Abstract
AIM: To investigate the probable role of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in the pathogenesis of inflammatory bowel disease (IBD).
METHODS: Fifty-eight patients were enrolled; nineteen healthy volunteers served as controls; 8 patients were diagnosed with Crohn’s disease, and 31 with ulcerative colitis. Clinical and endoscopic activity indexes of patients with Crohn’s disease and ulcerative colitis respectively were estimated. Upon admission blood was sampled; sTREM-1 and TNFα were measured by an immunoassay and malondialdehyde (MDA) by the thiobarbiturate assay, after passage through an HPLC system.
RESULTS: Median ± SE of TNFα of controls, patients with Crohn’s disease and patients with ulcerative colitis were 6.02 ± 3.94, 7.98 ± 5.08 (P = NS vs controls), and 8.45 ± 4.15 ng/L (P = 0.018 vs controls) respectively. Respective values of sTREM-1 were 53.31 ± 32.93, 735.10 ± 197.17 (P = 0.008 vs controls) and 435.82 ± 279.71 ng/L (P = 0.049 vs controls). sTREM-1 was positively correlated with Crohn’s disease activity index and clinical and endoscopic activity indexes of ulcerative colitis (P = 0.002, 0.001 and 0.009, respectively). sTREM-1 of patients with ulcerative colitis was positively correlated with TNFα (P = 0.001).
CONCLUSION: sTREM-1 seems to behave as a novel mediator in IBD in correlation with the degree of the inflammatory reaction of the intestinal mucosa.
Keywords: sTREM-1, Pro-inflammatory cytokines, Malondialdehyde, Inflammatory bowel disease
INTRODUCTION
Accumulated evidence over the last decade demonstrates that inflammatory bowel disease (IBD) is a heterogeneous group of diseases resulting from different pathogenetic mechanisms with a common symptomatic expression[1]. Dysfunction of the innate and adaptive immune systems associated with gut mucosa might involve impairment of mucosal barrier function and development of localized or systemic inflammatory and autoimmune processes[2,3]. Triggering receptor expressed on myeloid cells (TREM)-1 is a recently discovered receptor expressed on the surface of neutrophils and monocytes in the presence of microbial components. Engagement of TREM-1 has been reported to trigger the synthesis of proinflammatory cytokines, though its expression failed to elevate in several autoimmune diseases[4,5]. Expression of TREM-1 was not identified on cell membranes of macrophages of intestinal lamina propria; its absence was connected with prevention of excessive inflammatory reactions by bacterial flora, and thus, of any tissue damage of the intestine[6].
A soluble form of TREM-1, named sTREM-1, has been found; it is thought to be released from cell membranes as a result of the severity of inflammation. sTREM-1 was found elevated in samples of gastric juice of patients with peptic ulcer disease; its correlation with gastritis score leads to the assumption of a probable implication of sTREM-1 in the pathogenesis of gastritis[7]. Based on the latter findings in gastric diseases, the present study aimed to clarify the significance of sTREM-1, if any, in inflammatory bowel disease.
MATERIALS AND METHODS
Subjects
Fifty-eight patients were enrolled in the prospective study from January 2001 to June 2001; nineteen healthy volunteers served as controls; eight patients were diagnosed with Crohn’s disease; and 31 with ulcerative colitis. Exclusion criteria for the study were (1) gastric or colonic neoplasia, (2) liver cirrhosis, (3) any history of administration of monoclonal anti-TNFα antibodies (infliximab, adalimubab) or soluble TNFα receptors (etanercept), (4) concurrent existence of infectious or ischemic colitis and (5) patients with Crohn’s disease type L4B2 and L4B3 classified according to Vienna classification system[8].
Crohn’s disease was confirmed by findings on barium radiography, endoscopy, and histopathology, as described by others[9]. The activity of Crohn’s disease among patients was defined according to Crohn’s Disease Activity Index (CDAI). Patients with CDAI score ranging between 150 and 400 were thought to have mild activity of the disease and patients with CDAI score ranging between 400 and 600 were thought to have severe activity. Remission was considered when CDAI score was less than 150[10].
The diagnosis of ulcerative colitis relied on history, stool examination, the sigmoidoscopic or colonoscopic appearance and the histological assessment of rectal or colonic specimens. Activity of ulcerative colitis was defined by its clinical and endoscopic severity. According to its clinical severity patients were categorized as suffering from disease remission, or mild, moderate, and severe disease as described previously; the latter disease activity was graded between 0 and 3, respectively[11]. According to endoscopic findings a system scaling between 0 and 4 was applied. The latter system is as follows: 0: normal mucosa, 1: loss of vascular pattern, 2: granular mucosa, 3: friability on rubbing and 4: spontaneous bleeding or ulceration of the mucosa[12].
