Abstract
AIM: To investigate the relationship between ulcerative colitis (UC) clinical activity index (CAI) and circulating levels of IL-1ra, IL-10, IL-6 and IL-18.
METHODS: Blood levels of IL-1ra, IL-10, IL-6 and IL-18 were measured in 31 patients with active UC, the mean CAI was 11.1, ranging from 5-25; and 12 healthy individuals as controls. Patients were given granulocyte and monocyte adsorptive apheresis (GMA) with Adacolumn. Leucocytes which bear the FcγR and complement receptors were adsorbed to the column leucocytapheresis carriers. Each patient could receive up to 11 GMA sessions over 8 wk.
RESULTS: We found strong correlations between CAI and IL-10 (r = 0.827, P < 0.001), IL-6 (r = 0.785, P < 0.001) and IL-18 (r = 0.791, P < 0.001). IL-1ra was not correlated with CAI. Following GMA therapy, 24 of the 31 patients achieved remission and the levels of all 4 cytokines fell to the levels in healthy controls. Further, blood levels of IL-1ra and IL-10 increased at the column outflow and inflow at 60 min suggesting release from leucocytes that adhered to the carriers.
CONCLUSION: Elevated blood levels of IL-6 and IL-18 together with peripheral blood granulocytes and monocytes/macrophages in patients with active UC show activative behaviour and increased survival time can be pro-inflammatory and the targets of GMA therapy.
Keywords: Interleukin-1 receptor antagonist, Inter-leukin-6, Interleukin-10, Interleukin-18, Ulcerative colitis
INTRODUCTION
Factors which initiate and perpetuate ulcerative colitis (UC) are not well understood. However, the condition is often associated with elevated circulating granulocytes and monocytes/macrophages[1-5] that show activative behaviour and increased survival time[6-10]. Another feature of active UC is the increased generation and activities of inflammatory cytokines which can initiate and perpetuate the disease[11-15]. Furthermore, recent clinical and basic research data indicate that the clinical relapse of UC is mediated by an unchecked influx of granulocytes and monocytes/macrophages into the mucosal tissue[16-19]. The level of neutrophils in the mucosa was quantitatively related to the severity of intestinal inflammation and relapse both in UC and Crohn’s disease[16].
Granulocytes and monocytes/macrophages are major sources of inflammatory cytokines[20-24] and therefore, it is assumed that high and activated peripheral blood levels of these leucocytes can be a major pro-inflammatory condition. Factors believed to contribute to the elevated neutrophil counts and increased survival time include inflammatory cytokines[8] and paradoxically corticosteroids[9] that are given to most patients with severe active UC. The circulating levels of anti- and pro-inflammatory cytokines are likely to follow leucocyte levels or activities and are potentially related to disease activity.
However, leucocytes produce proinflammatory as well as anti-inflammatory cytokines. Thus, interleukin-6 (IL-6) produced by neutrophils and monocytes/macrophages (as well as other cell types including endothelial cells) is reported to play a major role in neutrophil and monocyte recruitment during inflammation[15]. Similarly, IL-18 produced by monocytes/macrophages is thought to be a potent inducer of interferon-γ (IFN-γ) production, hence contributing to the induction of Th1 responses[23,24] A similar scenario may be described for anti-inflammatory cytokines. Thus, elevated level of IL-1 receptor antagonist (IL-1ra) is considered to reflect a natural compensatory mechanism to counter the activities of the pro-inflammatory cytokine IL-1β in inflammatory diseases[25-26]. Similarly, during inflammation, production of IL-10 increases and potentially can switch off the production of pro-inflammatory cytokines including IFN-γ, IL-1β, IL-6, IL-8, IL-12 and tissue necrosis factor (TNF-α).
Therefore, there are cytokines which can initiate and perpetuate inflammation and others which mitigate the inflammation. Accordingly, cytokines currently represent the best validated therapeutic targets[25-30]. With this in mind, we studied the circulating levels of 2 major anti-inflammatory and 2 major pro-inflammatory cytokines during active UC and during remission. IL-10 and IL-1ra were chosen as two known anti-inflammatory cytokines while IL-6 and IL-18 were considered to represent two typical pro-inflammatory cytokines. Since peripheral blood granulocytes and monocytes/macrophages are thought to represent major sources of both pro- and anti-inflammatory cytokines[1-6], we targeted these cells using granulocyte and monocyte/macrophage adsorptive apheresis (GMA) with Adacolumn[4,5].
