Abstract
Breast metastases from gastric cancer are extremely rare. A case report of a 37-year-old female with breast inflammatory invasion and ascites is described. Breast biopsy revealed carcinomatous invasion of the lymphatics from adenocarcinoma cells with signet-ring features. Estrogen (ER) and progesterone receptors (PR) and c-erb-B2 were negative. Upper gastrointestinal endoscopy revealed a prepyloric ulcerative mass. Histopathologic examination of the lesion showed infiltration from a high-grade adenocarcinoma, identical with that of the breast. Immunostaining was positive for cytokeratins CK-7 and CK-20 and CEA and negative for ER and PR. Ascitic fluid cytology was positive for adenocarcinoma cells. Mammography was not diagnostic. Abdominal CT scanning revealed large ovarian masses suggestive of metastases (Krukenberg’s tumor). A cisplatin-based regimen was given but no objective response was observed. The patient died six months after initial diagnosis. A review of the literature is performed.
Keywords: Gastric adenocarcinoma, Signet-ring, Breast metastasis, Ovarian metastasis
INTRODUCTION
Breast metastases from extra-mammary neoplasms are extremely rare. The incidence ranges between 1%-2% in clinical and 1.7%-6.6% in autopsy series[1-2]. Less than 500 cases of secondary breast tumors have been reported so far in the English speaking literature[3]. The most common primary malignancies metastasizing to the breast are malignant melanoma, lung cancer, carcinoid tumors, ovarian cancer, renal cancer, gastrointestinal carcinomas and a variety of other primaries[2-4]. Herein, a case of a woman with gastric signet-ring adenocarcinoma presenting with breast metastasis is described.
CASE REPORT
A 37-year-old female presented to our department in September 2003 with abdominal distention and painful and edematous left breast. Her medical history was unremarkable except for three attempts of in vitro fertilization during the last two years. Physical examination revealed ascites and marked left breast inflammation. Neither discrete tumor nodules in the breast nor any enlarged axillary nor supraclavicular lymph nodes were palpable. Mammography showed diffuse increased density and skin thickening, without evidence of mass or microcalcifications. Abdominal ultrasonography established the presence of bilateral cystic adnexal masses with thickened walls and ascitic fluid. Abdominal CT confirmed the presence of large ovarian masses suggestive of metastases (Krukenberg’s tumor); however, there was no evidence of lung, liver or bone metastases. Serum tumor markers CEA, CA 15-3 and CA 19-9 were within normal limits and CA 125 was slightly elevated (63.6 U/L). Ascitic fluid cytology was found positive for adenocarcinoma. Core needle biopsy of the left breast revealed diffuse infiltration of the lymphatics in form of tumor emboli from neoplastic cells with signet-ring features (Figure 1). Immunohistochemical studies for estrogen receptor (ER) and progesterone receptor (PR) as well as for c-erb-B2 were negative. Upper gastrointestinal endoscopy demonstrated a large prepyloric ulcerative mass. Biopsy of the lesion revealed infiltration from a diffuse-type high-grade gastric adenocarcinoma with signet-ring features (Figure 2 and Figure 3). Malignant cells were positive for cytokeratins 7 and 20 (CK7, CK20) and CEA, and negative for ER and PR. Subsequent study of the breast biopsy revealed similar findings (positive staining for CK7, CK20 and CEA). The patient was treated with cisplatin-based chemotherapy; but she showed no objective response and died six months after the initial diagnosis.
Figure 1.
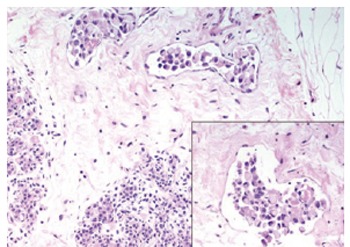
Breast biopsy showing breast lymphatic invasion from neoplastic cells with signet-ring features (Hematoxylin & eosin, 200 ×, 400 ×).
Figure 2.
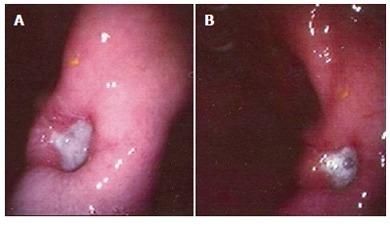
(A & B) Upper GI endoscopy of the patient, showing a large ulcerative lesion in the prepyloric region.