Patients with no clinical, laboratory, histological and endoscopic abnormalities who underwent colonoscopy served as controls. The study was conducted in accordance to the Helsinki Declaration. Upon admission, a total of 20 mL of blood were collected after puncture of one forearm vein. Ten mL was added into 40 mL of trypticase soy broth (Becton Dickinson) for quantitative blood culture. The other 10 mL of blood was collected in a sterile tube and centrifuged; the supernatant was kept at -70°C until assayed. Serum was applied for the estimation of sTREM-1, TNFα and malondialdehyde (MDA).
Estimation of sTREM-1
Estimation of sTREM-1 was performed by a home-made enzyme immunoassay. Capture antibody of sTREM-1 (R&D Inc, Minneapolis, USA) was diluted to 4000 mg/L and distributed in a 96-well plate at a volume of 0.1 mL per well. Samples of serum were centrifuged at 12 000 g for 10 min and the supernatants were removed. After overnight incubation at 25°C, wells were thoroughly washed with a 0.5 g/L solution of Tween in PBS (Merck) (pH: 7.2-7.4). Then 0.1 mL of standard concentrations of sTREM-1 (15.1-4000 ng/L, R&D Inc) diluted with reagent diluent (10 g/L BSA in PBS, pH 7.2-7.4, 0.2 micron filtered) serving as a buffer or of serum was added in wells. After incubation for two hours, wells were washed thrice, and 0.1 mL of one 400 ng/mL dilution of sTREM-1 detection antibody (R&D Inc) was added per well. The plate was then incubated for two hours, and attached antibodies were signalled by streptavidin. Concentrations of sTREM-1 in each well were estimated by the optical density detected at 450 nm after addition of one 1:1 solution of H2O2: tetramethylbenzidine as a substrate (R&D Inc). sTREM-1 concentration was expressed in ng/L. All determinations were performed in duplicate; the inter-day variation of the assay was 5.23%.
Estimation of TNFα
Tumor necrosis factor alpha was measured in serum with an enzyme immunoassay (Amersham, London, UK). Lowest limit of detection was 0.5 ng/L. All measurements were performed in duplicate and cytokine concentrations were expressed as ng/L.
Estimation of malondialdehyde (MDA)
Lipid peroxidation was estimated by the concentration of MDA, as already described[13]. Briefly, a 0.1 mL aliquot of each sample was mixed to 0.9 mL of trichloroacetic acid 200 g/L (Merck) and centrifuged at 12 000 g, 4°C for 10 min. The supernatant was removed and incubated with 2 mL of thiobarbituric acid 2 g/L (Merck) for 60 min at 90°C. After centrifugation, a volume of 10 μL of the supernatant was injected into a high-performance liquid chromatography system (HPLC, Agilent 1100 Series, Waldbronn, Germany) with the following characteristics of elution: Zorbax Eclipse XDB-C18 (4.6 mm × 150 mm, 5 μm) column below 37°C; mobile phase consisting of a 50 mmol/L K3PO4 (pH 6.8) buffer and methanol 990 g/L at a 60/40 ratio with a flow rate of 1 mL/min; fluorometric detection with signals of excitation at 515 nm and emission at 535 nm. The retention time of MDA was 3.5 min and it was estimated as μmol/L by a standard curve created with 1, 1, 3, 3-tetramethoxy-propane (Merck). All determinations were performed in duplicate.
Statistical analysis
Comparison between groups was made by Mann-Whitney U test. Correlations between concentrations of sTREM-1, TNFα and MDA and severity indexes were performed according to Spearman’s rank of order. P < 0.05 was considered as significant.
RESULTS
Patients’ clinical and demographic characteristics are shown in Table 1. Blood cultures obtained from patients of all study groups were sterile. Concentrations (Median ± SE) of sTREM-1 of controls, patients with Crohn’s disease, and patients with ulcerative colitis were 53.31 ± 32.93, 735.10 ± 197.17 (P = 0.008 vs controls), and 435.82 ± 279.71 (P = 0.049 vs controls) ng/L, respectively (Table 1). Respective concentrations of TNFα were 6.02 ± 3.94, 7.98 ± 5.08 (P = NS vs controls), and 8.45 ± 4.15 (P = 0.018 vs controls) ng/L (Table 1). Respective concentrations of MDA were 0.93 ± 0.23, 1.46 ± 0.56, and 1.49 ± 0.61 (P = 0.041 vs controls) μmol/L (Table 1). The concentrations of sTREM-1 were higher in patients with severe degree of Crohn’s disease than in patients in remission of Crohn’s disease (P = 0.032, Table 2).
