Abstract
AIM: To characterize the tumor suppressor gene p53 mutations and study the correlation of p53 gene mutation and the expression of P53 protein in cholangiocarcinoma.
METHODS: A total of 36 unselected, frozen samples of cholangiocarcinoma were collected. p53 gene status(exon 5-8) and P53 protein were examined by automated sequencing and immunohistochemical staining, combined with the clinical parameters of patients.
RESULTS: p53 gene mutations were found in 22 of 36 (61.1%) patients. Nineteen of 36 (52.8%) patients were positive for P53 protein expression. There were significant differences in extent of differentiation and invasion between the positive and negative expression of P53 protein. However, there were no significant differences in pathologic parameters between the mutations and non-mutations.
CONCLUSION: The alterations of the p53 gene evaluated by DNA sequence analysis is relatively accurate. Expression of P53 protein could not act as an independent index to estimate the prognosis of cholangiocarcinoma.
Keywords: Cholangiocarcinoma, p53 gene, Mutation, DNA sequence
INTRODUCTION
Tumor suppressor gene, besides oncogene, is involved in the development of cancer, which inhibits cell proliferation and formation of tumor. Normally tumor suppressor gene counteracts with oncogene to protect an organism against cancer. The p53 tumor suppressor gene is the most common mutated gene in human cancer[1-9], occurring in approximately 50% cancers[10-12]. Cholangiocarcinoma is among the most common malignant tumors. Mutation of p53 is one of the most frequently encountered genetic alterations in cholangiocarcinoma. p53 mutations play a central role in carcinogenesis of cholangiocarcinoma.
Alterations of the p53 gene can be evaluated by DNA sequencing analysis and immunohistochemistry(IHC). P53 protein overexpression is considered a poor prognosis of cholangiocarcinoma[13-17]. But IHC has shown controversial results over that of DNA sequencing analysis. We examined mutation status of p53 gene by automated sequencing and IHC in 36 cases of cholangiocarcinoma and studied the correlation of p53 gene mutation with clinical parameters and its clinical significance.
MATERIALS AND METHODS
Patients
A total of 36 unselected, frozen samples were obtained from patients with cholangiocarcinoma who had been treated by surgical resection from April 2000 to May 2005 in the Department of General Surgery of the First Affiliated Hospital of China Medical University and Hepatobiliary Surgery of the Affiliated Yantai Yuhuangding Hospital of Qingdao University Medical College. The types of cholangiocarcinoma included 18 cases of tubular adenocarcinomas, 9 cases of papillary adenocarcinomas, 4 cases of mucoid carcinomas and 5 cases of undifferentiated carcinoma. Among them, well-moderately differentiated was 25 and poorly differentiated was 11 cases respectively. There were 16 cases of T1 stage, 10 cases of T2 stage and 10 cases of T3 stage by the UICC standard. Lymph node metastasis was seen in 33 cases. Non- lymph node metastasis was seen in 3 cases. The patients contained 23 males and 13 females, with age ranging from 36 to 71 (median, 61.2) years. All of the samples were frozen at -80°C until DNA extraction and subjected to histological diagnosis by a pathologist.
DNA extraction
DNA was extracted from tissues using a QIAamp DNA Micro kit: QIA (Germany). Tissue samples weighing less than 10 mg were placed into a 1.5 mL microcentrifuge tube. Immediately 180 μL buffer ATL and 20 μL proteinase K were added and mixed by pulse-vortexing for 15 s. Then they were incubated at 56°C overnight. Two hundred microliter buffer AL and 200 μL ethanol (100%) were added and incubated for 5 min at room temperature. After that, all of the lysates were applied onto the QIAamp MinElute column. Five hundred microliter buffer AW1 and buffer AW2 were added. After centrifugation, 100 μL buffer AE was applied to get DNA. DNA quantity was determined by the ratio of A260/280.
