Abstract
AIM: To investigate if cisplatin alters vitamin status and if VR modulates cisplatin induced intestinal apoptosis and oxidative stress in Wistar/NIN (WNIN) male rats.
METHODS: Weanling, WNIN male rats (n = 12 per group) received adlibitum for 17 wk: control diet (20% protein) or the same with 50% vitamin restriction. They were then sub-divided into two groups of six rats each and administered cisplatin (2.61 mg/kg bodyweight) once a week for three wk or PBS (vehicle control). Intestinal epithelial cell (IEC) apoptosis was monitored by morphometry, Annexin-V binding, M30 cytodeath assay and DNA fragmentation. Structural and functional integrity of the villus were assessed by villus height / crypt depth ratio and activities of alkaline phosphatase, lys, ala-dipeptidyl amino-peptidase, respectively. To assess the probable mechanism(s) of altered apoptosis, oxidative stress parameters, caspase-3 activity, and expression of Bcl-2 and Bax were determined.
RESULTS: Cisplatin per se decreased plasma vitamin levels and they were the lowest in VR animals treated with cisplatin. As expected VR increased only villus apoptosis, whereas cisplatin increased stem cell apoptosis in the crypt. However, cisplatin treatment of VR rats increased apoptosis both in villus and crypt regions and was associated with higher levels of TBARS, protein carbonyls and caspase-3 activity, but lower GSH concentrations. VR induced decrease in Bcl-2 expression was further lowered by cisplatin. Bax expression, unaffected by VR was increased on cisplatin treatment. Mucosal functional integrity was severely compromised in cisplatin treated VR-rats.
CONCLUSION: Low intake of vitamins increases the sensitivity of rats to cisplatin and promotes intestinal epithelial cell apoptosis.
Keywords: Apoptosis, Cisplatin, Intestinal epithelium, Mucosal integrity, Oxidative stress, Vitamins.
INTRODUCTION
Although vitamin deficiency is encountered infrequently in developed countries, inadequate intake of several vitamins is associated with chronic diseases like cancer, coronary heart disease and osteoporosis[1]. About 35% of all cancers are associated with various nutritional deficiencies or excesses and a poor nutritional status of various water and fat-soluble vitamins has been demonstrated in populations more prone to cancers, without symptoms of clinical vitamin deficiencies[2]. In addition, the prospect that high intake of certain vitamins may confer protection against cancer and its side effects during chemo/radiotherapy has drawn substantial attention during the last decade[3]. In this context, we have recently shown that low intake of vitamins (50% reduction) per se can increase the intestinal epithelial cell apoptosis through increased oxidative stress and result in lowered functional integrity of the intestinal mucosa[4,5].
The cytotoxic actions of chemotherapeutic agents are not tumor specific, and injury to normal but rapidly proliferating cells in the bone marrow and intestinal crypt often complicates the treatment of patients with neoplastic disease[6,7]. Systemic chemotherapy exerts cytoablative actions via several different mechanisms, ultimately leading to cell cycle arrest and/or apoptosis and produces changes in the structure of the intestinal mucosa[8-10] that are associated with increased permeability of the intestine[11].
Cisplatin (cis-diamminedichloroplatinum, II) is one of the most frequently used anticancer drugs. The therapeutic efficacy of cisplatin derives from its ability to form complexes with DNA[12], which exert their cytotoxicity by directly inhibiting DNA and RNA synthesis and inducing apoptosis[13,14]. In addition, cisplatin has been shown to induce production of reactive oxygen species (ROS) that are reported to be important mediators of stress response in many cell types[15-17]. ROS accumulation and reduced glutathione (GSH) levels are critical to the bioactivity of cisplatin[18-20]. Importantly, inhibitors of ROS can block cisplatin-induced apoptosis indicating that pathways involved in and/or activated by oxidative stress are critical to cisplatin bioactivity[17]. In fact, increased intracellular GSH concentrations are found in cells resistant to cisplatin[18], and cisplatin-induced toxicity can be blunted by systemic administration of antioxidants like N-acetyl cysteine[20]. It has been demonstrated earlier that mitochondrial damage is an early event in the pathogenesis of gastric toxicity due to cisplatin [21]. However, cisplatin-induced apoptosis also involves events that are not ROS-dependent[22].
Whether vitamin status can modulate apoptosis in normal proliferating cells is particularly important for cancer chemotherapy as it has immediate clinical ramifications. Therefore, we have investigated the effect of sub-optimal intake of vitamins mimicking a general fall of vitamin status seen in cancer patients on the intestinal toxicity of cisplatin using rat as an animal model.