Table 1.
Demographic data of IBD patients
| Demographic data | Control group | Crohn’s disease | Ulcerative colitis |
| Gender (Male/Female) | 12/7 | 3/5 | 18/13 |
| Age (mean ± SD, yr) | 64.2 ± 14.8 | 29.4 ± 13.0 | 43.8 ± 16.4 |
| White blood cells (mean ± SD, x 109/μL) | 7.9 ± 2.3 | 9.0 ± 3.0a | 9.5 ± 3.6a |
| Platelets count (mean ± SD, x 109/μL) | 237 ± 56 | 187 ± 38a | 179 ± 33a |
| Blood cultures | sterile | sterile | sterile |
| Clinical activity index of UC (mean ± SD) | - | - | 1.52 ± 0.26 |
| Endoscopic activity index of UC (mean ± SD) | - | - | 1.65 ± 0.45 |
| Crohn’s disease activity index (mean ± SD) | - | 331.81±117.25 | - |
| TNFα (median ± SE, ng/L) | 6.0 ± 3.9 | 8.0 ± 5.1 | 8.4 ± 4.2a |
| sTREM-1 (median ± SE, ng/L) | 53 ± 33 | 735 ± 197b | 435 ± 279a |
| MDA (median ± SE, μmol/L) | 0.93 ± 0.23 | 1.46 ± 0.56 | 1.49 ± 0.61a |
P < 0.05,
P < 0.01 vs controls.
Table 2.
sTREM-1, TNFα and MDA in IBD patients
| Activity index | TNFα (ng/L) | sTREM-1 (ng/L) | MDA (μmol/L) |
| UC Clinical AI | |||
| Grade 0 | 4.19 ± 1.52 | 7.09 ± 4.90 | 1.74 ± 0.11 |
| 1 | 5.13 ± 0.98 | 18.91 ± 15.00 | 1.87 ± 1.05 |
| 2 | 4.98 ± 0.42 | 288.28 ± 133.82b | 1.83 ± 0.46 |
| 3 | 9.98 ± 1.23a | 1596.45 ± 397.38b | 1.97 ± 0.31 |
| UC endoscopic AI | |||
| Grade 0 | 4.33 ± 1.51 | 4.91 ± 3.21 | 1.36 ± 0.11 |
| 1 | 5.13 ± 0.98 | 18.91 ± 15.00 | 1.87 ± 1.05 |
| 2 | 4.86 ± 0.41 | 288.28 ± 133.82b | 1.66 ± 0.43 |
| 3 | 8.98 ± 0.42a | 1108.19 ± 309.95b | 1.74 ± 0.39 |
| 4 | 21.99 ± 10.50a | 2281.25 ± 556.77b | 1.91 ± 0.50 |
| CDAI | |||
| Score < 150 | 4.88 ± 1.44 | 4.91 ± 3.21 | 1.78 ± 0.11 |
| 150-400 | 5.21 ± 0.88 | 16.03 ± 4.19 | 1.88 ± 0.67 |
| 400-600 | 5.67 ± 0.91 | 2281.25 ± 556.77a | 1.95 ± 0.95 |
P < 0.05,
P < 0.01 vs grade 0 and score < 150.
Correlation between sTREM-1 and TNFα of patients with ulcerative colitis was significant (P = 0.001) (Figure 1). No correlation in patients with ulcerative colitis was found between sTREM-1 and MDA, and TNFα and MDA, consecutively; nor was correlation found in patients with Crohn’s disease between sTREM-1 and TNFα, sTREM-1 and MDA and TNFα and MDA.
Figure 1.
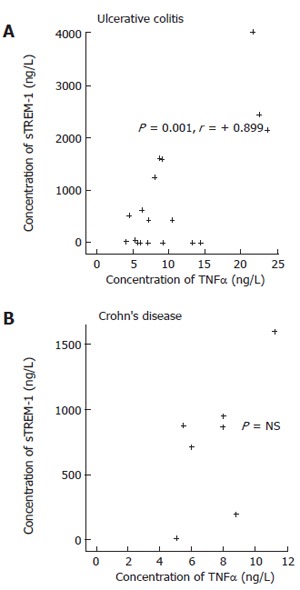
Correlations between sTREM-1, TNFα of IBD patients.