Primer sequences and PCR amplification
Table 1 shows primer sequences used for p53 exons 5-8, which was synthesized by Hokkaido Bioscience Co. (Japan). PCR used a 20 μL reaction volume containing 1 unit of Hot start EXTaq DNA polymerase (Takara, Biochemical, Japan), 2 μL of 10 × EXTaq buffer, 2 μL of dNTP mixture and each primer (8 pmol each for reaction) and 1 μL of DNA template. The condition of the first PCR is as follows: 96°C for 3 min for denaturation, 40 cycles of 96°C for 30 s, 60°C for 30 s, 72°C for 30 s with a final elongation step of 4 min at 72°C. Water was used as a negative control. Five microliters of PCR product were analyzed on 1% TBE gel electrophoresis. Each sample was repeated three times.
Table 1.
Primers sequences of p53 gene exons 5-8
| Primers |
Sequences |
|
| Sense | Anti-sense | |
| Exon 5 | 5′-TGT TCA CTT GTG CCC TGA CT-3′ | 5′-CAG CCC TGT CGT CTC TCC AG-3′ |
| Exon 6 | 5′-ACA GGG CTG GTT GCC CAG GGT-3′ | 5′-CTC CCAGAG ACC CCA GTT GC-3′ |
| Exon 7 | 5′-GGT CTC CCC AAG GCG CAC TGG-3′ | 5′-AGG CTG GGG GCA CAG CAG GCC-3′ |
| Exon 8 | 5′-ATT TCC TTA CTG CCT CTT GC-3′ | 5′-AAG TGA ATC TGA GGC ATA AC-3 |
DNA sequencing
All of the PCR products were purified using Auto seq TMG-50 (Amersham Biochemical Company, USA). BigDye Terminator Cycle sequencing Ready Reaction (Perkin Elmer, USA) was used. The primers of sequencing were the same as PCR primers. But its concentration was one tenth of PCR primers. The condition is as follows: 95°C for 4 min, 95°C for 30 s, 55°C for 30 s, 72°C for 30 s for 40 cycles with a final step of 4 min at 72°C. Both sense and antisense chains were analyzed on an ABI prism 310 Genetic Analyzer (Perkin Elmer). Each sample was repeated three times.
Immunohistochemistry
Five-micron sections were dewaxed in xylene and rehydrated. Endogenous peroxidase was destroyed by a 15-min treatment in 30 mL/L hydrogen peroxide(H2O2) in phosphate-buffered saline (PBS) at room temperature. The sections were blocked with a combination of normal mouse serum and then incubated with anti-p53 protein(dilution 1:50, mouse anti-p53 protein by Boster Co.), followed by biotinylated-conjugated sheep anti-mouse IgG (Boster Co.). The complex was visualized by diaminobenzidine(Boster Co.). The specificity of the reaction was confirmed by use of negative control, blank control and substitution control, in which PBS substituted the secondary antibody biotinylated-conjugated sheep anti-mouse IgG.
Statistical analysis
The results were analyzed with χ2 test. P < 0.05 was taken as significant.
RESULTS
p53 gene mutations and expression of P53 protein in cholangiocarcinoma tissues
Positive band of exons 5-8 was found in all samples after PCR amplification (Figures 1 A-D). p53 gene mutations were detected in 22 of 36 patients (61.1%) by DNA sequencing. Among them, there were 7 cases of exon 5 mutations, which were located on 161,175 and 196 codons. All were transition (G:C/A:T). Six cases of exon 6 mutations were located on 209, 213 and 215 codons, of which 4 cases were of transition(G:C/A:T) and 2 cases of tranversion (G-T ). Three cases of exon 7 mutations were located on 248, 252 codons. Six cases of exon 8 mutations were located on 282, 287, 289, 306 codons, of which 4 cases were of transition (G:C/A:T) and 2 cases of tranversion (G-T) (Figures 2 A-C).
Figure 1.
p53 exons 5, 6, 7, 8 PCR product. L : 100 bp DNA ladder; 1-7: Samples. A: Exons 5; B: Exons 6; C: Exons 7; D: Exons 8.
Figure 2.