MATERIALS AND METHODS
Study objectives
First, to see the relationship between CAI and circulating levels of the two major anti-inflammatory cytokines IL-10, IL-1ra and two pro-inflammatory cytokines IL-6 and IL-18 during active UC. Second, to investigate the effects of adsorptive granulocytes and monocyte/macrophage apheresis (GMA) on the levels of these cytokines and CAI. This was to provide an insight into the mechanisms of clinical efficacy of GMA with Adacolumn presently and hitherto reported[4,5,31]. Third, to identify predictors of response to GMA.
Patients
The demography of the 31 patients included in this study is presented in the Table 1. There were 17 males and 14 females, mean age 34.3 years, range 14-58 years. CAI was determined according to Rachmilewitz[32]. UC was severe in 11, moderate in 16 and mild in 4. All patients had been treated with conventional medications including 5-aminosalicylic acid (5-ASA) and prednisolone prior to entry. Eighteen of the 31 patients were steroid dependent , 9 were steroid refractory and 4 were steroid naive. The conventional medication was continued and the steroid dose was tapered when CAI score decreased to remission level (4 or less). Twelve healthy individuals of the same age range served as a control group.
Table 1.
Demography of 31 patients with active ulcerative colitis who were investigated in this study
| Backgroung | Measurement | |
| Male/Female | 17/14 | |
| Age, yr (range) | 34.5-15.3 | (14-58) |
| Age at first attack (yr) | 29.1-11.9 | (12-54) |
| Duration of UC (yr) | 5.9-7.2 | (0.5-26) |
| Number of relapses | 4.3-3.9 | (1-15) |
| Classification of severity | ||
| Mild | 4 | |
| Moderate | 16 | |
| Severe | 11 | |
| Mean (range) clinical activity index (CAI)[33] | 11.1(5-25) | |
| Total colitis | 24 | |
| Left sided colitis | 7 | |
Granulocyte and monocyte adsorptive apheresis
GMA was performed with Adacolumn precisely as described previously[4,5,20,31,33]. In Japan, GMA with Adacolumn has been approved by the Ministry of Health for public funding to treat patients with active UC. Adacolumns were purchased from Japan Immunoresearch Laboratories (Takasaki, Japan). Each patient could receive up to 11 GMA sessions. Patients with CAI > 12 received 2 sessions/wk in the first 3 wk and then one session/wk. During wk 6, CAI scores were determined and GMA was continued until patients had received up to 11 sessions. This therapy was added to the patients’ ongoing treatment following a relapse or worsening UC symptoms. No additional conventional medication was given during this study.
Assessment of response to therapy
CAI equal or greater than 5 was the entry criteria. Clinical remission was defined as a CAI decrease to 4 or less and mucosal vascular patterns had become visible (at least partly). When CAI had fallen, but was still above 4, the patient was considered to have improved.
Measurement of cytokines
Peripheral blood was sampled directly via a venepuncture in the antecubital vein and also from the Adacolumn inflow at the start of GMA and then at the end of the 60 minutes GMA session from the column inflow and outflow (blood which was returning to patients). IL-1ra, IL-10, IL-6 and IL-18 were measured using enzyme linked immuno-sorbent assays (ELISA). Human IL-1ra ELISA kit was from R&D systems (Minneapolis, USA); the ELISA kit for IL-10 was from Biosource Europe SA (Nivelles, Belgium); human IL-18 ELISA Kit was from MBL (Medical and Biological Laboratories, Nagoya Japan) and the ELISA kit for IL-6 was from FUJIREBIO Inc. (Tokyo). All assays were done at an special research laboratory (SRL) in blind manner and the data were processed by an individual who was unaware of the subjects’ clinical conditions or the purpose of the study.