MATERIALS AND METHODS
Chemicals and reagents
Cisplatin was obtained from Dabur Pharmaceuticals, India. Annexin V-Biotin, M30 CytoDEATH and Streptavidin peroxidase were procured from Roche Diagnostics, Mannheim, Germany. Primary antibodies for Bcl-2 and Bax were from Oncogene Research products, San Diego, CA, USA and the substrate for caspase-3 (Ac-DEVD-pNA) was from Calbiochem, San Diego, CA, USA. Biotinylated secondary antibodies, RNase, proteinase K, Nonidet NP-40, agarose, lys, ala- 7-amido-4-methyl coumarin and the vitamins used in the diets were from Sigma chemical company, St Louis, MO, USA. All other chemicals were of the highest analytical grade and procured from local sources.
Animals and study design
The animal studies were approved by the Ethical committee for animal experimentation at the National Institute of Nutrition, Hyderabad, India, which ensures that the guidelines set by the government of India in this regard are strictly implemented. A total of 24 Wistar/ NIN (WNIN) weanling male rats were obtained from the National Center for Laboratory of Animal Sciences at National Institute of Nutrition, Hyderabad. They were divided randomly into two groups of 12 animals each and were housed individually in polypropylene cages in a room maintained at 24 ± 2 °C, 50-60% relative humidity, with a 12 h light-dark cycle. They were fed for 17 wk, a casein based (20% protein) control diet or the same with 50% restriction of vitamin mixture [23]. After 17 wk, control and vitamin restricted (VR) rats were further divided into two groups of six rats each and administered cisplatin (CDDP - dissolved in PBS, at a dose of 2.61 mg/kg bodyweight) once in a week for three wk or PBS (vehicle control). The rats had free access to food and water and their food intake (daily) and body weights (weekly) were recorded periodically during the course of the experiment.
At the end of the feeding and treatment regimen, venous blood was collected from all rats after a 17 h fasting, through orbital sinus vein puncture into heparin containing vials. Plasma was separated immediately and stored at -20 °C for analysis of riboflavin, folic acid and vitamin E. Three rats from each group were euthanized in a CO2 chamber and sacrificed each day. A 20-cm segment of the jejunum, beginning 12-cm distal to the ligament of Treitz, was immediately excised via a midline abdominal incision and freed from mesentery and fat. It was then processed for evaluating the changes in apoptotic rates.
Processing of jejunum
The 20-cm segment of jejunum was gently washed with ice-cold phosphate buffered saline (PBS), divided randomly into three segments of 5, 5 and 10 cm each and processed as reported earlier [14]. They were used respectively for light microscopic observations, isolation of epithelial cells and extraction of DNA and determination of enzyme activities, parameters of oxidative stress and antioxidant status in addition to the expression of pro and anti apoptotic proteins, as described earlier [14,15].
Detection of apoptosis
Samples of rat jejunum fixed in buffered formalin for 24 h were dehydrated, embedded in paraffin and the number of apoptotic cells were scored in 4-µm thick serial sections under a light microscope, after: (i) staining with hematoxylin and eosin (ii) M30 staining and (iii) by Annexin V binding, as described by us earlier[14,15]. Also, the DNA isolated from the jejunal epithelial cells was resolved on an agarose gel and the DNA fragments visualized under a UV trans-illuminator after ethidium bromide staining (BioRad).
Oxidative stress, antioxidant status and expression of proteins modulating apoptosis
The frozen samples of the mucosal scrapings were processed and the activities of caspase-3, Cu, Zn-SOD, glutathione peroxidase, catalase were determined as described by us earlier[14,15]. Tissue oxidative stress was quantified by the estimation of TBARS and protein carbonyls and the expression of apoptotic modulatory proteins Bcl-2 and Bax was assessed by immuno-precipitation and western blotting[14,15].
Structural and functional integrity of villus
Structural integrity of the villus was assessed from the ratio of villus height: crypt depths, which were measured under a light microscope (100× magnification) using a calibrated ocular micrometer. The values of the villus height and crypt depth in each animal were obtained from the mean of measurements made in seven different fields of the tissue section as described earlier [14]. Activities of alkaline phosphatase and lys, ala dipeptidyl amino peptidase were measured 12000 g supernatant, as markers of functional integrity of the mucosal membrane [14,15].
Plasma vitamin status
Plasma vitamin E and riboflavin levels were quantified on an RP-HPLC (Agilent Tech Inc., CA, USA) [14], whereas folic acid was determined by a solid phase competitive binding assay as described earlier [15]
Statistical analysis
All the results are expressed as mean + SE. Data was analyzed statistically by one way analysis of variance (ANOVA) followed by Post Hoc multiple comparison tests of significance using the SPSS package (Version 10.0, Chicago, USA). Since no heterogeneity of variance was observed with any of the parameters tested, differences among the groups were tested by the parametric, least significant difference (LSD) test. The differences were considered significant only if P < 0.05.
RESULTS
Body weight
Feeding vitamin restricted diet for 17 wk showed no significant changes in body weight of rats compared to controls. However, cisplatin administration during the next 3 wk resulted in loss of body weight to an extent of 10 % in control rats and 18 % in VR rats, due to reduced food intake (data not given).
