Abstract
A 67-year-old man, who had undergone surgery to resect multiple gastric cancers 4 years ago, visited our hospital for surveillance colonoscopy. Colonoscopy revealed a discolored, 7-mm in diameter, flat-elevated lesion with central depression in the transverse colon near the splenic flexure. Although the findings of endoscopy and barium enema were suggestive of submucosal invasion, the patient chose to undergo endoscopic mucosal resection. Pathological examination of the resected specimen revealed signet-ring cell carcinoma and a positive surgical margin. A second operation was performed, and no residual tumor or metastasis to lymph nodes was found in the resected specimens. Primary colorectal cancers composed of signet-ring cell carcinoma detected and treated at an early stage are extremely rare. We present a case and review the literature.
Keywords: Signet-ring cell carcinoma, Early colorectal cancer, Endoscopic mucosal resection
INTRODUCTION
Signet-ring cell carcinoma is uncommon in the colon and rectum, with a reported incidence ranging from 0.1% to 0.9%[1,2]. In general, signet-ring cell carcinoma shows the characteristic appearance of “linitis plastica” and behaves more aggressively than carcinoma of other histological types[1,2]. As symptoms usually appeared late, signet-ring cell carcinomas are commonly detected at advanced stages. Therefore, cases detected and treated at early stage are rare. In this case report, we present our experience with a signet-ring cell carcinoma in the transverse colon detected at early stage and review the literature.
CASE REPORT
A 67-year-old man visited our hospital 4 years ago with a history of 4 synchronous primary cancers of the stomach. He underwent total gastrectomy, and the surgical specimen contained 2 early cancers which invaded the mucosa and submucosa, and 2 advanced cancers which penetrated serosa without invasion of adjacent structures. No components of signet-ring cell carcinoma were found on histology. He had a brother and a sister, and the family history included that his father had died of a gastric cancer at the age of 68, and his sister had an advanced sigmoid colon cancer and a uterine cancer at the age of 62 and 65, respectively. The patient’s past history included an operation for synchronous gastric cancers, however there was no history of inflammatory bowel disease. He also had a focal cancer in an adenoma with moderate atypia, which had been completely resected endoscopically one year before. Therefore, he underwent surveillance colonoscopy at our department of endoscopy. Findings on laboratory tests were unremarkable. Total colonoscopy, with a magnifying colonoscope (CF-200Z, Olympus Optical Co., Japan) was easily introduced into the cecum without complications. Colonoscopy revealed a discolored flat-elevated lesion with central depression, 7 mm in diameter, in the transverse colon near the splenic flexure (Figure 1A). The tumor bled easily from the depressed area without contact from the endoscope and had convergent mucosal folds suggesting submucosal cancer involvement. After spraying of 0.2% indigo-carmine dye, the depression was seen more clearly (Figure 1B). After chromoendoscopy with 0.2% indigo-carmine dye and 0.05% crystal violet staining, we attempted to observe the surface structure of this lesion, the so-called “pit patterns”, at a higher magnification to estimate the depth of invasion[3,4]. However, the pit patterns could not be detected, as dense mucus coated the surface and could not be removed by repeated washing (Figure 1C)[5]. The findings of barium enema and conventional colonoscopy suggested submucosal cancer invasion, and thus, surgical resection, including node dissection, was recommended for curative treatment. However, the patient refused surgical resection but consented to endoscopic mucosal resection (EMR). The lesion was successfully removed en bloc with EMR without complications. The resected specimen was 7 mm in diameter. The resected specimen was fixed with 20% formalin, and then the mucus on the surface was removed for better staining and observation. The fixed lesion was stained with 0.05% crystal violet and observed stereomicroscopically under water immersion. Stereomicroscopy revealed a type V (non structural) pit pattern in the depressed area. These findings suggested that this lesion had deeply invaded the submucosal layer[3,6]. Histologically, the tumor was composed of signet-ring cell carcinoma, without accompanying adenoma or other types of cancer cells, that had infiltrated deeply into the submucosal layer with invasion of lymphatic vessels (Figure 2A-B). Immunostaining was used to examine the expression of MLH1 and MSH2 of the resected specimen; the tumor was positive for MSH2 but negative for MLH1. A cut end of the resected specimen was positive for carcinoma cells. Systemic diagnostic imaging including abdominal computed tomography showed no evidence of distant metastasis or ascites. Thereafter, the patient consented to undergo an additional laparotomy. The resected specimen revealed no residual carcinoma at the EMR site and showed no metastasis to lymph nodes. The postoperative course was uneventful, and the patient is still alive and in apparent good health 5 years after the operation.
Figure 1.