Ethics
As indicated above, Adacolumn is officially approved in Japan for the treatment of patients with active UC. However, when blood samples were required for research other than for routine clinical laboratory, patients were informed of the extra volume of blood to be taken and the purpose of the blood sample. All patients we consulted agreed to donate blood samples for assaying cytokines. They were advised that refusal to donate blood will not jeopardize their future treatment and care.
Statistical analysis
The amounts of IL-1ra, IL-10, IL-6 and IL-18 are presented as individual observations and comparisons are made using the Stat View Software. Statistical tests were the Mann-Whitney U test, Scheffe’s test, the Turkey-Kramer test or the Spearman’s rank correlation test, indicated in the figure legends. A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied when appropriate.
RESULTS
Blood levels of anti- and pro-inflammatory cytokines during active UC
The relationship between CAI and blood levels of IL-10 and IL-1ra in 31 patients is presented in Figure 1. The Spearman’s rank correlation coefficient (Rs) showed a strong positive relationship between blood levels of IL-10 and CAI, while no correlation was found between blood levels of IL-1ra and CAI. Similarly, the majority of non-responders to GMA (albeit a few) had low blood levels of IL-1ra, while no obvious difference between responders and non-responders to GMA was seen for circulating IL-10 levels. Likewise, Figure 2 shows the relationship between blood levels of IL-6, IL-18 and CAI in the 31 patients. Also for these two pro-inflammatory cytokines, Rs shows a strong positive correlation with CAI. Most of the few non-responders to GMA had high blood levels of IL-6 and IL-18.
Figure 1.
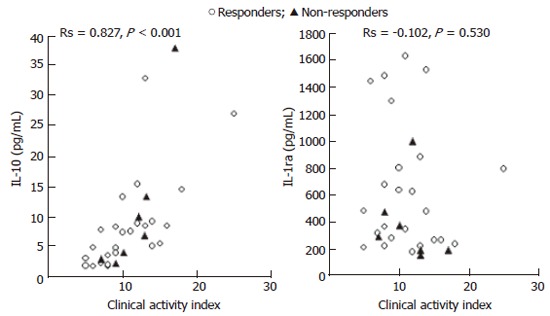
The relationship between blood levels of IL-10 and IL-1ra as measured in serum samples and the ulcerative colitis CAI in 31 patients with UC.
Figure 2.
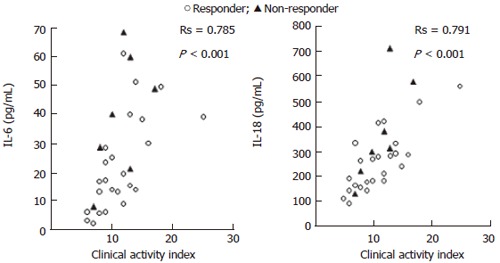
The relationship between blood levels of IL-6 and IL-18 and CAI in 31 patients with UC.
Blood levels of anti- and pro-inflammatory cytokines during GMA
Figure 3 shows the changes in blood levels of IL-10 and IL-1ra at the Adacolumn inflow (peripheral blood) at the start of GMA therapy and outflow at the end of the 60-min GMA and again peripheral blood just after the completion of GMA in the 31 patients. The dotted lines represent patients who did not respond to GMA during the observation period. This figure shows that blood levels of both IL-10 and IL-1ra are significantly higher in the column outflow relative to inflow and this accounts for the elevated levels of these cytokines in the peripheral blood during the GMA procedure. We found strong correlations between ulcerative clinical activity index (CAI) and circulating levels of IL-10, IL-6 and IL-18. In contrast, IL-1ra was high in most patients during active UC, but did not show correlation with CAI. However, the circulating levels of all 4 cytokines were low during remission, similar to the levels in healthy controls. It is inferred that the increments in IL-1ra and IL-10 in the column outflow are due to release from monocytes/macrophages and neutrophils that adhere to the Adacolumn leucocytapheresis carriers, with additional contributions from activated Th2 and B lymphocytes (IL-10). Blood levels of IL-6 and IL-18 did not change significantly during the 60 min GMA procedure. The likely explanation could be inactivation within the column by a strong flux of proteases and active oxygen derivatives released by adherent leucocytes.