Plasma vitamin status
Plasma concentration of folate decreased by 13 % in control rats treated with cisplatin compared to those treated with saline. On the other hand they decreased by 38% in VR rats treated with cisplatin compared to saline treatment. Plasma riboflavin concentration decreased by 14% in cisplatin treated compared to saline treated control rats, where as this decrease was 24% in cisplatin treated compared to saline treated VR rats. Plasma vitamin E level fell by 15% in control rats treated with cisplatin compared to those treated with saline, where as the decrease was 29 % in cisplatin treated compared to saline treated VR rats (Table 1).
Table 1.
Plasma levels of fat and water-soluble vitamins in control and vitamin restricted rats treated with cisplatin
| Group | Folate(µg/dL) | Riboflavin(µg/dL) | α -Tocopherol(µg/dL) |
| CON | 4.85a ± 0.10 | 4.74a ± 0.06 | 74.1a ± 2.59 |
| VR | 2.08b ± 0.50 | 2.42b ± 0.12 | 46.4b ± 2.74 |
| CON+CDDP | 4.23c ± 0.11 | 4.05c ± 0.07 | 63.2c ± 2.93 |
| VR+CDDP | 1.28d ± 0.11 | 1.85d ± 0.03 | 33.1d ± 2.61 |
Values are mean ± SE of 6 observations; Means in a column with different superscripts are significantly different from each other (P ≤ 0.05), by one-way ANOVA, followed by post-hoc least significant difference ( LSD ) test. CON - control rats; VR - vitamin restricted rats; CON+CDDP - control rats treated with cisplatin and VR+CDDP - vitamin restricted rats treated with cisplatin.
Jejunal mucosal apoptosis
Vitamin restriction per se increased the jejunal villus apoptosis by 2 - fold, but not the crypt apoptosis. Cisplatin increased jejunal villus apoptosis by 1.7 and 2.4 fold respectively in control and VR rats (Figure 1A). However, crypt apoptosis in jejunum increased by 4 and 5 fold in control and VR rats respectively by cisplatin treatment (Figure 1B). Vitamin restriction per se increased Caspase - 3 activity by 2.4-fold. Cisplatin treatment increased caspase-3 activity by 3.5-fold in control and VR rats, compared to their saline treated controls (Figure 1C). There were 14% Annexin V positive cells in VR rats. Cisplatin treatment resulted in 13 and 25 % Annexin V positive cells in control and VR rats respectively (data not given). On the other hand M 30 positive cells were 25% in VR rats. Cisplatin treatment resulted in 30% of cells positive for M 30 in control rats whereas M 30 positive cells were 70% in VR rats (Figure 2). VR rats showed a clear inter-nucleosomal DNA fragmentation pattern characteristic of apoptosis. Interestingly DNA fragmentation increased further with cisplatin treatment. However, control rats treated with cisplatin showed relatively less fragmentation of DNA compared to VR rats (Figure 3).
Figure 1.
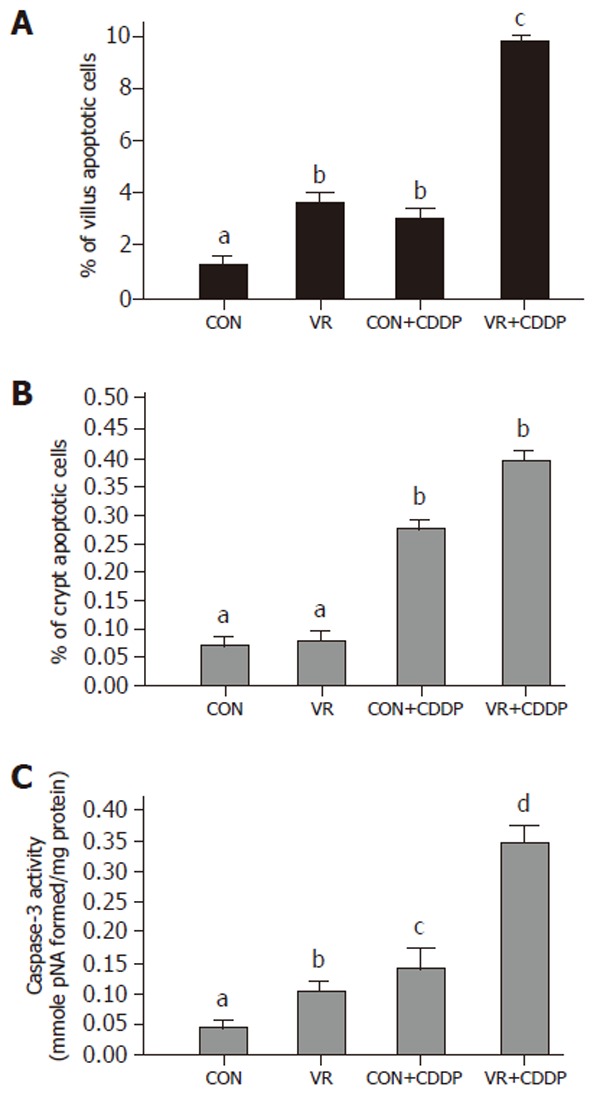
Apoptotic index and caspase-3 activity of the intestinal mucosa of control and vitamin restricted rats treated with cisplatin or saline (vehicle). Vertical bars are means with standard error, of IEC apoptotic indices in villus (Panel A) and crypt (Panel B) regions of control, vitamin restricted rats treated with saline and cisplatin (n = 6 per group). Intestinal sections were stained with hematoxylin and eosin and studied by morphometry under a light microscope. Caspase-3 activity (Panel C) was measured in 12000 g supernatant using a colorimetric substrate.