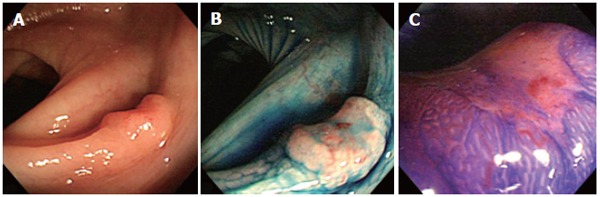
A: Colonoscopy revealed a discolored, flat-elevated lesion with central depression, 7 mm in diameter, in the transverse colon near the splenic flexure; B: Chromoendoscopy with 0.2% indigo-carmine dye showed the depressed area more clearly; C: Magnifying colonoscopy after 0.05% crystal violet staining was attempted but failed to detect the pit pattern for depth estimation, as the depressed area was covered by dense mucus, which could not be removed by repeated water washing.
Figure 2.
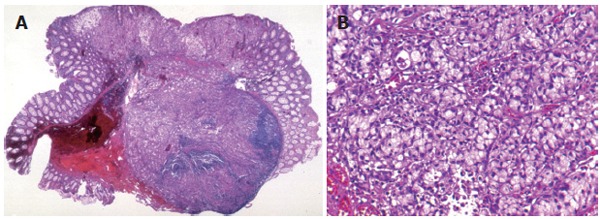
A, B: Histologically, the resected specimen showed a signet-ring cell carcinoma that had deeply invaded into the submucosal layer, and the vertical cut end of the resected tumor was positive for carcinoma cells.
DISCUSSION
More than 96% of signet-ring cell carcinomas arise in the stomach, and the rest occurred in other organs, including the colon, rectum, gallbladder, pancreas, urinary bladder, and breast[7]. The case described here was believed to be a primary lesion of the transverse colon because a thorough workup did not reveal any other sites of involvement. Although the patient’s history included surgical resection of an advanced gastric carcinoma, a secondary carcinomatous deposit should also be excluded. Metastasis from gastric carcinoma is probably the most common secondary carcinoma in the colon and rectum[8]. It may present itself as single or multiple strictures mimicking primary colorectal carcinoma or inflammatory bowel diseases[8,9]. Furthermore, multiple flat and depressed lesions or polyps have also been reported as rare presentations of colonic metastasis from gastric signet-ring cell carcinoma[10-13]. Our case presented a solitary depressed lesion without metastasis or lymphatic, hematogenous or peritoneal involvement, and the patient is still alive 5 years after surgery; therefore, on the basis of these findings, this case could be judged as a primary lesion. Crystal violet staining is commonly used to evaluate pit patterns, especially for lesions in which there is the suspicion of submucosal invasive carcinoma. Unfortunately, the pit patterns could not be detected in this case, as dense mucus coated the surface and failed to be removed by repeated washing endoscopically. This might reveal that signet-ring cell carcinoma produces abundant mucus.
Primary signet-ring cell carcinoma of the colon and rectum, as first described by Laufman and Saphir in 1951, is rare[14]. The reported incidence is about 1%[1,2]. The histological appearance of the tumor is characterized by cells with abundant intracytoplasmic mucin and peripherally placed nuclei. Signet-ring cell carcinomas of the colon and rectum are usually diagnosed at an advanced stage, because symptoms usually develop late. Thereafter, cancers limited to the mucosal and submucosal layers are rarely detected; to the best of our knowledge, only 26 cases, including ours, have been reported[15-20]. The clinicopathological details of the reported cases are summarized in Table 1. The patients included 21 males and 5 females with a mean age of 57.1 years (range of 6-79 years). The mean size of the tumors was 16.0 mm (range, 2 to 45 mm). Macroscopically, 16 (61.5%) cases were flat or depressed. The smallest tumor was a tiny, discolored, flat lesion 2 mm in diameter. Despite the small size, it surprisingly metastasized to lymph nodes[18]. Tsujinaka et al have described the first case of early signet-ring cell carcinoma of the colon without an adenomatous component. It was a depressed lesion that was believed to have arisen via a de novo pathway[15]. This tumor only showed signet-ring cancer cells without accompanying adenoma or other types of cancer cells. It is unknown whether signet-ring cell carcinoma usually arises from a pre-existing adenomatous polyp or as a so-called de novo carcinoma[21]. However, according to the reviewed articles, there are 3 reported cases with an adenomatous component; therefore, an adenoma-signet-ring cell carcinoma sequence also exists[16,17].
Table 1.
