Abstract
AIM: To investigate the dynamic functional and ultrastructural changes of gastric parietal cells induced by water immersion-restraint stress (WRS) in rats.
METHODS: WRS model of Sprague-Dawley (SD) rats was established. Fifty-six male SD rats were randomly divided into control group, stress group and post-stress group. The stress group was divided into 1, 2 and 4 h stress subgroups. The post-stress group was divided into 24, 48 and 72 h subgroups. The pH value of gastric juice, ulcer index (UI) of gastric mucosa and H+, K+-ATPase activity of gastric parietal cells were measured. Ultrastructural change of parietal cells was observed under transmission electron microscope (TEM).
RESULTS: The pH value of gastric juice decreased time-dependently in stress group and increased in post-stress group. The H+, K+-ATPase activity of gastric parietal cells and the UI of gastric mucosa increased time-dependently in stress group and decreased in post-stress group. Compared to control group, the pH value decreased remarkably (P = 0.0001), the UI and H+, K+-ATPase activity increased significantly (P = 0.0001, P = 0.0174) in 4 h stress subgroup. UI was positively related with stress time (r = 0.9876, P < 0.01) but negatively with pH value (r = -0.8724, P < 0.05). The parietal cells became active in stress group, especially in 4 h stress subgroup, in which plenty of intracellular canalicular and mitochondria were observed under TEM. In post-stress group, the parietal cells recovered to resting state.
CONCOUSION: The acid secretion of parietal cells is consistent with their ultrastructural changes during the development and healing of stress ulcer induced by WRS and the degree of gastric mucosal lesions, suggesting gastric acid play an important role in the development of stress ulcer and is closely related with the recovery of gastric mucosal lesions induced by WRS.
Keywords: Stress ulcer; Gastric parietal cells; Ultrastructure; H+, K+-ATPase activity; Sprague-Dawley rat
INTRODUCTION
Stress ulcer is a common complication of severe brain trauma and psychosis in clinical practice. Critically ill patients are at increased risk of developing stress-related gastric mucosal lesions and gastrointestinal bleeding[1,2]. But the pathogenesis of stress-related gastric mucosal lesions is not fully elucidated. The development and healing of stress ulceration are a complex process affected by many factors. It had been shown that gastric acid plays an important role in the development of stress-induced ulcer and is the most common endogenous destructive factor[3-5].
It is reported that water immersion-restraint stress (WRS) may lead to changes of gastric acid secretion. Some studies indicate that WRS increases acid secretion[6,7], while other studies showed that gastric acid secretion decrease during WRS[8,9]. Gastric acid secretion is intimately related with the ultrastructure of parietal cells, but the dynamic ultrastructure change of parietal cells during WRS has not been reported yet.
Moreover, changes of parietal cells ultrastructure and gastric acid secretion during the healing of stress-induced ulceration are poorly understood so far. The purpose of the present study was to investigate the dynamic ultrastructural and functional of gastric parietal cells induced by WRS in order to demonstrate the role of gastric acid in the development of stress ulcer and in the healing of gastric mucosal lesions.
MATERIALS AND METHODS
Animals and water immersion-restraint stress model
Fifty-six male SD rats, weighing 180-220 g were purchased from Xipuer-Bikai Experimental Animal Co. LTD, Shanghai, China. The animals were fasted for 24 h with free access to water and deprived of water 1 h before the experiment. Animals were randomly divided into control group (n = 8), stress group (n = 24) and post-stress group (n = 24), and exposed to various periods of stress and immersed in 19 ± 1°C water as previously described[10]. Rat in control group were sacrified after lightly anesthetized with ether. Rats in stress group were killed after 1, 2 and 4 h of WRS respectively. Rats in post-stress group were killed after 24, 48 and 72 h of WRS respectively[11].
Measurement of gastric juice pH
All rats were anesthetized with pentobarbital sodium (30 mg/kg ip). A midline laparotomy was performed and the stomach was exposed. The pylorus and cardia were ligated, and the pH electrode of pH/mV meter (59 002-00, Cole-Parmer Instrument Company, USA) was inserted into stomach lumen to measure the pH value of gastric juice[12].