Figure 3.
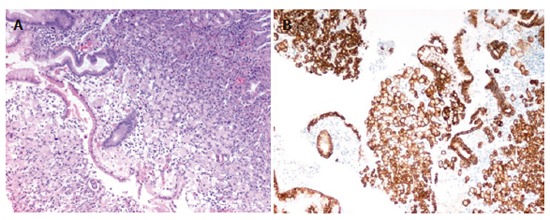
Gastric biopsy showing infiltration from neoplastic cells with signet-ring features (Hematoxylin & eosin, 100 ×). Right picture shows immunohistochemical stain for CK-7 (Hematoxylin & eosin, 100 ×).
DISCUSSION
Metastatic breast involvement from extra-mammary primaries is unusual. Most cases are related with extensive disease burden and carry an unfavorable prognosis[5]. Breast invasion is the initial manifestation of the disease in 25%-40% of the cases[2]. Mammographically, metastatic tumors in the breast present as well-circumscribed nodules without microcalcifications, mimicking benign breast lesions[5]. Calcifications may be seen in metastatic deposits from ovarian carcinoma with psammoma bodies[6]. The most common localization of metastatic lesions is the upper-outer quadrant of the left breast. Breast involvement is bilateral in 25% of the cases, and there is concomitant axillary lymph node enlargement in up to 15%[5]. The occurrence of multiple tumor nodules is unusual and diffuse involvement mimicking inflammatory breast carcinoma is rare[7,8]. The latter clinical manifestation, due to lymphatic invasion, is rapidly progressive, and may produce clinical signs of breast involvement before a firm metastatic nodule is formed, which is even more common in hematological malignancies (leukemias, lymphomas).
Breast metastases from gastric cancer are extremely rare. To date, only 25 cases have been reported in the English speaking literature and are mentioned in Table 1. Signet-ring features have been reported in 13 cases. From reports with available data, it seems that breast involvement usually follows the detection of gastric carcinoma. Breast metastasis as initial manifestation of a primary gastric tumor, as in our case, has been reported in 10 cases. In half of these cases breast involvement had the clinical appearance of inflammatory breast cancer.
Table 1.
Patients’ characteristics1 in reported cases of breast metastasis from gastric cancer
| n | Reference2 | Age | Histology | Presentation after initial diagnosis (mo) | Clinical presentation | Localization | Other metastatic sites |
| 1 | Reitmann 1908 | 33 | Scirhous | - | - | R+L3 | - |
| 2 | Kreibich 1909 | 65 | Scirhous | - | - | R | Skin |
| 3 | Mourier 1910 | 31 | Mucinous | - | - | L | Liver, pancreas |
| 4 | Stahr 1922 | 46 | Anaplastic | - | - | R+L | - |
| 5 | Dawson 1936 | 25 | Mucinous | - | - | R+L | Ovaries |
| 6 | Abrams 1949 | - | - | - | - | - | - |
| 7 | Sandison 1959 | 56 | Signet-ring cell | - | - | L | - |
| 8 | Hajdu 1972 | - | Adenocarcinoma | - | - | L | - |
| 9 | Silverman 1974 | - | Mucin-producing | Concomitant4 | - | - | - |
| 10 | Toombs 1977 | - | - | - | - | - | - |
| 11 | Satake 1980 | 39 | Signet-ring cell | Concomitant | Nodule | L | - |
| 12 | Togo 1980 | 70 | Signet-ring cell | Concomitant | Nodule | L | - |
| 13 | Nielsen 1981 | 59 | Mucinous | Concomitant | Nodule | L | - |
| 14 | Champault 1982 | 65 | Adenocarcinoma | Concomitant | Nodule | L | - |
| 15 | Kasuga 1986 | 48 | Signet-ring cell | 31 | Nodules | R+L | - |
| 16 | Alexander 1989 | 28 | Mucinous differentiation | Concomitant | Nodules | R+L | Lymph nodes |
| 17 | Hamby 1991 | 31 | Signet-ring cell | Concomitant | Nodule | R | Lymph nodes, ovaries |
| 18 | Cavazzini 1993 | - | Signet-ring cell | - | Inflammatory | - | - |
| 19 | Domanski 1996 | 48 | Signet-ring cell | Concomitant | Nodule | L upper-outer | L supraclavicular |
| 20 | de la Cruz Mera 1998 | 61 | Signet-ring cell | 13 | Nodule | L upper-outer | Pleura |
| 21 | Briest 1999 | 46 | Signet-ring cell | 2 | Inflammatory | R+L | Bilateral axillary nodes |
| 22 | Kwak 2000 | 41 | Signet-ring cell | Concomitant | Inflammatory | L | Ovaries5 |
| 23 | Kwak 2000 | 23 | Signet-ring cell | Concomitant | Inflammatory | R | Axillary nodes |
| 24 | Madan 2002 | 39 | Signet-ring cell | 3 | Nodule | R | Ovaries, peritoneum |
| 25 | Di Cosimo 2003 | 39 | Signet-ring cell | 1 | Nodules | R+L | Ovaries, peritoneum, skin |
| 26 | Boutis 2005 | 37 | Signet-ring cell | Concomitant | Inflammatory | L | Ovaries5, ascites |
