoimmune mechanism that extended to retroperitoneal fibrosis. Pancreas. 2003;26:92–94. doi: 10.1097/00006676-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Fukui T, Okazaki K, Yoshizawa H, Ohashi S, Tamaki H, Kawasaki K, Matsuura M, Asada M, Nakase H, Nakashima Y, et al. A case of autoimmune pancreatitis associated with sclerosing cholangitis, retroperitoneal fibrosis and Sjögren's syndrome. Pancreatology. 2005;5:86–91. doi: 10.1159/000084494. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 15.Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP and MRI findings in 9 patients with autoimmune pancreatitis. World J Gastroenterol. 2006;12:2919–2922. doi: 10.3748/wjg.v12.i18.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]