Clinicopathological details in patients with primary early colorectal signet ring cell carcinomas
| Mean age (range) (yr) | 57.1 (6-79) |
| Sex (male/female) | 21/5 |
| Mean size (range) (mm) | 16.0 (2-45) |
| Site of lesions | |
| Right-sided colon (cecum/ascending/transverse) | 13 (3/1/9) |
| Left-sided colon (descending/sigmoid) | 7 (3/4) |
| Rectum | 6 |
| Depth of invasion | |
| Intramucosal | 6 |
| Submucosal | 20 |
| Macroscopic features | |
| Polypoid | 10 |
| Flat | 3 |
| Depressed | 13 |
| Lymph node metastasis | |
| Positive | 2 |
| Negative/unknown | 17/7 |
The sites of reported cases are mostly in the right-sided colon (50%, 13 cases), but 7 are in the left-sided colon and 6 are in the rectum (Table 1). In our case, the transverse colon was the most commonly involved site. Eight of the reported cases also had multiple synchronous colorectal cancers. The right-sided predilection, histological characteristics, and the tendency to multiplicity are now well documented in hereditary non-polyposis colorectal cancer. However, in the present case, the family history and past history did not fulfill the diagnostic criteria[22,23]. Considering the clinicopathological characteristics of this case, immunostaining was used to examine expression of MLH1 and MSH2 of the resected specimen; the tumor was positive for MSH2 but negative for MLH1. Signet-ring cell carcinomas have been analyzed for microsatellite instability, which is present in approximately 30% of tumors[24]. Furthermore, although not available in our case, mutations of K-ras and p53 gene have been reported in signet-ring cell carcinomas of the colon and rectum; however, the frequency of K-ras gene mutation in signet-ring cell carcinomas is significantly lower than that of well and moderately differentiated carcinomas[24]. These results suggest that the genetic background of signet-ring cell carcinomas might differ from that of well or moderately differentiated carcinomas of the colon and rectum. DNA-replication errors are suggested to be at least partly involved in the carcinogenesis of signet ring cell colorectal carcinoma[24]. Although, our patient did not have inflammatory bowel disease, an association of inflammatory bowel disease and signet-ring cell carcinoma of up to 14% has been reported[25]. Psathakis et al. reported two (14.3%) of 14 patients with signet-ring cell carcinoma, who had a long history of ulcerative colitis[26]. Anthony et al. reported two cases of Crohn’s disease that developed signet-ring cell carcinoma[27].
In conclusion, we have reported a rare case of primary signet-ring cell carcinoma of the colon detected and treated at early stage, and reviewed the literature.
Footnotes
S- Editor Wang J L- Editor Ma JY E- Editor Bi L
References
- 1.Giacchero A, Aste H, Baracchini P, Conio M, Fulcheri E, Lapertosa G, Tanzi R. Primary signet-ring carcinoma of the large bowel. Report of nine cases. Cancer. 1985;56:2723–2726. doi: 10.1002/1097-0142(19851201)56:11<2723::aid-cncr2820561137>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52:1453–1457. doi: 10.1002/1097-0142(19831015)52:8<1453::aid-cncr2820520819>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, Fujii T, Koba I, Sano Y, Fu KI, Parra-Blanco A, Tajiri H, Yoshida S, Rembacken B. Assessment of colorectal lesions using magnifying colonoscopy and mucosal dye spraying: can significant lesions be distinguished. Endoscopy. 2001;33:306–310. doi: 10.1055/s-2001-13700. [DOI] [PubMed] [Google Scholar]
- 5.Fujii T, Iishi H, Tatsuta M, Hirasawa R, Uedo N, Hifumi K, Omori M. Effectiveness of premedication with pronase for improving visibility during gastroendoscopy: a randomized controlled trial. Gastrointest Endosc. 1998;47:382–387. doi: 10.1016/s0016-5107(98)70223-8. [DOI] [PubMed] [Google Scholar]
- 6.Kawano H, Tsuruta O, Ikeda H, Toyonaga A, Tanikawa K. Diagnosis of the level of depth in superficial depressed-type colorectal tumors in terms of stereomicroscopic pit patterns. Int J Oncol. 1998;12:769–775. doi: 10.3892/ijo.12.4.769. [DOI] [PubMed] [Google Scholar]
- 7.Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol. 1996;91:2195–2199. [PubMed] [Google Scholar]