Evaluation of gastric mucosal ulcer index (UI)
The ulcer index (UI) of gastric mucosal lesions was evaluated by the score system reported by Nie SN et al[13]. Briefly, after measurement of the pH value of gastric juice, the stomach was opened along the greater curvature and rinsed with 0.1 mol/L ice-cold phosphate-buffered saline (PBS). The stomach was then examined with a 10 × magnifier to observe erosions and make scores as 1-5: 1: small round hemorrhagic erosion, 2: hemorrhagic erosion < 1 mm, Grade 3: hemorrhagic erosion = 1-2 mm, 4: hemorrhagic erosion = 2-3 mm, 5: hemorrhagic erosion > 4 mm. The score was multiplied by 2 when the width of erosion was larger than 1 mm.
H+, K+-ATPase assay
The oxyntic mucosa was scraped with glass slides, immediately frozen in liquid nitrogen and stored at -80 °C for H+, K+-ATPase assay, at which time they were thawed at 4°C. Microsomal gastric H+, K+-ATPase was prepared after homogenization of gastric mucosa and sucrose-Ficoll gradient centrifugation[14,15]. H+, K+-ATPase was assessed with a kit ( Nanjing Jiancheng Biotechnology Company), and protein was quantified using a UV/spectrophotometer (PE)[16]. The gastric mucosal H+, K+-ATPase activity was expressed as μmol Pi/mg protein/h (U/mg prot).
Electron microscopy
Tissue specimens of the oxyntic mucosa for electron microscopy, were immediately fixed in a solution containing 1% glutaraldehyde and 3% formaldehyde buffered at pH 7.4 with 0.1 mol/L sodium PBS for 2 h at 4°C. Secondary fixation was carried out in 1% osmium tetroxide solution buffered at pH 7.4 with 0.1 mol/L sodium phosphate buffer for 1 h at 4°C. The specimens were then dehydrated in graded acetone solutions and embedded in epoxy resin (Epon 812). Ultrathin sections (60-80 nm) were made for double staining with aqueous uranyl acetate and lead citrate, and observed under transmission electron microscope[17]. Parietal cells were recognized by the presence of secretory canaliculi and/or tubulovesicles. Secreting parietal cells were defined as cells having secretory canaliculi. Non-secreting cells had tubulovesicles but no secretory canaliculi[18].
Statistical analysis
All data were expressed as mean ± SD. The statistical differences between different groups were analyzed by Student’s t-test. The relationship between two variants was analyzed by linearly relevant analysis. P < 0.05 was considered significant.
RESULTS
Change of gastric juice pH value
The pH value of gastric juice was 2.56 ± 0.14 in normal group and gradually declined after 1, 2 and 4 h of WRS in rats (2.26 ± 0.04, 1.81 ± 0.25 and 1.31 ± 0.24, respectively) (Figure 1). The pH value was significantly lower (2.0-fold) in stress 4 h group than that in normal group (P < 0.001). The pH values of gastric juice after 24, 48, 72 h of WRS in post-stress groups was significantly higher (1.9-fold, 1.8-fold and 1.8-fold, respectively) than that in stress group (P < 0.001), but there was no statistical significance compared to normal group (P > 0.05, Figure 1). These data demonstrated that the pH value of gastric juice decreased time-dependently in stress group and increased in post-stress group.
Figure 1.
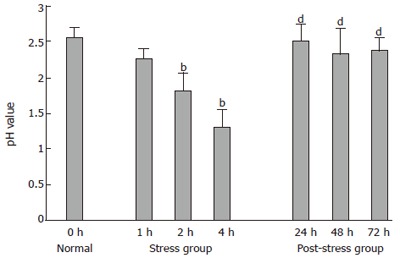
pH value of gastric juice in all groups. bP < 0.01 vs normal group; dP < 0.01 vs stress 4 h group.
