Abstract
Sarcoidosis is a systemic disease of unknown aetiology that may affect any organ in the body. The gastrointestinal tract however is only rarely affected outside the liver. Symptoms may be non-specific. Irritable bowel syndrome (IBS) is a common diagnosis. The recognition of IBS is aided by the use of the Rome II criteria - in the absence of organic disease. We describe the first case of a patient with gastric sarcoidosis who presented with IBS symptoms but subsequently responded to immunosuppressive therapy.
Keywords: Gastrointestinal, Sarcoidosis, Irritable bowel syndrome
INTRODUCTION
Sarcoidosis is a systemic disease of unknown aetiology characterised by the presence of non-caseating epithelioid granulomas. The lungs, eyes and skin are the most commonly affected organs[1]. Liver involvement is well recognised but symptomatic involvement of the gastrointestinal (GI) tract occurs in less than 1% of cases[2-6]. Presenting GI symptoms in order of frequency are abdominal pain, haematemesis, nausea and vomiting and weight loss[1,2,4-6]. This observation is only based on the case reports and case series that have been reported[2,4-6]. Irritable bowel syndrome (IBS) is a common diagnosis and the symptom based Rome II criteria may be used to support the diagnosis[7]. However it is essential when investigating patients who present with IBS type symptoms that organic disease is excluded[7]. We describe the first case of a patient with gastric sarcoidosis who presented with IBS symptoms but subsequently responded to immunosuppressive therapy.
CASE REPORT
A 27 year old Caucasian man presented to the respiratory physicians with chest discomfort and ‘flu-like symptoms in 1983. A plain radiograph of the chest showed bilateral hilar lymphadenopathy and left middle zone infiltrates. Gallium scanning and a positive Kveim test confirmed the diagnosis of pulmonary sarcoidosis. Remission was achieved with oral steroids. Apart from a chronic irritable cough, he had no recurrence of his respiratory symptoms over the following 22 years. His previous annual serum angiotensin converting enzyme (ACE) and a recent high resolution computed tomography (CT) of the chest was normal.
He presented to a gastroenterology department with a 6-mo history of upper/central abdominal pain relieved on defaecation. There was a change in frequency of bowel movement - he now opened his bowels less than three times per week (prior to this presentation he opened his bowels daily). The stool varied between normal and pellet-like. He described bloating, distension and tenesmus. There was no weight loss or any other sinister symptoms. His symptoms fulfilled the Rome II criteria for constipation predominant IBS. His inflammatory markers (ESR and CRP) were normal. Further investigations including an upper GI endoscopy, colonoscopy, ultrasound and CT of the abdomen were all normal. The diagnosis of IBS was suggested but the patient still had concerns about the cause of his symptoms. With the consent of the clinicians from the initial centre the patient sought a second opinion. Although he had no pulmonary symptoms, (apart from a chronic cough) we wondered if his IBS symptoms could be due to gastrointestinal sarcoidosis. Further investigations in our centre showed a raised serum ACE level of 67 IU/mL (Range 18-55 IU/mL). In addition, he also had a positive IgG antigliadin antibody and a serum IgA of 0.52 G/L (0.8-4.0 G/L) but negative IgA antigliadin and anti-endomysial antibody (EMA). We performed a gastroscopy, which showed antral erythema (Figure 1) and a polypoid lesion at the gastro-oesophageal junction (Figure 2). Duodenal biopsies were normal. Biopsy of the antrum revealed granulomas consistent with sarcoidosis (Figure 3). Biopsy of the polypoid lesion showed chronic inflammatory cell infiltrate only (Figure 4). His CLO test was negative and there was no evidence of H pylori or alcohol-acid fast bacilli in any of the biopsy specimens.
Figure 1.
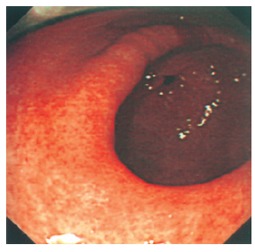
Gastric antrum viewed from mid body of stomach showing widespread erythematous changes consistent with gastritis.