Figure 3.
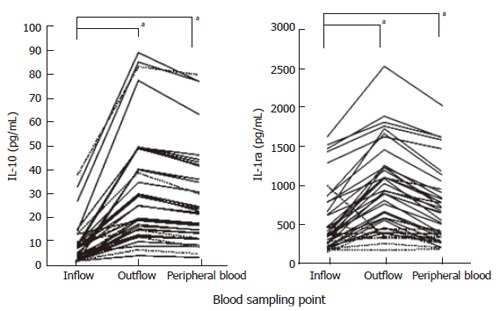
Blood levels of IL-10 and IL-1ra at the Adacolumn inflow (peripheral blood) at the start of GMA therapy and outflow at the end of the 60 min GMA and again peripheral blood just after the completion of 60 minutes GMA therapy in 31 patients with active UC. aP < 0.05 vs inflow by Turkey-Kramer test.
Blood levels of anti- and pro-inflammatory cytokines during active UC and remission
Figure 4 shows blood levels of IL-10 and IL-1ra in patients with UC during active disease (n = 31), when in remission (n = 12) and in age matched controls (n = 12). The results show wide variations in the blood levels of these two anti-inflammatory cytokines during active disease, but for both cytokines, the levels are very low during remission, similar to the levels in healthy controls. The 12 patients in remission were from the 31 patients with active disease who achieved remission following GMA (wk 12).
Figure 4.
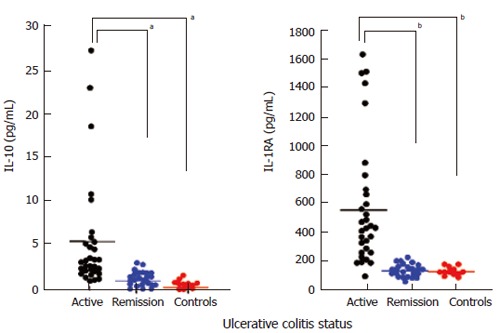
Blood levels of IL-10 and IL-1ra in patients with UC during active disease (n = 31), when in remission (n = 24) and in age matched controls (n = 12). aP < 0.05, bP < 0.01 vs active by Scheffe’s test.
Blood levels of IL-6 and IL-18 are presented in Figure 5. The data for these two pro-inflammatory cytokines are mirror images of the data in Figure 4 for two anti-inflammatory cytokines. The results show wide variations in the blood levels of these two cytokines during active disease, but for both cytokines, the levels are very low during remission, similar to the levels in healthy controls.
Figure 5.
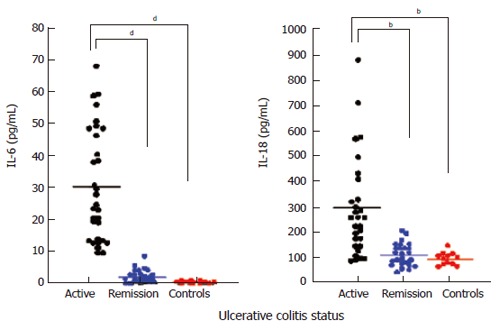
Blood levels of IL-6 and IL-18 (measured in serum samples) in patients with UC during active disease (n = 31), when in remission (n = 24) and in age matched controls (n = 12). bP < 0.01, dP < 0.001 vs active by Scheffe’s test.
Blood levels of anti- and pro-inflammatory cytokines during GMA course
Figure 6 shows the changes in blood levels of IL-10 and IL-1ra in one typical case during an 8 wk GMA course. Two prominent features can be seen: higher levels during active disease; in the column outflow and decline with the number of GMA sessions. This is a case who responded to GMA and achieved remission during this therapy.
Figure 6.
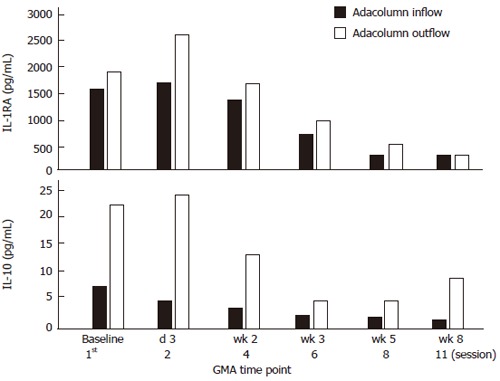
Changes in blood levels of IL-10 and IL-1ra in one typical case during an 8 wk GMA course (up to 11 GMA sessions).