Change of gastric mucosal ulcer index (UI)
There was no gastric mucosal lesion in normal group. Scattered spot or lineal erosions, hemorrhage and ulcers were observed in oxyntic mucosa in stress group. The gastric mucosal UI gradually increased after 1, 2 and 4 h of WRS in rats (9.5 ± 2.98, 22.5 ± 3.16 and 35.0 ± 3.93) (Figure 2).
Figure 2.
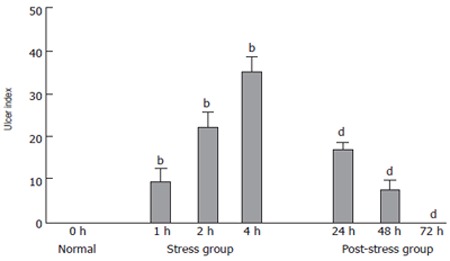
Ulcer index (UI) of gastric mucosa in all groups. bP < 0.01 vs normal group; dP < 0.01 vs stress 4 h group.
The UI decreased significantly (2.0-fold and 4.7-fold) after 24 and 48 h of WRS in post-stress group compared to that in stress group (P < 0.001). No lesion occurred in the oxyntic mucosa of rats 72 h after WRS (Figure 2). These data demonstrated that the UI of gastric mucosa increased time-dependently in stress group and decreased in post-stress group. UI was positively related with stress time (r = 0.9876, P < 0.01) but negatively with pH value (r = -0.8724, P < 0.05).
H+, K+-ATPase activity
The H+, K+-ATPase activity of gastric parietal cells was 7.48 ± 0.59 U/mg prot in normal group and gradually increased after 1, 2 and 4 h of WRS in rats (7.72 ± 0.41, 8.28 ± 0.52 and 9.50 ± 1.63 U/mg prot, respectively) (Figure 3). The H+, K+-ATPase activity was significantly higher (1.1-fold and 1.3-fold) in stress group than in normal group (P < 0.05). The H+, K+-ATPase activity decreased in post-stress group, but there was no statistical significance (P > 0.05). The H+, K+-ATPase activity was significantly lower (1.3-fold, respectively) after 48 and 72 h of WRS in post stress group than in stress group (P < 0.05, Figure 3). These data demonstrated that the H+, K+-ATPase activity of gastric parietal cells increased time-dependently in stress group and decreased in post-stress group.
Figure 3.
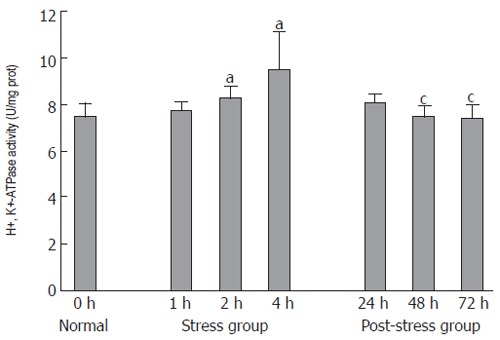
H+, K+-ATPase activity of parietal cells in all groups. aP < 0.05 vs normal group; cP < 0.05 vs stress 4 h group.