Figure 2.
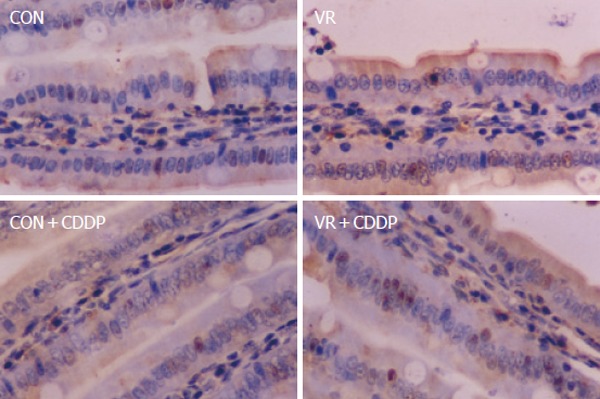
Photomicrographs of apoptotic cells showing positivity for M30 antibody. Representative photomicrographs of intestinal sections immuno-histochemically stained with M 30 antibody. De-paraffinized, intestinal sections were hydrated and probed with M 30 antibody and the positive cells detected by enzymatic color reaction. Number of the brown colored positive cells were counted in a total of 1000 cells and expressed as the percentage.
Figure 3.
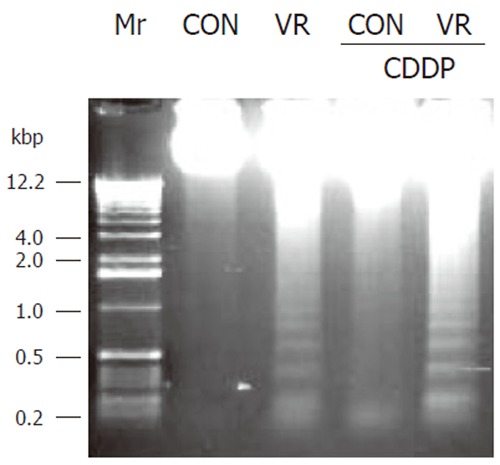
DNA fragmentation pattern in intestinal epithelial cells of control and vitamin restricted treated with saline or cisplatin. Epithelial cells were separated from a portion of rat jejunum, DNA extracted and resolved on a 1.5% agarose gel and stained with ethidium bromide.
Tissue oxidative stress
Levels of TBARS and protein carbonyls in intestinal epithelial cells increased significantly with vitamin restriction compared to control rats. Cisplatin administration increased the levels of TBARS and protein carbonyls both in control and VR rats, but to a lesser extent in control than VR rats (Figure 4A).
Figure 4.
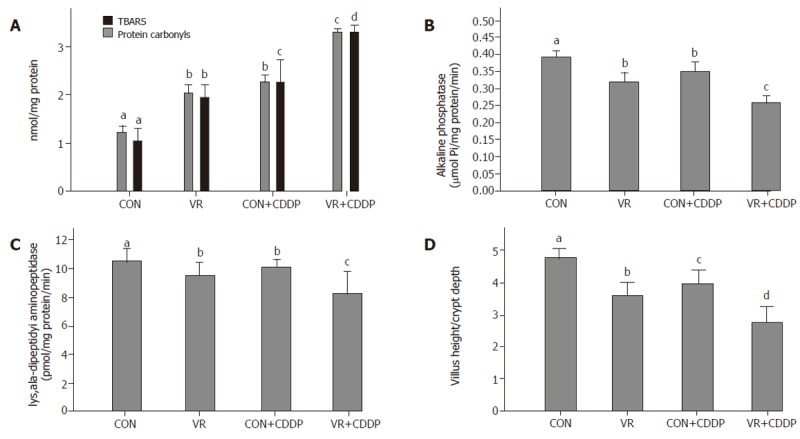
Effect of vitamin restriction and cisplatin on oxidative stress, structural and functional integrity of the intestinal mucosa. Levels of lipid peroxides (TBARS) and protein carbonyls are shown in panel A. Lowered activities of alkaline phosphatase (Panel B), lys, ala-dipeptidyl aminopeptidase (Panel C), are indicative of compromised functional integrity. Panel D indicates the ratio of villus height: crypt depth in control and vitamin restricted rats treated with saline and cisplatin, indicating structural integrity of the mucosa.
