Abstract
AIM: To clone and characterize the porcine aquaporins (AQPs) in the gastrointestinal system.
METHODS: A PCR-based cloning strategy and RACE were used to clone full-length AQP coding sequence from reversely transcribed pig liver cDNA. Stopped-flow light scattering and a YFP-based fluorescence method were used to measure the osmotic water permeability of erythrocytes and the stably transfected CHO cells. RT-PCR, Northern blot, and immunohistochemistry were used to determine the gastrointestinal expression and localization of cloned AQPs. Protein expression in transfected cells and red blood cells was analyzed by Western blot.
RESULTS: An 813 bp cDNA encoding a 271 amino acid porcine aquaporin (designated pAQP1) was cloned from liver mRNA (pAQP1 has a 93% identity with human AQP1 and contains two NPA motifs conserved in AQP family, one consensus sequence for N-linked glycosylation, and one mercury-sensitive site at cysteine 191). RT-PCR analysis revealed extensive expression of pAQP1 mRNA in porcine digestive glands and gut. Northern blot showed a single 3.0 kb transcript in selected digestive organs. pAQP1 protein was localized at central lacteals of the small intestine, microvessles of salivary glands, as well as epithelium of intrahepatic bile ducts by immunoperoxydase. High osmotic water permeability that is inhibitable by HgCl2 was detected in porcine erythrocytes and CHO cells stably transfected with pAQP1 cDNA. Immunoblot analysis of porcine erythrocytes and pAQP-transfected CHO cells revealed an unglycosylated 28 ku band and larger glycosylated proteins.
CONCLUSION: pAQP1 is the first porcine aquaporin that can be molecularly identified so far. The broad distribution of pAQP1 in epithelium and endothelium of porcine digestive organs may suggest an important role of channel-mediated water transport in fluid secretion/absorption as well as in digestive function and pathophysiology of the gastrointestinal system.
Keywords: Aquaporin, Molecular cloning, Porcine gastrointestinal organs, Water transport, Digestive function
INTRODUCTION
Aquaporins (AQPs) are water channel proteins located on membranes of various cell types where they create high water permeability. So far at least 12 mammalian members of AQP family have been molecularly localized in diverse fluid transporting tissues have been molecularly identified. Recent studies on human subjects with AQP gene mutations using transgenic AQP knockout mice indicate that AQPs have important functions in multi-tissue physiology and pathophysiology[1,2]. In the gastrointestinal (GI) system, several aquaporins (including AQPs 1, 3-5, 7-10) are localized on various epithelial and endothelial cell membranes of human and rodent GI organs and provide a trans-cellular pathway for fast water movement during fluid secretion and absorption[3-6]. Studies on AQP knockout mice demonstrated that AQP5 plays a role in saliva secretion[7], AQP1 a role in dietary fat processing[8], and AQP4 a role in colonic fluid absorption and fecal dehydration[9]. On the other hand, localization of AQPs in some mouse GI organs does not indicate physiological importance. For example, AQP4 deletion in gastric parietal cells does not affect gastric acid secretion[10], AQP1 deletion in micro-vessels of salivary glands does not affect saliva secretion, AQP1 deletion in intra-hepatic bile ducts does not affect the flow rate and components of bile[8]. GI phenotype in AQP1-null human subjects has not been reported so far, which may indicate species differences of AQP involvement in digestive physiology.
Although significant progresses have been made in molecular biology and physiology of AQP family in the GI system, identification and characterization of AQPs in GI organs of mammalian species other than in those of human and rodents have been poorly studied. The pig GI system more resembles the human GI system both structurally and physiologically in model animals[11]. However, no porcine AQP member has been identified molecularly so far. In the present study, we cloned the first porcine aquaporin, pAQP1 from pig liver, by a PCR-based homologous cloning strategy. Its functional properties and distribution in pig GI system were analyzed.
MATERIALS AND METHODS
cDNA cloning of pAQP1
Full-length coding sequence of pig AQP1 cDNA was cloned using a PCR-based homologous cloning strategy[12]. Total RNA was extracted from pig liver using TRIZOL reagent (Invitrogen). mRNA was isolated from the total liver RNA using an Oligotex mRMA kit (Qiagen). cDNA was reversely transcribed from the mRNA using a first strand cDNA amplification kit (Invitrogen) and used as template for 30 cycles of PCR amplification at 94 oC for 30 s, at 55 oC for 30 s, at 72 oC for 1 min with degenerate oligonucleotide primers designed according to amino acid sequences around the two NPA motifs of aquaporin family: sense: 5’-CA(C-T)IT(CA)AA(CT)CCIGCIGTIAC-3’; antisense: 5’-(GC)CI(AG)(AG)I(A-G)(AT)GC(TG)IGC(AT)GG(AG)TT-3’. PCR products of about 360 bp were subcloned into pCR2.1TOPO TA cloning vector (Invitrogen) and sequenced. A sequence with open reading frame that is most homologous to dog AQP1 water channel was identified. To clone the full length coding sequence of the candidate water channel, 5’- and 3’-RACE (rapid amplification of cDNA ends) were performed using the Marathon cDNA amplification kit (BD Biosciences-Clontech) following the provided protocol. The sequences of gene-specific primers (GSP) are as follows: 5’-RACE GSP: 5’-CACACACTGGGCAATGATGTACATG-3’; 5’-RACE NGSP: 5’-GACACTGATCTGGCAGCTGAGCAG-3’; 3’-RACE GSP: 5’-CCTTGCCATCGGCTTCTCTGTGGC-3’; 3’-RACE NGSP: 5’-CTGGGACACCTGCTGGCGATTGAC-3’. An Expond long PCR kit (Roche) was used for 30 cycles of RACE amplification at 94 oC for 30 s, at 65 oC for 30 s, at 68 oC for 4 min. PCR products were subcloned to pCR2.1TOPO TA cloning vector and sequenced. The longest open reading frame was obtained by joining the 5’-RACE and 3’-RACE sequence and the sequence between the two NPA motifs. The protein encoded by the longest open reading frame was designated as pAQP1.