Ultrastructural change of parietal cells
As shown in Figure 4A, gastric parietal cells in normal group presented resting state, a large number of tubulovesicles and few intracellular canaliculi lined with rare microvilli could be found in cytoplasm. As shown in Figure 4B, parietal cells in 1 h WRS subgroup presented pre-secreting state, intracellular canaliculi lined with short microvilli were observed, and plenty of vesicles in the cytoplasm were located around the canaliculi and accumulated on the apical plasma membrane, and the secretory surface was more elaborate than that of the non-secreting cells. As shown in Figure 4C, parietal cells in 2 h WRS subgroup presented secreting state, where decreased vesicles and increased intracellular canaliculi lined with elongated microvilli were observed. The remaining vesicles were in the region adjacent to the surface and frequently fused with the plasma membrane. In addition, there were abundant mitochondria in the cytoplasm. As shown in Figure 4D, parietal cells in 4 h WRS subgroup presented active secreting state. The cytoplasm was almost devoid of vesicles, whereas the intracellular canalicular lumen contained numerous elongated microvilli. The cytoplasm in these secreting cells was extremely dense owing to the close packing of mitochondria. As shown in Figure 4E-G, in 24, 48 and 72 h WRS subgroups, the parietal cells returned to resting state, abundant tubulovesicles and few canaliculi were observed in the cytoplasm in post-stress group after 24, 48 and 72 h WRS. Besides, Swollen mitochondria, vacuolar degeneration and crista-fragmentation were observed in some parietal cells. Concentrated karyotin, roughness of nuclear envelopes and dilated perinuclear space were observed in other cells, which might be due to the damaged parietal cells induced by stress.
Figure 4.
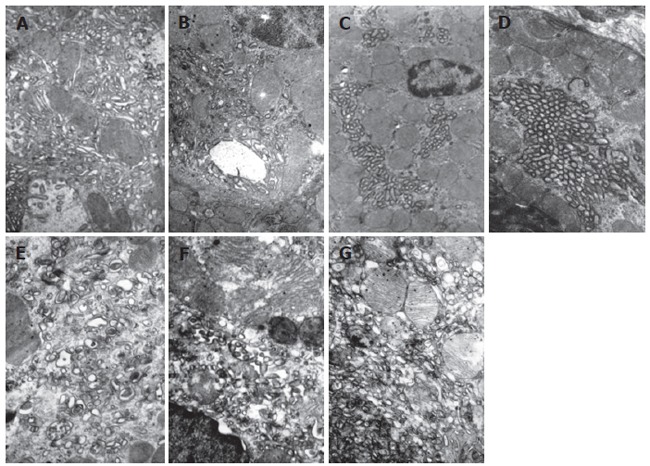
Ultrastructural change of parietal cells. A: Gastric parietal cells presented resting state, abundant tubulovesicles and few intracellular canaliculi lined with rare microvilli could be found in the cytoplasm in normal group (×12 000); B: Parietal cells presented pre-secreting state, intracellular canaliculi lined with short microvilli were observed, plenty of vesicles were located around the canaliculi and accumulated on the apical plasma membrane in 1 h WRS group (×12 000); C: Parietal cells presented secreting state with decreased vesicles and increased intracellular canaliculi lined with elongated microvilli in 2 h WRS group; D: Parietal cells presented active secreting state and cytoplasm was almost devoid of vesicles and intracellular canalicular lumen contained numerous elongated microvilli in 4 h WRS group (×12 000); E - G: at 24, 48 and 72 h at the end of WRS for 4 h, Parietal cells returned to resting state with abundant tubulovesicles and few canaliculi in cytoplasm in post-stress group swollen mitochondria, vacuolar degeneration, crista-fragmentation, concentrated karyotin, roughness of nuclear envelopes and dilated perinuclear space were observed ( ×15 000).
DISCUSSION
Stress ulcers are the superficial mucosal lesions located predominantly in fundus of the stomach. They occur mainly in severe trauma and sepsis as well as in acute severe psychological stress. It has become apparent that the physiological stress of critical illness increases the risk of developing stress-related mucosal injury and gastrointestinal bleeding. The pathogenesis of stress ulcers is not completely understood. however, recent studies indicate that the origin may be multifactorial, and there is an imbalance between protective and destructive factors[19-21]. Major destructive factors include acid, pepsin, bile, reperfusion injury and free oxygen radicals[22-24]. Protective factors include adequate mucosal blood flow, mucus-bicarbonate layer, epithelial cell renewal and prostaglandins[25-27]. It is generally agreed that mucosal ischemia is the major inciting event in the pathogenesis of acute stress ulceration of the stomach and that the presence of luminal acid and pepsin is required in the development of overt ulceration[28]. Back-diffusion of acid occurs in the absence of overt disruption of the mucosal barrier, and is closely related to the formation of ulcers.