Structural and functional integrity of the jejunal villi
Activities of alkaline phosphatase and lys, ala - dipeptidyl amino-peptidase decreased significantly in the intestinal epithelial cells of rats fed vitamin restricted diet. Cisplatin treatment decreased their activities not only in control rats but also decreased it further in VR rats, compared to their saline treated controls (Figure 4B, C). Ratio of Villus height to Crypt depth, indicative of structural integrity of the intestinal mucosa, significantly decreased with vitamin restricted diet and showed a further decline with cisplatin treatment (Figure 4D).
Tissue antioxidant status
Vitamin restriction significantly decreased the intestinal epithelial cell GSH concentration compared to control rats. Cisplatin treatment per se lowered GSH levels in control rats compared to saline treated ones. Cisplatin treatment further lowered the GSH levels in VR rats compared to saline treated VR rats (Figure 5A).
Figure 5.
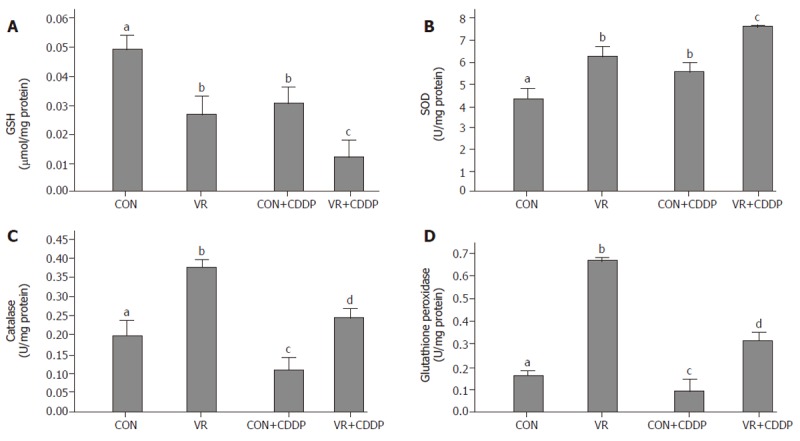
Intestinal glutathione levels and antioxidant enzyme activities in control and vitamin restricted rats treated with saline or cisplatin. Glutathione (GSH) levels are shown in panel A, whereas activities of SOD, catalase and glutathione peroxidase (GPX) are shown in panels B to D, respectively.
Vitamin restriction as well as cisplatin administration increased the SOD activity significantly (Figure 5B). Catalase activity increased significantly in VR rats compared to control rats. Cisplatin administration in control and VR rats significantly decreased the catalase activity compared to saline treated rats (Figure 5C). Glutathione peroxidase activity increased significantly with vitamin restriction. Similar to catalase, cisplatin administration in control and VR rats significantly decreased the glutathione peroxidase activity compared to saline treated rats (Figure 5D).
Expression of pro- and anti-apoptotic proteins in intestinal epithelial cells
Figure 6 shows the levels of Bcl-2 and Bax in control and vitamin restricted rats treated with saline or cisplatin. Cisplatin treatment as also vitamin restriction decreased Bcl-2 levels significantly. The levels decreased further in VR rats on cisplatin treatment. On the other hand Bax levels increased significantly in VR rats compared to controls. Cisplatin treatment elevated the levels of Bax protein not only in control rats but also increased them further in VR rats.
Figure 6.
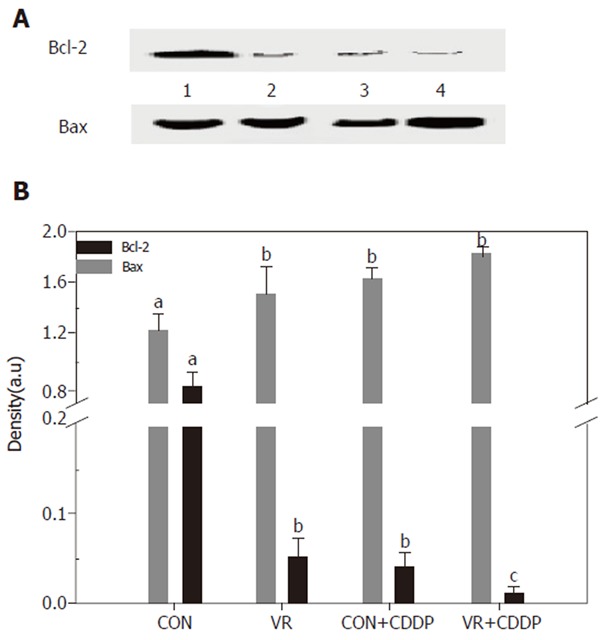
Expression of apoptosis regulatory proteins (Bcl-2 and Bax) in the intestinal epithelial cells Bcl-2 and Bax were immune-precipitated from the 12,000 g supernatant of the intestinal mucosal scrapings using monoclonal Bcl-2 / Bax antibodies. The immuno-precipitates were resolved on 12% SDS-PAGE, transferred electrophoretically to PVDF membrane and the immuno-blots developed using the same antibodies and detected / quantified with HRP conjugated anti - rabbit antibodies. Lane 1 - 4 are: CON, VR, CON+CDDP, VR+CDDP respectively. Upper panel shows Bcl-2 expression and bottom panel shows Bax expression. Panel B: Quantification of the IP / Western blots of Bcl-2 and Bax proteins. Immuno-blot was scanned using a densitometer (BioRad) and densities quantified using the Quantity One software from BioRad. Each bar represents the mean ± SE of three immuno-blots. Bars with different superscripts are significantly different from one another (P≤ 0.05) by one-way ANOVA and post-hoc least significant difference test.