Figure 7 shows IL-6 and IL-18 levels in one typical case during an 8 wk GMA course. Also for these two pro-inflammatory cytokines, the levels were high during active disease and fell rapidly (IL-6) during the course of GMA therapy. However, unlike IL-10 and IL-1ra, the levels of these two cytokines did not increase in the column outflow.
Figure 7.
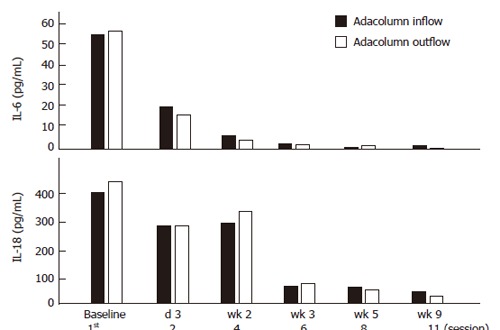
Similar to the data shown in Figure 6, here we see changes in blood levels of IL-6 and IL-18 were seen in one typical case during an 8 wk GMA course.
Changes in CAI associated with GMA
Patients received up to 11 GMA sessions to deplete the activated and excessive peripheral blood granulocytes and monocytes/macrophages. There was a significant (P < 0.05) fall in CAI at wk 6 and after 11 sessions, 24 of the 31 patients were in clinical remission (CAI ≤ 4). The mean CAI value at entry was 11.1, ranging 5-25. The corresponding values one week after the last treatment session were 2.4, ranging 0-15.
Safety of GMA
All patients completed their GMA therapy, compliance was excellent. GMA was safe, no severe side effects were seen during or after the GMA procedures. Transient flushing and mild headache were seen in a small number of patients, similar to the side effects reported in previous GMA studies[4,31,33].
DISCUSSION
Factors which initiate and perpetuate or exacerbate UC are yet to be characterized. The current view is that treatment strategies based on understanding of the factors that initiate or exacerbate UC (targeted therapy) should have greater efficacy margins and less treatment-related side effects[11,19,34,35]. However, UC is an inflammatory disorder reflecting unphysiological activities of pro-inflammatory cytokines and an over-exuberant intestinal inflammatory response[11,34,35]. Hence, it is assumed that cytokines can initiate and perpetuate inflammation and currently represent the best therapeutic targets. With this in mind, we studied their circulating levels during active disease and remission. IL-10 and IL-1ra were chosen as two typical anti-inflammatory cytokines while IL-6 and IL-18 were considered to represent two typical pro-inflammatory cytokines. The circulating levels of all 4 cytokines were high in most patients during active UC, but low during remission, similar to the levels in healthy controls. Further more, most non-responders to GMA had low blood levels of IL-1ra. This study did not measure mucosal tissue levels of these 4 cytokines which might be more appropriate to intestinal inflammation. Since monocytes/macrophages and granulocytes are major sources of both pro- and anti-inflammatory cytokines, this might suggest that these leucocytes contribute to the circulating levels of cytokines. Accordingly, we targeted these leucocytes using GMA with Adacolumn. The column carriers adsorb about 65% of granulocytes, 55% of monocytes and a small fraction of lymphocytes from the blood in the column (FcγR and complement receptors bearing leucocytes). The procedure is followed by a marked increase in peripheral blood lymphocyte counts, notably CD4+ T cells[5] and CD10 negative (immature) neutrophils[36].