In this study, we found that gastric mucosal lesions gradually aggravated after 1, 2 and 4 h of WRS in rats. Gastric mucosa lesions progressed to apparent erosions and hemorrhage in 4 h WRS group. There positive relevance between the ulcer index UI was significantly related with the exposure time to WRS. We also found that gastric juice pH value decreased and H+, K+-ATPase activity of parietal cells gradually increased after 1, 2 and 4 h of WRS, indicating that gastric acid secretion increases with the exposure time to WRS. The pH value was significantly lower (2.0-fold) and the H+, K+-ATPase activity was significantly higher (1.3-fold) in stress group than in normal group. UI was negatively related with pH value. These results strongly suggest that gastric acid can mediate acute gastric mucosal lesions induced by WRS.
The present study assessed the dynamic ultrastructural change of gastric parietal cells in rats induced by WRS. Gastric parietal cells also known as oxyntic cells, have two systems in the cytoplasm under transmission electron microscope: intracellular secretory canaliculus system and tubulovesicular system[29]. In normal group, abundant tubulovesicles and few intracellular canaliculi lined with rare microvilli were found in cytoplasm and the gastric parietal cells presented resting state. Tubulovesicles gradually decreased while intracellular canaliculi lined with elongated microvilli gradually increased after exposed to WRS for 1, 2 and 4 h. In 4 h WRS group, parietal cell cytoplasm was almost devoid of vesicles and the intracellular canalicular lumen contained numerous elongated microvilli. Our observation showed that parietal cells presented a series of states from resting, pre-secreting, secreting to active secreting after 0, 1, 2 and 4 h of WRS. Our study also showed that gastric mucosal lesions induced by WRS paralleled to time-course changes of ultrastructure of gastric parietal cells. These results indicate that WRS can time-dependently activate parietal cells and induce gastric acid secretion.
The mechanism of the recovery of gastric mucosa after stress is not fully understood. the healing of stress ulcer is a very complex process affected by multi-factors. It has been reported that the mucosal integrity and repair involved enhancement of gastric mucosal blood flow and increased mucosal cell proliferation mediated by epidermal growth factor (EGF) and transforming growth factor alpha (TGFα)[30,31]. The importance of gastric acid in the process of restructuring gastric mucosa after WRS has not yet been evaluated. In present study, we found that the injured gastric mucosa was gradually restructured after the stress was removed and no lesion was observed after 72 h at the end of WRS for 4 h. We also found the pH value increased significantly at 24, 48 and 72 h of WRS. These results strongly demonstrate that the decrease of gastric acid is beneficial for restructuring injured gastric mucosa.
In the present study, we found that the parietal cells returned to resting state while swollen mitochondria, vacuolar degeneration, crista-fragmentation, concentrated karyotin, roughness of nuclear envelopes and dilated perinuclear space were observed after 24, 48 and 72 h of WRS, suggesting that damaged parietal cells induced by WRS.
The major founding of our study is that the ultrastructural changes of parietal cells is consisitent with the change of H+, K+-ATPase activity in the development and healing of stress ulceration induced by WRS. Moreover, the recovery of ultrastructural and functional changes of parietal cells was earlier than the restructuring of damaged gastric mucosa after WRS, suggesting that the ultrastructural and functional changes of parietal cells may be an important pathophysiologic mechanism in restructuring stress ulceration.
In conclusion, ultrastructural and functional changes of gastric parietal cells are induced by WRS in rats. Increased gastric acid secretion induced by WRS is consistent with the ultrastructural changes of parietal cells and is significantly correlated with the degree of gastric mucosa lesions. Gastric acid not only plays an important role in the development of stress ulcer, but also is closely related to the recovery of gastric mucosal lesions induced by WRS.