Figure 2.
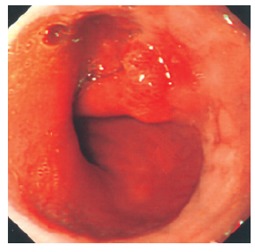
Gastro-oesophageal junction showing polypoid lesion.
Figure 3.
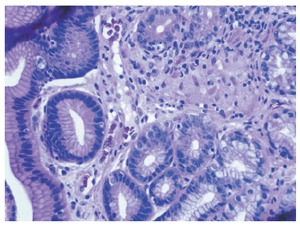
Gastric antral biopsy (HE x 400 magnification) showing surface epithelium on the left of the image. The glands are displaced by a central granuloma formed from loosely aggregated epithelioid histiocytes, with a few surrounding lymphocytes.
Figure 4.
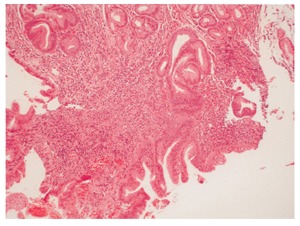
Biopsy of polypoid lesion at gastro-oesophageal junction showing glandular tissue with active chronic inflammation and reactive epithlelial changes (HE x 200 magnification).
Oral prednisolone initially achieved some symptomatic benefit, but he responded subjectively to a short course of intravenous hydrocortisone. During this time his serum ACE normalised and he was discharged on 30 mg prednisolone. He has subsequently had an octreotide scan showing no activity in the stomach (whilst on prednisolone) and was started on oral azathioprine with progressive resolution of his GI symptoms.
DISCUSSION
Sarcoidosis of the GI tract is rare but when involved the stomach is the commonest site. Gastric granulomas have coincidentally been reported in up to 10% of patients with pulmonary sarcoidosis[8,9]. However, whether the presence of gastrointestinal sarcoid accounts for GI symptoms is controversial. Previous investigators have described endoscopic and radiological findings of peptic ulceration, inflammation and gastric outlet obstruc-tion[2,3,5,10-12]. Diagnosis is achieved by biopsy confirming the presence of granulomas without evidence of any other granulomatous disorders. One series of 67 patients with sarcoidosis described 7.5% of patients as having abdominal pains at diagnosis. Further evaluation revealed 15% had liver involvement (biopsy proven) and 36% had luminal GI disease; reflux oesophagitis (21%), IBS (10%) and inflammatory bowel disease (4.5%)[13]. Both reflux and irritable bowel syndrome symptoms are frequent amongst the general population, however, this study suggests that the presence of such symptoms may require investigation. The optimal approach would be upper GI endoscopy and biopsy of any abnormal areas.
This is the first reported case of a patient who fulfilled the RomeIIcriteria for IBS but was subsequently found to have gastric sarcoidosis. Although we performed antigliadin antibodies (and EMA) to exclude coeliac disease-IgG antigliadin lacks specificity and has previously been reported in association with gastrointestinal sarcoidosis[14,15]. If we had not performed antral biopsies having seen macroscopic evidence of inflammation, then the diagnosis may have been missed. It is possible that in our case both gastric sarcoid and IBS co-exist. We believe that this is unlikely given the symptomatic and serological response to immunosuppression[16,17]. In addition, a normal octreotide scan whilst on prednisolone (with no areas of increased uptake) also suggests remission and further corroborates our view[16].
In conclusion, we report the first case of gastric sarcoid presenting with RomeIIcriteria for IBS. Our case suggests that patients with suspected functional GI symptoms in the presence of pulmonary sarcoid, may have sarcoidosis of the GI tract. It is imperative that the endoscopist is aware of this possibility and performs a biopsy of any macroscopically abnormal areas. By taking this approach patients with symptomatic GI sarcoid may benefit from early diagnosis and symptomatic relief with prednisolone or immunosuppressive therapy.