The major cellular sources of IL-1ra are known to be monocytes/macrophages and polymorphonuclear granulocytes[37] while the major cellular sources of IL-10 are reported to be monocytes/macrophages, also Th2 cells and B lymphocytes[28]. Similarly, IL-6 is produced by neutrophils, monocytes/macrophages as well as endothelial cells[15] while IL-18 is produced by monocytes/macrophages, epithelial and Kupffer cells[23,24]. Each of these cytokines has a different profile of inflammatory or anti-inflammatory action. Both IL-1ra and IL-10 are reported to have strong anti-inflammatory actions[26,27,29,38-42]. Overproduction of the pro-inflammatory cytokine, IL-1β is thought to be involved in the perpetuation of inflammatory bowel disease, while IL-1ra, the naturally occurring antagonist of IL-1β is believed to counteract the actions of IL-1β; an imbalance of IL-1β and IL-1ra production is thought to be a major factor in the perpetuation of intestinal inflammation[27,38]. The production of IL-1ra is reported to be stimulated by several substances including adherent IgG, certain cytokines like IL-10[26,37,39,42,43]. IgGs adsorb to cellulose acetate carriers of Adacolumn (see below) and this may be one explanation for the elevation of IL-1ra during GMA. Similarly, IL-10 is reported to be a major Th2 cytokine that is known to inhibit the production of IFN-γ, IL-1β, IL-6, IL-8, IL-12 and TNF-α limiting the emergence of Th1 cytokine field[30,44]. In contrast, IL-6 is recently reported to have complex inflammatory actions. Thus it has a major role in leucocyte extravasation by activating endothelial cells and stimulating the production of chemokines including IL-8 and monocyte chemoattractant protein[15]. This indicates that circulating IL-6 can be a major pro-inflammatory factor. IL-18 has been recognized to be a potent inducer of IFN-γ production by T-cells[24,45]. Either independently or in synergy with IL-12, the effects of IL-18 via induction of IFN-γ can lead to a rapid activation of the monocyte/macrophage system with an upregulation of these cells’ innate immune capabilities[46,47] that may lead to a Th1 type inflammatory response[23,24]. Hence high levels of circulating IL-18 should be considered pro-inflammatory.
One obvious question which has been drawing our attention in this study was why a consistent increase in IL-6 and IL-18 at the Adacolumn outflow could not be detected as was seen for IL-1ra and IL-10. It is not to say that they were not released from cells which adhere to the column carriers, rather to say that no increase was detected. However, it is logical to view the inside the column as an environment in which very high levels of active oxygen derivatives and proteases (released from adherent leucocytes) prevail. It is likely that the cytokines we have measured have different stability within the column environment and those which survive in appreciable amounts show an increase at the column outflow.
Patients with autoimmune diseases are known to have circulating immune complexes (ICs) in their plasma[49,50]. The Adacolumn adsorptive leucocytapheresis carriers are made of cellulose acetate and cellulose acetate is known to adsorb IgG and ICs from plasma[50]. Upon adsorption, the binding sites on IgG and ICs become available for the Fcγ receptors on neutrophils and monocytes/macrophages[50-53]. Further more, cellulose acetate with adsorbed IgG and ICs generates active complement fragments including C3a, C4a and C5a[5,50,53]. The opsonins, C3b, C3bi C4b, C4bi, C5b and others derived from active complement fragments also adsorb onto the carriers[5,50,53-55] and serve as binding sites for the leucocyte complement receptors, CR1, CR2, CR3 (Mac-1, CD11b/CD18) and CR4[54-57]. Leucocyte adsorption to the cellulose acetate carriers of Adacolumn is governed by the Fcγ receptors (FcγRs) and the complement receptors on the leucocytes. The expression of these two sets of receptors is common on neutrophils and monocytes/macrophages. In contrast, the expression of complement receptors is not a common feature of lymphocytes except on small subsets of B, T and NK (natural killer) cells[55,58]. Similarly, FcγRs are not widely expressed on lymphocytes except on a small population of CD19+B cells[58] and CD56+NK cells[59]. These basic processes should explain why the carriers selectively adsorb granulocytes and monocytes/macrophages, but not lymphocytes.
In conclusion, elevated blood levels of IL-6 and IL-18 together with peripheral blood granulocytes and monocytes/macrophages which in patients with UC show activative behaviour and increased survival time can be pro-inflammatory and are not counteracted by anti-inflammatory cytokines during active UC. Depletion of these leucocytes by GMA was associated with remission of UC in most of the treated patients and a decline in circulating cytokines. These observations support the assumption that peripheral blood granulocytes and monocytes/macrophages influence the circulating levels of cytokines, hence UC disease and should be appropriate targets of the GMA therapy.