Footnotes
Supported by the Key Project of Military Medicine during the 10th five-year Plan period, PLA, China, No. 01Z059
S- Editor Guo SY L- Editor Wang XL E- Editor Bi L
References
- 1.Yang YX, Lewis JD. Prevention and treatment of stress ulcers in critically ill patients. Semin Gastrointest Dis. 2003;14:11–19. [PubMed] [Google Scholar]
- 2.Steinberg KP. Stress-related mucosal disease in the critically ill patient: risk factors and strategies to prevent stress-related bleeding in the intensive care unit. Crit Care Med. 2002;30:S362–S364. doi: 10.1097/00003246-200206001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Peterson WL. The role of acid in upper gastrointestinal haemorrhage due to ulcer and stress-related mucosal damage. Aliment Pharmacol Ther. 1995;9 Suppl 1:43–46. doi: 10.1111/j.1365-2036.1995.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin RA. Utility of proton pump inhibitors in the treatment of gastrointestinal hemorrhage. Conn Med. 2004;68:435–438. [PubMed] [Google Scholar]
- 5.van Rensburg CJ, Hartmann M, Thorpe A, Venter L, Theron I, Lühmann R, Wurst W. Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003;98:2635–2641. doi: 10.1111/j.1572-0241.2003.08723.x. [DOI] [PubMed] [Google Scholar]
- 6.Al Moutaery AR. Protective effect of ketoconazole against experimentally induced gastric ulcers in rats. Res Commun Mol Pathol Pharmacol. 2003;113-114:5–23. [PubMed] [Google Scholar]
- 7.Brzozowski T, Konturek PC, Konturek SJ, Kwiecień S, Drozdowicz D, Bielanski W, Pajdo R, Ptak A, Nikiforuk A, Pawlik WW, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39–51. doi: 10.1016/j.regpep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Hayase M, Takeuchi K. Gastric acid secretion and lesion formation in rats under water-immersion stress. Dig Dis Sci. 1986;31:166–171. doi: 10.1007/BF01300703. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Cabano CA. Luminal acid in water-immersion stress and the antiulcer effect of axetazolamide in the rat gastric mucosa. Acta Gastroenterol Latinoam. 1999;29:25–31. [PubMed] [Google Scholar]
- 10.Konturek PC, Brzozowski T, Ptak A, Kania J, Kwiecień S, Hahn EG, Konturek SJ. Nitric oxide releasing aspirin protects the gastric mucosa against stress and promotes healing of stress-induced gastric mucosal damage: role of heat shock protein 70. Digestion. 2002;66:160–172. doi: 10.1159/000066762. [DOI] [PubMed] [Google Scholar]
- 11.Li YM, Zhou XP, Li ZS, Peng GY, Fang DC. Changes of function and ultrastructure of gastric parietal cells during restructure of stress ulcer in rats. Zhonghua Jizhen Yixue Zazhi. 2004;13:169–171. [Google Scholar]
- 12.Li YM, Li ZS, Zhou XP, Xie ZF, Zhan XB, Tu ZX, Peng GY, Fang DC. Effect of acid inhibitor on the function and ultrastructure of gastric parietal cells in rats under stress. Jiefangjun Yixue Zazhi. 2002;27:1078–1080. [Google Scholar]
- 13.Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ, Xu BH, Huang F, Lin X, Sun DY, Sun HC, et al. Role of TFF in healing of stress-induced gastric lesions. World J Gastroenterol. 2003;9:1772–1776. doi: 10.3748/wjg.v9.i8.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabon EC, Bin Im W, Sachs G. Preparation of gastric H+,K+-ATPase. Methods Enzymol. 1988;157:649–654. doi: 10.1016/0076-6879(88)57112-4. [DOI] [PubMed] [Google Scholar]