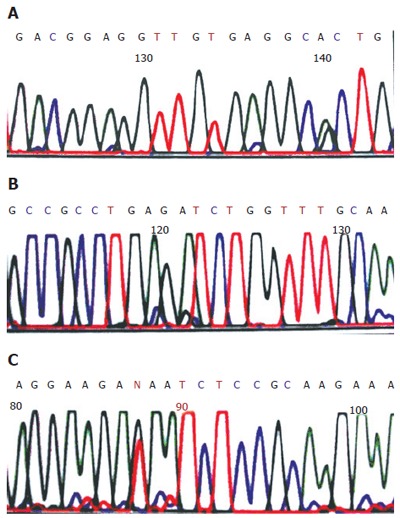
Tranversion of p53 exons 5, 6 , 8 in cholangiocarcinoma. A: Exons 5 (G: C/A: T); B: Exons 6 (G: C/A: T); C: Exons 8 (G→T).
Nineteen cases (52.8%) were positive for P53 protein. P53 protein localized in the nuclei of cholangic epithelial cells. Moreover, it was crisp and finely granular (Figure 3).
Figure 3.
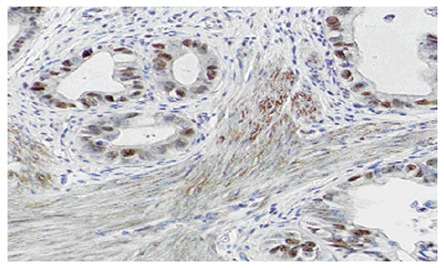
P53 protein was positive in cholangiocarcinoma (SABC × 400).
Correlation of p53 gene mutation and clinical parameters
There were significant differences in degree of differentiation and invasion between the positive and negative samples of P53 protein expression (P < 0.05, P < 0.05). However, there were no significant differences in age, gender, degree of differentiation and invasion, lymph node metastasis, stage between the mutations and non-mutations (P > 0.05) (Table 2).
Table 2.
Correlation of mutation status of p53 gene and P53 protein expression with pathologic parameters in cholangiocarcinoma
| Pathologic character | n |
p53 gene mutation |
P53 protein expression |
||
| Positive (n, %) | P vaule | Positive (n, %) | P vaule | ||
| Pathologic type | |||||
| Tubular | 18 | 11 (61.1) | 10 (55.6) | ||
| carcinoma | |||||
| Papillary | 9 | 6 (66.7) | 5 ( 55.6) | ||
| carcinoma | |||||
| Mucoid | 4 | 2 (50.0) | 2 (50.0) | ||
| carcinoma | |||||
| Non-differentiated | 5 | 3 (60.0) | > 0.05 | 2 (40.0) | > 0.05 |
| carcinoma | |||||
| Differentiation | |||||
| Well -Moderate | 25 | 13 (52.0) | 9 (36.0) | ||
| Poor | 11 | 9 (81.8) | > 0.05 | 10 (90.9) | < 0.05a |
| Invasion | |||||
| T1 | 16 | 7 (43.8) | 2 (12.5) | ||
| T2 | 10 | 8 (80.0) | 8 (80.0) | ||
| T3 | 10 | 7 (70.0) | > 0.05 | 9 (90.0) | < 0.05c |
| Lymph node metastasis | |||||
| N0 | 3 | 2 (66.7) | 0 (0) | ||
| N1 | 33 | 20 (60.6) | > 0.05 | 19 (57.5) | > 0.05 |
DISCUSSION
Cholangiocarcinoma is the second most common cancer of the hepatobiliary system. In recent years, the incidence and mortality of cholangiocarcinoma have been increasing in China[18,19]. In most patients, the disease is only diagnosed at a late stage. Patients with obstructive jaundice are frequently at the advanced stage of the disease, which is contraindicated for operation[20-26]. The development of molecular biology, the identification of molecular factors involved in cholangiocarcinoma carcinogenesis, and the elucidation of the mechanisms will significantly impact prevention, diagnosis, treatment and prognosis. p53 gene is located on the short arm of chromosome 17, which consists of 11 exons. There are four mutation hot-spots(132-143, 174-179, 236-248, 272-281) within the core domain (exon 5-8), which are the key sites of biological activity of P53 protein[27,28]. p53 gene is an important regulator factor of cell proliferation. It is related to cell cycles, DNA repair, cell differentiation and apoptosis. In the presence of DNA damage the expression of p53 is enhanced and induces G1 cell cycle arrest until DNA is repaired. If repair is insufficient, p53 gene promotes apoptotic cell death. However, p53 gene mutation cannot block cell proliferation. Through cooperation with inactivation of tumor suppressor genes and activation of oncogenes, cells transform into malignant ones and become a tumor. p53 tumor suppressor gene is the most common mutated gene in human cancer and is frequently seen in cholangiocarcinoma. Recent studies have found that positive expression of P53 protein is related to invasion and lymph node metastasis in cholangiocarcinoma[15,16]. p53 gene mutation could act as an index to estimate the prognosis of cholangiocarcinoma.