- 8.Amorn Y, Knight WA Jr. Primary linitis plastica of the colon: report of two cases and review of the literature. Cancer. 1978;41:2420–2425. doi: 10.1002/1097-0142(197806)41:6<2420::aid-cncr2820410648>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Tanakaya K, Takeuchi H, Yasui Y, Takeda A, Umeda Y, Murakami I. Metastatic carcinoma of the colon similar to Crohn's disease: a case report. Acta Med Okayama. 2004;58:217–220. doi: 10.18926/AMO/32085. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Yang MT, Lin KY, Tu HY, Zhang TA, Chen PH. Metastases from gastric carcinoma to colon in the form of multiple flat elevated lesions: a case report. Kaohsiung J Med Sci. 2004;20:552–557. doi: 10.1016/S1607-551X(09)70257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraga Y, Yasui W, Kumamoto T, Watanabe M, Kobayakawa M, Eguchi N, Nakamura T, Kawamura H, Nakai S, Kamei F. [Colonic metastases of signet-ring cell carcinoma presenting as multiple small depressed lesions like erosions: report of a case] Nihon Shokakibyo Gakkai Zasshi. 2002;99:615–621. [PubMed] [Google Scholar]
- 12.Ogiwara H, Konno H, Kitayama Y, Kino I, Baba S. Metastases from gastric adenocarcinoma presenting as multiple colonic polyps: report of a case. Surg Today. 1994;24:473–475. doi: 10.1007/BF01427044. [DOI] [PubMed] [Google Scholar]
- 13.Metayer P, Antonietti M, Oumrani M, Hemet J, Lemoine F, Basuyau J. Metastases of a gastric adenocarcinoma presenting as colonic polyposis. Report of a case. Dis Colon Rectum. 1991;34:622–623. doi: 10.1007/BF02049905. [DOI] [PubMed] [Google Scholar]
- 14.Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62:79–91. doi: 10.1001/archsurg.1951.01250030082009. [DOI] [PubMed] [Google Scholar]
- 15.Tsujinaka Y, Tsuchiya S, Ooki S, Oomi Y, Kaneko H, Eguchi H, Kikkou T. IIc type of early carcinoma of the rectum originating "de novo", report of a case. Stmomach and Intestine. 1983;18:211–217. [Google Scholar]
- 16.Nakamura T, Nakano G, Sakamoto K. Adenoma of the rectum with multiple foci of signet-ring cell carcinoma. Report of a case. Dis Colon Rectum. 1983;26:529–532. doi: 10.1007/BF02563747. [DOI] [PubMed] [Google Scholar]
- 17.Hamazaki M, Kono S, Mimaya J, Ishihara A. Signet ring cell carcinoma in a polyp of the colon. A case report of a six-year-old boy. Acta Pathol Jpn. 1987;37:1679–1684. doi: 10.1111/j.1440-1827.1987.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 18.Urabe T, Kuroda Y, Urushihara T, Amano H, Yonehara S, Arihiro K. Two-mm diameter signet-ring cell carcinoma of the rectum with lymph node metastasis, report of a case. Stmomach and Intestine. 1998;33:1179–1183. [Google Scholar]
- 19.Masubuchi S, Konishi F, Togashi K, Shitoh K, Kashiwagi H, Kanazawa K. A case of early signet-ring cell carcinoma of the colon. J Jpn Soc Coloproctol. 1999:52; 128–132. [Google Scholar]
- 20.Toyota J, Sugimoto K, Shimomura T, Ashida K, Fukuchi T, Nishide T, Takahashi H, Nagamatsu R, Hashimoto Y, Azumi Y, et al. [A case of colon sm cancer IIa + IIc type converted well differentiated adenocarcinoma into signet-ring cell carcinoma] Nihon Shokakibyo Gakkai Zasshi. 2002;99:1220–1225. [PubMed] [Google Scholar]
- 21.Jass JR. Do all colorectal carcinomas arise in preexisting adenomas. World J Surg. 1989;13:45–51. doi: 10.1007/BF01671153. [DOI] [PubMed] [Google Scholar]
- 22.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 23.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata Y, Tomita N, Monden T, Ohue M, Ohnishi T, Sasaki M, Sekimoto M, Sakita I, Tamaki Y, Takahashi J, et al. Molecular characteristics of poorly differentiated adenocarcinoma and signet-ring-cell carcinoma of colorectum. Int J Cancer. 1999;84:33–38. doi: 10.1002/(sici)1097-0215(19990219)84:1<33::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Ooi BS, Ho YH, Eu KW, Seow Choen F. Primary colorectal signet-ring cell carcinoma in Singapore. ANZ J Surg. 2001;71:703–706. doi: 10.1046/j.1445-1433.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- 26.Psathakis D, Schiedeck TH, Krug F, Oevermann E, Kujath P, Bruch HP. Ordinary colorectal adenocarcinoma vs. primary colorectal signet-ring cell carcinoma: study matched for age, gender, grade, and stage. Dis Colon Rectum. 1999;42:1618–1625. doi: 10.1007/BF02236218. [DOI] [PubMed] [Google Scholar]
- 27.Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol. 1996;3:344–348. doi: 10.1007/BF02305663. [DOI] [PubMed] [Google Scholar]