- 15.Beil W, Sewing KF, Busche R, Wagner S. Helicobacter pylori augments the acid inhibitory effect of omeprazole on parietal cells and gastric H(+)/K(+)-ATPase. Gut. 2001;48:157–162. doi: 10.1136/gut.48.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XQ, Zhang YF, Liu TT, Hsiao KJ, Zhang JM, Gu XF, Bao KR, Yu LH, Wang MX. Correlation of ATP7B genotype with phenotype in Chinese patients with Wilson disease. World J Gastroenterol. 2004;10:590–593. doi: 10.3748/wjg.v10.i4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Yasunaga Y, Nishibayashi H, Yamamori K, Higashimoto Y, Matsuzawa Y. Morphological and functional restoration of parietal cells in helicobacter pylori associated enlarged fold gastritis after eradication. Gut. 1999;45:653–661. doi: 10.1136/gut.45.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito S, Schofield GC. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974;63:364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther. 2004;26:197–213. doi: 10.1016/s0149-2918(04)90019-7. [DOI] [PubMed] [Google Scholar]
- 20.Fennerty MB. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: rationale for the therapeutic benefits of acid suppression. Crit Care Med. 2002;30:S351–S355. doi: 10.1097/00003246-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 21.Duerksen DR. Stress-related mucosal disease in critically ill patients. Best Pract Res Clin Gastroenterol. 2003;17:327–344. doi: 10.1016/s1521-6918(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 22.Andriulli A, Annese V, Caruso N, Pilotto A, Accadia L, Niro AG, Quitadamo M, Merla A, Fiorella S, Leandro G. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol. 2005;100:207–219. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 23.Daley RJ, Rebuck JA, Welage LS, Rogers FB. Prevention of stress ulceration: current trends in critical care. Crit Care Med. 2004;32:2008–2013. doi: 10.1097/01.ccm.0000142398.73762.20. [DOI] [PubMed] [Google Scholar]
- 24.Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39–50. [PubMed] [Google Scholar]
- 25.Qui BS, Mei QB, Liu L, Tchou-Wong KM. Effects of nitric oxide on gastric ulceration induced by nicotine and cold-restraint stress. World J Gastroenterol. 2004;10:594–597. doi: 10.3748/wjg.v10.i4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cryer B. Mucosal defense and repair. Role of prostaglandins in the stomach and duodenum. Gastroenterol Clin North Am. 2001;30:877–94, v-vi. doi: 10.1016/s0889-8553(05)70218-1. [DOI] [PubMed] [Google Scholar]
- 27.Komoike Y, Nakashima M, Nakagiri A, Takeuchi K. Prostaglandin E receptor EP1 subtype but not prostacyclin IP receptor involved in mucosal blood flow response of mouse stomachs following barrier disruption. Digestion. 2003;67:186–194. doi: 10.1159/000072057. [DOI] [PubMed] [Google Scholar]
- 28.Brzozowski T, Konturek PC, Konturek SJ, Drozdowicz D, Kwiecieñ S, Pajdo R, Bielanski W, Hahn EG. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia-reperfusion into gastric ulcers. Eur J Pharmacol. 2000;398:147–158. doi: 10.1016/s0014-2999(00)00287-9. [DOI] [PubMed] [Google Scholar]
- 29.Ogata T, Yamasaki Y. Morphological studies on the translocation of tubulovesicular system toward the intracellular canaliculus during stimulation of the gastric parietal cell. Microsc Res Tech. 2000;48:282–292. doi: 10.1002/(SICI)1097-0029(20000301)48:5<282::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 30.Konturek PC, Brzozowski T, Konturek SJ, Taut A, Sliwowski Z, Stachura J, Hahn EG. Activation of genes for growth factors and cyclooxygenases in rat gastric mucosa during recovery from stress damage. Eur J Pharmacol. 1998;342:55–65. doi: 10.1016/s0014-2999(97)01408-8. [DOI] [PubMed] [Google Scholar]
- 31.Vongthavaravat V, Mesiya S, Saymeh L, Xia Y, Ward A, Harty RF. Transforming growth factor alpha-mediated gastroprotection against stress ulceration in the rat: involvement of capsaicin-sensitive sensory neurons. Life Sci. 2003;72:1803–1811. doi: 10.1016/s0024-3205(02)02504-3. [DOI] [PubMed] [Google Scholar]