DISCUSSION
Low intake of vitamins increased cisplatin action on epithelial cell apoptosis
Cisplatin is a free radical-producing drug that significantly decreases the plasma concentrations of antioxidants (24) and induces intestinal epithelial cell apoptosis. Earlier, we showed that chronic low intake of vitamins decreased the plasma vitamin status and significantly increased intestinal villus epithelial cell apoptosis compared to control rats (14). In line with these findings, feeding 50% vitamin restricted diet for 20 wk significantly lowered the plasma levels of both fat and water soluble vitamins, with no significant effect either on food intake or body weight (data not shown). Interestingly, cisplatin administration per se decreased the plasma vitamin levels to about 85% of controls and it reduced further, the plasma vitamin levels in VR rats to 30-40% of controls. These results indicate that cisplatin treatment per se impairs the vitamin status, but also worsens the vitamin status in VR rats further.
Therefore, to understand if reduced vitamin status has any role in cisplatin induced intestinal toxicity, we assessed IEC apoptosis in control and VR rats treated with cisplatin. Unlike vitamin restriction, which increased the villus apoptosis alone, cisplatin increased apoptosis both in villus and crypt regions by 2.3 and 4.0- fold respectively (over saline treated controls) as assessed by morphometry. On the other hand, cisplatin treatment of VR rats increased villus and crypt apoptosis by 2.7 and 5.0- fold (over saline treated VR rats) respectively (Figure1A and B). In line with earlier reports that chemotherapy causes intestinal damage with apoptosis of intestinal crypts, which precedes hypo proliferation resulting in crypt hypoplasia [25], the stem cell region (crypt) showed greater sensitivity to cisplatin both in control and VR rats. Although VR per se had no effect on crypt apoptosis, it appeared to sensitize the crypt region to cisplatin induced apoptosis and in the villus region, VR appeared to synergistically increase cisplatin induced apoptosis. These results show for the first time to the best of our knowledge, different effects of VR on cisplatin induced apoptosis in proliferating and differentiated cells of the intestinal mucosa. Higher rates of apoptosis as assessed by morphometry correlated well with increased staining of IECs with Annexin V (data not shown) and M 30 antibodies (Figure 2). Indeed, 70% of the IECs were M 30 positive in VR rats treated with cisplatin while only 30% were positive in control rats treated with cisplatin.
Caspase 3 is an effector caspase activated by many apoptotic stimuli. To confirm that the increased IEC death in cisplatin treated VR rats is truly apoptotic and not necrotic, we assessed whether cisplatin and VR induced increase in IEC apoptosis correlated with an increase in effector caspase activation. Indeed caspase - 3 in VR rats was activated about 2.4 fold over controls whereas it increased about 3.0 fold both in control and VR rats treated with cisplatin (Figure 1C). Further, the increased Caspase 3 activity is in line with the synergistic effect of VR on cisplatin induced IEC apoptosis. That cisplatin treated VR rats showed 8-fold increase in caspase-3 activity compared to saline treated controls (Figure 1C) corroborates the increased IEC apoptosis in cisplatin treated VR rats and confirms that low intake of vitamins increases the sensitivity of intestinal epithelium to cisplatin induced apoptosis.
Modulation of the sensitivity of IECs to cisplatin-induced apoptosis by VR was further assessed by DNA fragmentation in these cells. Despite a constitutive low rate of apoptosis in IECs of control rats, no DNA fragmentation was detectable, where as low amounts of fragmented DNA were observed in VR rats (Figure 3). Cisplatin treatment per se significantly increased DNA fragmentation, while priming with low vitamin intake further increased the abundance of DNA fragments.
Increased apoptosis is associated with increased oxidative damage of protein and lipids in intestinal epithelial cells
Since VR increases IEC apoptosis by increasing oxidative stress, we determined whether cisplatin also affects the IEC oxidative stress. The significantly higher levels of TBARS and protein carbonyls, the oxidative products of lipids and proteins, in the IECs of cisplatin treated control rats indicates increased oxidative stress in them (Figure 4A). That their levels increased further and were the highest in cisplatin treated VR rats indicates that VR modulated cisplatin induced oxidative stress. The data suggests that cisplatin treatment substantially increased the ROS formation in the intestinal mucosa.