DISCUSSION
Triggering receptor expressed on myeloid cells (TREM-1) is a recently discovered receptor on cell membranes of neutrophils and monocytes involved in inflammatory processes and activated by microbial components. Engagement of TREM-1 has been reported to trigger the synthesis of proinflammatory cytokines[4]. sTREM-1 is the soluble counterpart of TREM-1 that is increased in gastric juice of patients with peptic ulcer disease[7]. Based on the latter involvement of sTREM-1 in diseases of the gastric mucose, it was hypothesized that sTREM-1 might be implicated in inflammation of intestinal mucosa. Our data revealed that sTREM-1 was increased in the sera of patients with ulcerative colitis and Crohn’s disease (Tables 1 and 2). Various hypotheses could be made to explain the mechanism of increase of sTREM-1 based on the role of bacteria and fungi to trigger TREM-1[14]. It might be assumed that sTREM-1 was increased as a consequence of two probable conditions related with a microbial infection;
(1) bacteremia as a result of bacterial translocation through the inflamed intestinal mucosa, and (2) infectious colitis. Both mechanisms are unfavorable for the following two reasons: (1) patients with infectious colitis were excluded from the study, and (2) quantitative blood cultures obtained from patients of all study groups were found sterile. Furthermore no differences were found for white blood cell and platelet counts between controls and patients with ulcerative colitis as well as between controls and patients with Crohn’s disease (Table 2). So, it might be presumed that sTREM-1 increase was not a result of a microbial infection.
The similar kinetics of sTREM-1 and TNFα might indicate the implication of sTREM-1 in the pathogenetic mechanisms leading to IBD. Evolution of IBD seems to result from the derangement of balance between pro-inflammatory cytokines and mediators with anti-inflammatory activity. The latter imbalance contributes to impairment of mucosal barrier function leading to colonic inflammation[15]. sTREM-1 was increased only in IBD of moderate and severe degree signifying the possibility that sTREM-1 might be an indicator of the severity of the colonic inflammation (Table 2) reflecting the degree of the inflammatory reaction taking place in the intestinal mucosa.
The significance of MDA increase in IBD has already been described by others[16,17]. MDA is a compound functioning as an indicator for the severity of oxidative stress. Oxidative stress seems to be involved in the pathogenesis of several inflammatory processes[16,17]. In the present study, MDA was not correlated with sTREM-1. The latter finding could be explained due to the fact that MDA is an indicator of lipid peroxidation that is not specific to IBD. The present data show for the first time, to our knowledge, that sTREM-1 levels correlate with clinical disease degree and TNFα levels in ulcerative colitis, raising the possibility that it is a mediator in this condition. Further research is mandatory to elucidate the role of sTREM-1 in the evolution from the initial immunologic events occurring in the inflamed intestinal mucosa to the development of inflammatory bowel disease.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
References
- 1.Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev. 2005;206:296–305. doi: 10.1111/j.0105-2896.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 2.Tlaskalová-Hogenová H, Tucková L, Stepánková R, Hudcovic T, Palová-Jelínková L, Kozáková H, Rossmann P, Sanchez D, Cinová J, Hrncír T, et al. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann N Y Acad Sci. 2005;1051:787–798. doi: 10.1196/annals.1361.122. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 5.Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenk M, Bouchon A, Birrer S, Colonna M, Mueller C. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J Immunol. 2005;174:517–524. doi: 10.4049/jimmunol.174.1.517. [DOI] [PubMed] [Google Scholar]
- 7.Koussoulas V, Vassiliou S, Demonakou M, Tassias G, Giamarellos-Bourboulis EJ, Mouktaroudi M, Giamarellou H, Barbatzas C. Soluble triggering receptor expressed on myeloid cells (sTREM-1): a new mediator involved in the pathogenesis of peptic ulcer disease. Eur J Gastroenterol Hepatol. 2006;18:375–379. doi: 10.1097/00042737-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer M, Petras RE, Hunt LE, Janosky JE, Galandiuk S. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol. 2000;95:3184–3188. doi: 10.1111/j.1572-0241.2000.03199.x. [DOI] [PubMed] [Google Scholar]
- 10.Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17 Suppl 2:11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 11.Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61:105–110. [PubMed] [Google Scholar]
- 12.de Lange T, Larsen S, Aabakken L. Inter-observer agreement in the assessment of endoscopic findings in ulcerative colitis. BMC Gastroenterol. 2004;4:9. doi: 10.1186/1471-230X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R, Chase SD. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;775:121–126. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 14.Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2:181–187. doi: 10.3121/cmr.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croitoru K, Zhou P. T-cell-induced mucosal damage in the intestine. Curr Opin Gastroenterol. 2004;20:581–586. doi: 10.1097/00001574-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 17.Tüzün A, Erdil A, Inal V, Aydin A, Bağci S, Yeşilova Z, Sayal A, Karaeren N, Dağalp K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–572. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]