Footnotes
S- Editor Wang J L- Editor Ma JY E- Editor Ma WH
References
- 1.Meuret G, Bitzi A, Hammer B. Macrophage turnover in Crohn's disease and ulcerative colitis. Gastroenterology. 1978;74:501–503. [PubMed] [Google Scholar]
- 2.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, Iwaoka Y, Saniabadi A, Matsushita I, Sato Y, Tozawa K, et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35. doi: 10.1053/jcgh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 5.Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Lofberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy DA, Rampton DS, Liu YC. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clin Exp Immunol. 1991;86:489–493. doi: 10.1111/j.1365-2249.1991.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannigan AE, O'Connell PR, Hurley H, O'Neill A, Brady HR, Fitzpatrick JM, Watson RW. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13:361–366. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 9.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 10.D'Odorico A, D'Inca R, Mestriner C, Di Leo V, Ferronato A, Sturniolo GC. Influence of disease site and activity on peripheral neutrophil function in inflammatory bowel disease. Dig Dis Sci. 2000;45:1594–1600. doi: 10.1023/a:1005521212948. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 12.Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998;42:470–476. doi: 10.1136/gut.42.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 14.Lampinen M, Carlson M, Sangfelt P, Taha Y, Thörn M, Lööf L, Raab Y, Venge P. IL-5 and TNF-alpha participate in recruitment of eosinophils to intestinal mucosa in ulcerative colitis. Dig Dis Sci. 2001;46:2004–2009. doi: 10.1023/a:1010659803912. [DOI] [PubMed] [Google Scholar]
- 15.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 16.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 17.Bjarnason I, Macpherson A, Menzies IS. Intestinal permeability: The basics. In: Sutherland LR, Collins SM, Martin F, McLeod RS, Targan SR, et al., editors. Inflammatory bowel disease. Basic research, clinical implications and trends in therapy. Dordrech: Kluwer Academic Press; 1994. pp. 53–70. [Google Scholar]
- 18.Allison MC, Dhillon AP, Lewis WG, Pounder RE (eds) Inflammatory Bowel Disease. London: Mosby; 1998. pp. 15–95. [Google Scholar]
- 19.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 20.Hanai H, Watanabe F, Saniabadi AR, Matsushitai I, Takeuchi K, Iida T. Therapeutic efficacy of granulocyte and monocyte adsorption apheresis in severe active ulcerative colitis. Dig Dis Sci. 2002;47:2349–2353. doi: 10.1023/a:1020159932758. [DOI] [PubMed] [Google Scholar]
- 21.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 22.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 23.Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63. doi: 10.1016/s0952-7915(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 24.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 25.Chomarat P, Vannier E, Dechanet J, Rissoan MC, Banchereau J, Dinarello CA, Miossec P. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432–1439. [PubMed] [Google Scholar]
- 26.Freeman BD, Buchman TG. Interleukin-1 receptor antagonist as therapy for inflammatory disorders. Expert Opin Biol Ther. 2001;1:301–308. doi: 10.1517/14712598.1.2.301. [DOI] [PubMed] [Google Scholar]
- 27.Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:435–442. doi: 10.1046/j.1365-2249.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 30.Kourilsky P, Truffa-Bachi P. Cytokine fields and the polarization of the immune response. Trends Immunol. 2001;22:502–509. doi: 10.1016/s1471-4906(01)02012-9. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565–571. doi: 10.1023/b:ddas.0000026299.43792.ae. [DOI] [PubMed] [Google Scholar]
- 32.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka T, Maruyama Y, Matsushita I, et al. Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitis. Am J Gastroenterol. 2004;99:1532–1538. doi: 10.1111/j.1572-0241.2004.30432.x. [DOI] [PubMed] [Google Scholar]
- 34.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622–635. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- 35.Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3:81–92. [PubMed] [Google Scholar]