Vitamin restriction and cisplatin administration compromised the structural and functional integrity of villi
In view of our earlier finding that VR induced increase in IEC apoptosis affected the structural and functional integrity of the intestinal mucosa [14,15], we assessed next, whether the increased IEC apoptosis induced by cisplatin in control and VR rats has similar effects. Cisplatin administration significantly decreased the ratio of villus height / crypt depth (Figure 4D) as well as mucosal marker enzyme activities (Figures 4B & C) in IECs of control rats and these indices were decreased further in cisplatin treated VR rats, indicating altered structural and functional integrity of mucosa and the synergism between cisplatin treatment and VR in this regard. These results indicate that cisplatin per se increases oxidative stress in general and lipid peroxidation in particular, which damage the membranes facilitating the leakage of solutes and VR appears to enhance this effect further. This appears to be responsible at least in part, for the lowered vitamin status in cisplatin treated rats.
Lowered antioxidant status and an imbalance in antioxidant enzyme activities with vitamin restriction increased mucosal sensitivity to cisplatin action
To gain insight into the oxidant-antioxidant balance in rats treated with cisplatin and the effect of VR on it, we monitored the GSH levels in addition to determining the antioxidant enzyme activities. Elevated intracellular levels of GSH, the cellular antioxidant involved in free radical scavenging activity, is associated with resistance to chemotherapeutic agents [26]. VR per se decreased the GSH levels in the IECs and cisplatin further decreased them markedly. (Figure 5A). Endogenous antioxidant systems are of singular importance in limiting oxidative cellular damage. To assess the increased sensitivity if any, of intestinal epithelium to cisplatin in VR rats was also associated with altered activities of anti-oxidant enzymes, we monitored the activities of antioxidant enzymes. Vitamin restriction per se increased the activities of all the three enzymes. While cisplatin treatment increased SOD and decreased catalase and GPX activities in control rats, it increased SOD further, and significantly decreased catalase and GPX activities in VR rats. These changes in the antioxidant enzymes observed in this study are in general agreement with the following literature reports: Up regulation / over expression of SOD and catalase are known to decrease the toxicity of cisplatin [27], whereas down regulation of GPX is associated with mitochondrial dysfunction in the kidney epithelial cells [28]. It appears that the possible increase in the accumulation of H2O2 or R2O2 as a consequence of the imbalance in the antioxidant enzyme activities could enhance the intrinsic sensitivity of the intestinal mucosa to cisplatin-induced apoptosis.
Vitamin restriction and cisplatin administration lowered Bcl-2 and increased Bax expression
To determine, if the increased IEC apoptosis seen in vitamin restriction and cisplatin treatment is associated with altered expression of the pro and anti-apoptotic proteins, the levels of Bax and Bcl-2 were measured by IP - Western blotting. Whereas Bcl-2 expression significantly decreased in VR rats (than controls), it was barely detectable in cisplatin treated control rats (Figure 6A). On the other hand, Bax expression was not altered in VR rats, but cisplatin increased its expression both in control and VR rats, albeit the increase was not significant (Figure 6B). The increased Bcl-2 expression is shown to stabilize mitochondria, prevent cytochrome c release and thereby reduce the cytotoxic potential of most anticancer drugs [29]. These results thus seem to suggest that up-regulation of Bax and the down-regulation of Bcl-2 were involved in cisplatin induced IEC apoptosis and that VR may not affect the action of cisplatin in this regard.
Anticancer drugs are known to operate principally by “forcing” the actively proliferating tumor cells into apoptosis[30]. However, these drugs also affect other proliferating cells like stem cells in the crypt of the intestinal mucosa and bone marrow either due to altered cellular redox status and / or deregulation of pro and anti-apoptotic protein expression. Since our studies indicate such effects to be more pronounced in vitamin restriction and that cisplatin treatment impairs vitamin status, it appears possible that intestinal toxicity of anti-cancer drugs can at least partly be due to a fall in vitamin status caused by cancer chemotherapy. Further, as VR potentiates the apoptotic response of IECs to cisplatin, vitamin deficiencies common among cancer patients may alter their intestinal tissue homeostasis and when they are exposed to a stress like chemical or radiation insult, result in frank tissue injury and loss of functional integrity. Our results provide qualitative and quantitative evidence, which support the hypothesis that vitamin restriction markedly amplifies the toxicity of cisplatin in the intestine. Therefore the need to fix the dose of anticancer drug based on the subjects’ vitamin status appears imperative to avoid the toxicity of the drug to the normal IECs. Further, it appears pertinent to study whether vitamin supplementation either singly or as a mixture can reverse such changes associated with cisplatin administration.
ACKNOWLEDGMENTS
We are grateful to Dr. N. Balakrishna for his help in the statistical analysis of the data. We thank Mr. B. Sreedhar for his valuable suggestions and critical comments during manuscript preparation.