We examined mutation status of p53 gene exons 5-8 by automated sequencing in 36 cases of cholangiocarcinoma. We found p53 gene mutations in 22 of 36 (61.1 %) patients. Nineteen of 36 (52.8%) patients were positive for P53 protein expression. There were significant differences in extent of differentiation and invasion between positive and negative expression of P53 protein. However, there were no significant differences in age, gender, extent of differentiation, invasion, lymph node metastasis, and stage between the mutations and non-mutations.
Wild-type p53 (non-mutated) has a short half-life of about 20 min. Mutant P53 protein has a greater stability with half-life prolonged up to 1.4-7 h. It can be detected by IHC method. But the use of different P53 antibodies and methods can result in a marked difference in the degree of overexpression, and varying levels of overexpression may also be noted in the same tumor specimen. IHC has been shown to have discordancy rates of 30%-35% compared with DNA sequencing. Thus, the determination of p53 overexpression is not an accurate measure of p53 function. Although DNA sequence analysis is a cumbersome, time-consuming and difficult method on archived material, it could provide a more accurate means of detecting p53 mutations. Thus, we think p53 gene mutation could not act as an independent index to estimate the prognosis of cholangiocarcinoma.
Footnotes
Supported by a grant from Outstanding Youth Foundation of Shandong Province, China, No. 2005BS02008
S- Editor Pan BR L- Editor Zhu LH E- Editor Ma WH
References
- 1.Forslund A, Kressner U, Lönnroth C, Andersson M, Lindmark G, Lundholm K. P53 mutations in colorectal cancer assessed in both genomic DNA and cDNA as compared to the presence of p53 LOH. Int J Oncol. 2002;21:409–415. [PubMed] [Google Scholar]
- 2.Gasco M, Shami S, Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4:70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 4.Caldeira S, Filotico R, Accardi R, Zehbe I, Franceschi S, Tommasino M. p53 mutations are common in human papillomavirus type 38-positive non-melanoma skin cancers. Cancer Lett. 2004;209:119–124. doi: 10.1016/j.canlet.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Baroni TE, Wang T, Qian H, Dearth LR, Truong LN, Zeng J, Denes AE, Chen SW, Brachmann RK. A global suppressor motif for p53 cancer mutants. Proc Natl Acad Sci USA. 2004;101:4930–4935. doi: 10.1073/pnas.0401162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JT, Wang JS, Jiann BP, Yu CC, Tsai JY, Huang JK, Wu TT. Correlation of p53 protein accumulation and Bcl-2 overexpression with histopathological features in prostatic cancer. J Formos Med Assoc. 2005;104:864–867. [PubMed] [Google Scholar]
- 7.El Far MA, Atwa MA, Yahya RS, El Basuni MA. Evaluation of serum levels of p53 in hepatocellular carcinoma in Egypt. Clin Chem Lab Med. 2006;44:653–656. doi: 10.1515/CCLM.2006.091. [DOI] [PubMed] [Google Scholar]
- 8.Saetta AA. K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder cancer. J Surg Oncol. 2006;93:644–649. doi: 10.1002/jso.20532. [DOI] [PubMed] [Google Scholar]
- 9.Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192–198. doi: 10.3748/wjg.v12.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harms K, Nozell S, Chen X. The common and distinct target genes of the p53 family transcription factors. Cell Mol Life Sci. 2004;61:822–842. doi: 10.1007/s00018-003-3304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir MM, Dar NA, Gochhait S, Zargar SA, Ahangar AG, Bamezai RN. p53 mutation profile of squamous cell carcinomas of the esophagus in Kashmir (India): a high-incidence area. Int J Cancer. 2005;116:62–68. doi: 10.1002/ijc.21002. [DOI] [PubMed] [Google Scholar]