1 Clinical information is mentioned in the table only if available by the authors.
References Reitmann 1908, Kreibich 1909, Mourier 1910, Stahr 1922, Dawson 1936, Satake 1980, Togo 1980 are included in Hamby 1991[2].
R: right; L: left.
Breast metastasis was the initial manifestation of disease.
No histological confirmation.
Breast metastases from gastric carcinoma differ from primary breast cancer in their histopathologic characteristics. Immunostaining is usually negative for estrogen and progesterone receptors[9,10] as well as for c-erbB-2[3]. GCDFP-15 (gross cystic disease fluid protein-15) is also absent[10]. There is no evidence of an in situ component[3,5] and a lack of desmoplastic response[3]. On the contrary, lymphatic tumor emboli and epithelial markers like CK7, CK20 and CEA are usually present[3,5].
In our case, the presence of signet-ring cells within breast lymphatics, the absence of mass or microcalcifications in the mammography, the lack of an in situ component and hormone receptor negativity strongly suggested an extra-mammary primary. It is of noted interest that Steinbrecher and Silverberg in 1976[11] first described primary signet-ring carcinoma of the breast, as a subtype of lobular carcinoma, with aggressive biological behavior and an increased tendency to metastasize in the abdomen and in the gastrointestinal tract compared to other histological subtypes[9,10]. Signet-ring carcinoma of the breast metastatic to the stomach has also been reported[12,13].
The presence of cystic ovarian masses suggested metastatic ovarian involvement (Krukenberg’s tumor). Unfortunately, the patient’s unfavorable clinical condition did not allow histological confirmation. Metastatic involvement of the breast and the ovaries is extremely rare and has been reported in only 5 cases so far. Selective invasion of hormone-dependent organs seems quite intriguing, especially in pre-menopausal women. Increased blood supply of the breast has been proposed as the mechanism for the increased incidence of breast metastasis in pre-menopausal women[14-15]. On the other hand, gastric cancer seems to have a more aggressive biologic behavior in younger age groups, where hormonal factors are implicated[16]. The appearance of breast metastases in men with gynecomastia supports the latter hypothesis[17]. In our patient, hormonal therapy for the induction of ovulation may have influenced the natural history of the disease[1]. However, no conclusions about the pathogenesis of the phenomenon can be drawn.