Footnotes
Supported by The Indian Council of Medical Research
S- Editor Guo SY L- Editor Kumar M E- Editor Liu WF
References
- 1.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002;287:3116–3126. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves MK, Baquet C, Gamshadzahi A. Diet, nutritional status, and cancer risk in American blacks. Nutr Cancer. 1989;12:1–28. doi: 10.1080/01635588909513997. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults: clinical applications. JAMA. 2002;287:3127–3129. doi: 10.1001/jama.287.23.3127. [DOI] [PubMed] [Google Scholar]
- 4.Bodiga VL, Boindala S, Putcha U, Subramaniam K, Manchala R. Chronic low intake of protein or vitamins increases the intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition. 2005;21:949–960. doi: 10.1016/j.nut.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Vijayalakshhmi B, Sesikeran B, Udaykumar P, Kalyanasundaram S, Raghunath M. Effects of vitamin restriction and supplementation on rat intestinal epithelial cell apoptosis. Free Radic Biol Med. 2005;38:1614–1624. doi: 10.1016/j.freeradbiomed.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Duong Van Huyen JP, Bloch F, Attar A, Levoir D, Kreft C, Molina T, Bruneval P. Diffuse mucosal damage in the large intestine associated with Irinotecan (CPT-11) Dig Dis Sci. 1998;43:2649–2651. doi: 10.1023/a:1026647110060. [DOI] [PubMed] [Google Scholar]
- 7.Wadler S, Benson AB, Engelking C, Catalano R, Field M, Kornblau SM, Mitchell E, Rubin J, Trotta P, Vokes E. Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol. 1998;16:3169–3178. doi: 10.1200/JCO.1998.16.9.3169. [DOI] [PubMed] [Google Scholar]
- 8.Baskerville A, Batter-Hatton D. Intestinal lesions induced experimentally by methotrexate. Br J Exp Pathol. 1977;58:663–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Levin RJ. Anatomical and functional changes of the small intestine induced by 5-fluorouracil. J Physiol. 1968;197:73P–74P. [PubMed] [Google Scholar]
- 10.Slavin RE, Dias MA, Saral R. Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients. Cancer. 1978;42:1747–1759. doi: 10.1002/1097-0142(197810)42:4<1747::aid-cncr2820420413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Siber GR, Mayer RJ, Levin MJ. Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res. 1980;40:3430–3436. [PubMed] [Google Scholar]
- 12.Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0. [DOI] [PubMed] [Google Scholar]
- 13.Meyn RE, Stephens LC, Hunter NR, Milas L. Kinetics of cisplatin-induced apoptosis in murine mammary and ovarian adenocarcinomas. Int J Cancer. 1995;60:725–729. doi: 10.1002/ijc.2910600526. [DOI] [PubMed] [Google Scholar]
- 14.Sorenson CM, Barry MA, Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990;82:749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- 15.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 16.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001;21:6913–6926. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyajima A, Nakashima J, Tachibana M, Nakamura K, Hayakawa M, Murai M. N-acetylcysteine modifies cis-dichlorodiammineplatinum-induced effects in bladder cancer cells. Jpn J Cancer Res. 1999;90:565–570. doi: 10.1111/j.1349-7006.1999.tb00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyajima A, Nakashima J, Yoshioka K, Tachibana M, Tazaki H, Murai M. Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br J Cancer. 1997;76:206–210. doi: 10.1038/bjc.1997.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh-Hamad D, Timmins K, Jalali Z. Cisplatin-induced renal toxicity: possible reversal by N-acetylcysteine treatment. J Am Soc Nephrol. 1997;8:1640–1644. doi: 10.1681/ASN.V8101640. [DOI] [PubMed] [Google Scholar]
- 21.Yáñez JA, Teng XW, Roupe KA, Fariss MW, Davies NM. Chemotherapy induced gastrointestinal toxicity in rats: involvement of mitochondrial DNA, gastrointestinal permeability and cyclooxygenase -2. J Pharm Pharm Sci. 2003;6:308–314. [PubMed] [Google Scholar]
- 22.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- 23.Reddy GB, Reddy PY, Vijayalakshmi A, Kumar MS, Suryanarayana P, Sesikeran B. Effect of long-term dietary manipulation on the aggregation of rat lens crystallins: role of alpha-crystallin chaperone function. Mol Vis. 2002;8:298–305. [PubMed] [Google Scholar]
- 24.Weijl NI, Hopman GD, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, Osanto S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann Oncol. 1998;9:1331–1337. doi: 10.1023/a:1008407014084. [DOI] [PubMed] [Google Scholar]
- 25.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Mack P, Wong KP. Glutathione-related mechanisms in cellular resistance to anticancer drugs. Int J Oncol. 1998;12:871–882. doi: 10.3892/ijo.12.4.871. [DOI] [PubMed] [Google Scholar]
- 27.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- 29.Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann SH. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989;49:5870–5878. [PubMed] [Google Scholar]