- 12.Moreno M, Pimentel F, Gazdar AF, Wistuba II, Miquel JF. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder carcinoma. Ann Hepatol. 2005;4:192–199. [PubMed] [Google Scholar]
- 13.Wang X, Zhang J, Chen J. [Result of p53, ki-67 protein expression in cholangiocarcinoma with in situ hybridization and immunohistochemistry methods] Zhongguo Yixue Kexueyuan Xuebao. 2000;22:57–60. [PubMed] [Google Scholar]
- 14.Ahrendt SA, Rashid A, Chow JT, Eisenberger CF, Pitt HA, Sidransky D. p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J Hepatobiliary Pancreat Surg. 2000;7:426–431. doi: 10.1007/s005340070039. [DOI] [PubMed] [Google Scholar]
- 15.Horie S, Endo K, Kawasaki H, Terada T. Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma: relationship with p53 overexpression, Ki-67 labeling, and clinicopathological features. Virchows Arch. 2000;437:25–30. doi: 10.1007/s004280000201. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, Imaoka S, Tsujimoto M, Matsuura N. Expression and clinical significance of the G1-S modulators in intrahepatic cholangiocellular carcinoma. Oncology. 2001;60:242–251. doi: 10.1159/000055325. [DOI] [PubMed] [Google Scholar]
- 17.Attallah AM, Abdel-Aziz MM, El-Sayed AM, Tabll AA. Detection of serum p53 protein in patients with different gastrointestinal cancers. Cancer Detect Prev. 2003;27:127–131. doi: 10.1016/s0361-090x(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 18.Jan YY, Yeh CN, Yeh TS, Chen TC. Prognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:1779–1784. doi: 10.3748/wjg.v11.i12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XF, Zhou XT, Zou SQ. An analysis of 680 cases of cholangiocarcinoma from 8 hospitals. Hepatobiliary Pancreat Dis Int. 2005;4:585–588. [PubMed] [Google Scholar]
- 20.Reddy SB, Patel T. Current approaches to the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2006;8:30–37. doi: 10.1007/s11894-006-0061-1. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:294–299. doi: 10.1097/01.mog.0000218967.60633.64. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield SD, Barakat O, Charnley RM, Jaques BC, O'Suilleabhain CB, Atherton PJ, Manas D. Management of hilar cholangiocarcinoma in the North of England: pathology, treatment, and outcome. World J Gastroenterol. 2005;11:7625–7630. doi: 10.3748/wjg.v11.i48.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 25.Sakata J, Shirai Y, Wakai T, Nomura T, Sakata E, Hatakeyama K. Catheter tract implantation metastases associated with percutaneous biliary drainage for extrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:7024–7027. doi: 10.3748/wjg.v11.i44.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubert C, Sempoux C, Berquin A, Deprez P, Jamar F, Gigot JF. Bile duct carcinoid tumors: an uncommon disease but with a good prognosis. Hepatogastroenterology. 2005;52:1042–1047. [PubMed] [Google Scholar]
- 27.Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Hum Mutat. 2003;21:217–228. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- 28.Khan SA, Taylor-Robinson SD, Carmichael PL, Habib N, Lemoine NR, Thomas HC. Analysis of p53 mutations for a mutational signature in human intrahepatic cholangiocarcinoma. Int J Oncol. 2006;28:1269–1277. [PubMed] [Google Scholar]