Metastatic disease of the breast is rare, and usually has atypical clinical and mammographic presentation. In cases of breast inflammation or lumps, biopsy should be performed, even in the presence of an extra-mammary neoplasm. Likewise, peculiar histological features from malignant breast lesions should be co-estimated with other clinical parameters toward the diagnosis of metastatic disease. Thus, avoidance of unnecessary radical surgical procedures and an adequate treatment is more probable to be given in these patients.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Bi L
References
- 1.Di Cosimo S, Ferretti G, Fazio N, Mandalà M, Curigliano G, Bosari S, Intra M, Latronico A, Goldhirsch A. Breast and ovarian metastatic localization of signet-ring cell gastric carcinoma. Ann Oncol. 2003;14:803–804. doi: 10.1093/annonc/mdg218. [DOI] [PubMed] [Google Scholar]
- 2.Hamby LS, McGrath PC, Cibull ML, Schwartz RW. Gastric carcinoma metastatic to the breast. J Surg Oncol. 1991;48:117–121. doi: 10.1002/jso.2930480209. [DOI] [PubMed] [Google Scholar]
- 3.Madan AK, Ternovits C, Huber SA, Pei LA, Jaffe BM. Gastrointestinal metastasis to the breast. Surgery. 2002;132:889–893. doi: 10.1067/msy.2002.126013. [DOI] [PubMed] [Google Scholar]
- 4.Vizcaíno I, Torregrosa A, Higueras V, Morote V, Cremades A, Torres V, Olmos S, Molins C. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol. 2001;11:1659–1665. doi: 10.1007/s003300000807. [DOI] [PubMed] [Google Scholar]
- 5.Alexander HR, Turnbull AD, Rosen PP. Isolated breast metastases from gastrointestinal carcinomas: report of two cases. J Surg Oncol. 1989;42:264–266. doi: 10.1002/jso.2930420412. [DOI] [PubMed] [Google Scholar]
- 6.Barai S, Kumar R, Haloi AK, Dhanpati G, Malhotra A. Bone scan demonstrating metastasis to the breast from an ovarian carcinoma and a review of the literature. Clin Nucl Med. 2004;29:167–170. doi: 10.1097/01.rlu.0000113854.98190.a9. [DOI] [PubMed] [Google Scholar]
- 7.Domanski HA. Metastases to the breast from extramammary neoplasms. A report of six cases with diagnosis by fine needle aspiration cytology. Acta Cytol. 1996;40:1293–1300. doi: 10.1159/000334024. [DOI] [PubMed] [Google Scholar]
- 8.Kwak JY, Kim EK, Oh KK. Radiologic findings of metastatic signet ring cell carcinoma to the breast from stomach. Yonsei Med J. 2000;41:669–672. doi: 10.3349/ymj.2000.41.5.669. [DOI] [PubMed] [Google Scholar]
- 9.Raju U, Ma CK, Shaw A. Signet ring variant of lobular carcinoma of the breast: a clinicopathologic and immunohistochemical study. Mod Pathol. 1993;6:516–520. [PubMed] [Google Scholar]
- 10.Merino MJ, Livolsi VA. Signet ring carcinoma of the female breast: a clinicopathologic analysis of 24 cases. Cancer. 1981;48:1830–1837. doi: 10.1002/1097-0142(19811015)48:8<1830::aid-cncr2820480821>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Steinbrecher JS, Silverberg SG. Signet-ring cell carcinoma of the breast. The mucinous variant of infiltrating lobular carcinoma. Cancer. 1976;37:828–840. doi: 10.1002/1097-0142(197602)37:2<828::aid-cncr2820370231>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Yim H, Jin YM, Shim C, Park HB. Gastric metastasis of mammary signet ring cell carcinoma--a differential diagnosis with primary gastric signet ring cell carcinoma. J Korean Med Sci. 1997;12:256–261. doi: 10.3346/jkms.1997.12.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park CH, Whang HS, Park HB. Bilateral signet-ring cell carcinoma of the breast: scintigraphic findings. Clin Nucl Med. 1996;21:115–117. doi: 10.1097/00003072-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Howarth CB, Caces JN, Pratt CB. Breast metastases in children with rhabdomyosarcoma. Cancer. 1980;46:2520–2524. doi: 10.1002/1097-0142(19801201)46:11<2520::aid-cncr2820461134>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Kwan WH, Choi PH, Li CK, Shing MK, Chik KW, Yuen P, Chow LT. Breast metastasis in adolescents with alveolar rhabdomyosarcoma of the extremities: report of two cases. Pediatr Hematol Oncol. 1996;13:277–285. doi: 10.3109/08880019609030828. [DOI] [PubMed] [Google Scholar]
- 16.Maeta M, Yamashiro H, Oka A, Tsujitani S, Ikeguchi M, Kaibara N. Gastric cancer in the young, with special reference to 14 pregnancy-associated cases: analysis based on 2,325 consecutive cases of gastric cancer. J Surg Oncol. 1995;58:191–195. doi: 10.1002/jso.2930580310. [DOI] [PubMed] [Google Scholar]
- 17.Cappabianca S, Grassi R, D'Alessandro P, Del Vecchio A, Maioli A, Donofrio V. Metastasis to the male breast from carcinoma of the urinary bladder. Br J Radiol. 2000;73:1326–1328. doi: 10.1259/bjr.73.876.11205680. [DOI] [PubMed] [Google Scholar]