- 36.Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, Shimoyama T. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334–1341. doi: 10.1023/a:1015330816364. [DOI] [PubMed] [Google Scholar]
- 37.Muzio M, Re F, Sironi M, Polentarutti N, Minty A, Caput D, Ferrara P, Mantovani A, Colotta F. Interleukin-13 induces the production of interleukin-1 receptor antagonist (IL-1ra) and the expression of the mRNA for the intracellular (keratinocyte) form of IL-1ra in human myelomonocytic cells. Blood. 1994;83:1738–1743. [PubMed] [Google Scholar]
- 38.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–2440. [PubMed] [Google Scholar]
- 39.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 40.Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473–1482. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 41.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 42.Ishizuka K, Sugimura K, Homma T, Matsuzawa J, Mochizuki T, Kobayashi M, Suzuki K, Otsuka K, Tashiro K, Yamaguchi O, et al. Influence of interleukin-10 on the interleukin-1 receptor antagonist/interleukin-1 beta ratio in the colonic mucosa of ulcerative colitis. Digestion. 2001;63 Suppl 1:22–27. doi: 10.1159/000051906. [DOI] [PubMed] [Google Scholar]
- 43.Andersen LS, Petersen J, Svenson M, Bendtzen K. Production of IL-1beta, IL-1 receptor antagonist and IL-10 by mononuclear cells from patients with SLE. Autoimmunity. 1999;30:235–242. doi: 10.3109/08916939908993804. [DOI] [PubMed] [Google Scholar]
- 44.Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fickenscher H, Hör S, Küpers H, Knappe A, Wittmann S, Sticht H. The interleukin-10 family of cytokines. Trends Immunol. 2002;23:89–96. doi: 10.1016/s1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 46.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, et al. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 47.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 48.Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 49.Hay FC, Nineham LJ, Perumal R, Roitt IM. Intra-articular and circulating immune complexes and antiglobulins (IgG and IgM) in rheumatoid arthritis; correlation with clinical features. Ann Rheum Dis. 1979;38:1–7. doi: 10.1136/ard.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Arrigo C, Candal-Couto JJ, Greer M, Veale DJ, Woof JM. Human neutrophil Fc receptor-mediated adhesion under flow: a hollow fibre model of intravascular arrest. Clin Exp Immunol. 1995;100:173–179. doi: 10.1111/j.1365-2249.1995.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF 3rd, Petty HR. CR3 (alphaM beta2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective Fc gammaRIIA (CD32) tail-minus mutant. J Immunol. 1996;157:5660–5665. [PubMed] [Google Scholar]
- 52.Moldovan I, Galon J, Maridonneau-Parini I, Roman Roman S, Mathiot C, Fridman WH, Sautès-Fridman C. Regulation of production of soluble Fc gamma receptors type III in normal and pathological conditions. Immunol Lett. 1999;68:125–134. doi: 10.1016/s0165-2478(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 53.Hiraishi K, Takeda Y, Shiobara N, Shibusawa H, Jimma F, Kashiwagi N, Saniabadi AR, Adachi M. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–340. doi: 10.1046/j.1526-0968.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 54.Ross GD, Medof ME. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- 55.Newman SL, Devery-Pocius JE, Ross GD, Henson PM. Phagocytosis by human monocyte-derived macrophages. Independent function of receptors for C3b (CR1) and iC3b (CR3) Complement. 1984;1:213–227. [PubMed] [Google Scholar]
- 56.Ross GD, Vĕtvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muto S, Vĕtvicka V, Ross GD. CR3 (CD11b/CD18) expressed by cytotoxic T cells and natural killer cells is upregulated in a manner similar to neutrophil CR3 following stimulation with various activating agents. J Clin Immunol. 1993;13:175–184. doi: 10.1007/BF00919970. [DOI] [PubMed] [Google Scholar]
- 58.de Andres B, Mueller AL, Verbeek S, Sandor M, Lynch RG. A regulatory role for Fcgamma receptors CD16 and CD32 in the development of murine B cells. Blood. 1998;92:2823–2829. [PubMed] [Google Scholar]
- 59.Sulica A, Morel P, Metes D, Herberman RB. Ig-binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001;20:371–414. doi: 10.3109/08830180109054414. [DOI] [PubMed] [Google Scholar]