Footnotes
S- Editor Wang J L- Editor Zhao JB E- Editor Bai SH
References
- 1.Mitchell D. Sarcoidosis. Medicine. 1998;26:43–49. [Google Scholar]
- 2.Fireman Z, Sternberg A, Yarchovsky Y, Abu-Much S, Coscas D, Topilsky M, Fireman E, Groisman GM. Multiple antral ulcers in gastric sarcoid. J Clin Gastroenterol. 1997;24:97–99. doi: 10.1097/00004836-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Moretti AM, Sallustio G, Attimonelli R, Arezzo C, Neri D, Losito A. [Gastric localization of sarcoidosis] Recenti Prog Med. 1993;84:750–755. [PubMed] [Google Scholar]
- 4.Sprague R, Harper P, McClain S, Trainer T, Beeken W. Disseminated gastrointestinal sarcoidosis. Case report and review of the literature. Gastroenterology. 1984;87:421–425. [PubMed] [Google Scholar]
- 5.Chinitz MA, Brandt LJ, Frank MS, Frager D, Sablay L. Symptomatic sarcoidosis of the stomach. Dig Dis Sci. 1985;30:682–688. doi: 10.1007/BF01308419. [DOI] [PubMed] [Google Scholar]
- 6.Tinker MA, Viswanathan B, Laufer H, Margolis IB. Acute appendicitis and pernicious anemia as complications of gastrointestinal sarcoidosis. Am J Gastroenterol. 1984;79:868–872. [PubMed] [Google Scholar]
- 7.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer ED. Note on silent sarcoidosis of the gastric mucosa. J Lab Clin Med. 1958;52:231–234. [PubMed] [Google Scholar]
- 9.Bratkovskiĭ MV, Magalif NI. [Sarcoidosis of the stomach] Probl Tuberk. 1990:28–30. [PubMed] [Google Scholar]
- 10.Farman J, Ramirez G, Rybak B, Lebwohl O, Semrad C, Rotterdam H. Gastric sarcoidosis. Abdom Imaging. 1997;22:248–252. doi: 10.1007/s002619900182. [DOI] [PubMed] [Google Scholar]
- 11.Hogg SG. Case report: gastric sarcoid simulating linitis plastica--a 5-year follow-up study. Clin Radiol. 1991;44:277–278. doi: 10.1016/s0009-9260(05)80198-9. [DOI] [PubMed] [Google Scholar]
- 12.Roth D, West B, Madison J, Cooper D. Gastric carcinoma in a patient with sarcoidosis of the gastrointestinal tract. Am J Gastroenterol. 1994;89:1589–1591. [PubMed] [Google Scholar]
- 13.Reynolds HY. Sarcoidosis: impact of other illnesses on the presentation and management of multi-organ disease. Lung. 2002;180:281–299. doi: 10.1007/s004080000104. [DOI] [PubMed] [Google Scholar]
- 14.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 15.McCormick PA, Feighery C, Dolan C, O'Farrelly C, Kelliher P, Graeme-Cook F, Finch A, Ward K, Fitzgerald MX, O'Donoghue DP. Altered gastrointestinal immune response in sarcoidosis. Gut. 1988;29:1628–1631. doi: 10.1136/gut.29.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhagen PM, Krenning EP, Reubi JC, Kwekkeboom DJ, Bakker WH, Mulder AH, Laissue I, Hoogstede HC, Lamberts SW. Somatostatin analogue scintigraphy in granulomatous diseases. Eur J Nucl Med. 1994;21:497–502. doi: 10.1007/BF00173035. [DOI] [PubMed] [Google Scholar]
- 17.Sachar DB, Rochester J. The myth of gastrointestinal sarcoidosis: a case of guilt by association. Inflamm Bowel Dis. 2004;10:441–443. doi: 10.1097/00054725-200407000-00019. [DOI] [PubMed] [Google Scholar]