RT-PCR and Northern blot
Total RNA was extracted from pig salivary glands, liver, pancreas, esophagus, stomach, small intestine and colon respectively, using TRIZOL reagent. For RT-PCR analysis, cDNAs were reversely transcribed from 5 µg of each total RNA using first strand cDNA amplification kit and 30 cycles of PCR amplification were performed at 94 oC for 30 s, at 62 oC for 30 s, at 72oC for 2 min with primers flanking the coding sequence of pAQP1 (sense: 5’-CGGATCCATGGCCAGCGAGTTCAAGAAG-3’; antisense: 5’-GCTCTAGATTATTTGGGCTTCATCTCCACC-3’. For Northern blot, total RNA (20 µg) from liver, salivary gland, small intestine and colon was resolved on 1.2% formaldehyde gel and transferred to a Hybond-N+ nylon membrane (Amersham). The membrane was prehybridized at 68 oC for 1 h in a rapid hybridization buffer (Amersham) and then hybridized for 1 h with a 32P-labled probe corresponding to the full-length pAQP1 coding sequence. The membrane was washed three times in 0.2 x SSC, 0.1% SDS at 68 oC, each for 15 min, and autoradiographed for 16 h with double intensifying screens. The membrane was boiled in water and re-probed with a 600 bp pig β-actin cDNA sequence.
Transfection and water permeability measurement of pAQP1 in CHO cells
A cell-based fluorescence microassay employing a Cl--sensitive EYFP mutant[13] was developed to measure the channel-mediated water permeability of plasma membrane. The 720 bp EYFP-H148Q-V163S DNA fragment encoding the Cl--sensitive EYFP mutant was PCR-amplified using Vent DNA polymerase (New England Biolabs) and primers with engineered restriction sites HindIII (5’ primer) and XbaI (3’ primer). The fragment was digested with HindIII/XbaI and ligated to the mammalian expression vector pcDNA3.1 Hygro (hygromycin-resistant, Invitrogen) predigested with HindIII/XbaI to form expression plasmid pcDNA3.1 Hygro-EYFP-H148Q-V163S. For pAQP1 expression, the 813 bp pAQP1 full length coding sequence was PCR-amplified using Vent DNA polymerase and primers with engineered restriction sites BamHI (5’ primer) and XbaI (3’ primer). The amplified pAQP1 fragment was digested with BamHI/XbaI and ligated to the mammalian expression vector pcDNA3.1(+) (G418-resistant, Invitrogen) predigested with BamHI/XbaI to form expression plasmid pcDNA3.1 pAQP1.
CHO-K1 cells were first transfected with expression plasmid pcDNA3.1Hygro-EYFP-H148Q-V163S using Lipofectin reagent (Invitrogen). After selection by hygromycin (Roche) at 500 μg/mL, highly expressed CHO clones were isolated by judging brightness of survived cell colonies under fluorescence microscope. The YFP-expressing cell clone, CHO-K1/EYFP-H148Q-V163S, was then transfected with pcDNA3.1 pAQP1 and selected with G418 (Invitrogen) at 500 μg/mL. pAQP1 expression in survived CHO cell clones was analyzed by immunoblot. CHO cell clones with high cytoplasmic expression of Cl--sensitive EYFP mutant and plasma membrane expression of pAQP1 were selected for water permeability measurement.
For water permeability measurement, the CHO-K1/EYFP-H148Q-V163S/pAQP1 cells were plated in 96-well clear bottom of black-walled microplates (Costar) at the density of 20000 cells per well in F-12 Ham’s medium supplemented with 10% FBS and 0.5 mg/mL G418. After incubation at 37 oC in an atmosphere containing 5% CO2 with 90% humidity for 18 h, the cells in 96-well plates were washed twice in PBS buffer (200 μL/wash), leaving 100μL PBS after the last wash. Measurement was performed on FLUOstar Optima plate reader (BMG Technology) equipped with syringe pumps and HQ500/20X (500 + 10 nm) excitation and HQ535/30M (535 + 15 nm) emission filters. Cells in each well of the plate were assayed individually for osmotically driven pAQP1-mediated water influx across the plasma membrane that dilutes the cytoplasmic Cl- by recording fluorescence increase continuously (0.2 s per point) for 2 s (baseline) and then for 21 s (water transport into the cells) after rapid injection (< 1 s) of 100 μL distilled water. Water permeability was expressed as half time (t1/2) needed from water injection to the point when the cytoplasmic fluorescence reached the maximum. The smaller t1/2 represented the higher water permeability.
Measurement of erythrocyte water permeability
Pig blood was drawn from the ear by venipuncture, and the washed erythrocytes were diluted into phosphate buffered saline (PBS) at a 0.1 vol %. Osmotic water permeability was measured by a stopped-flow light scattering technique in which the diluted erythrocyte suspension was mixed rapidly with PBS containing 100 mmol/L sucrose[14]. Erythrocyte volume was measured continuously by 90 s light scattering for 90 s at a 520-nm wavelength.
Immunohistochemistry
Paraffin-embedded blocks were prepared from fresh normal pig salivary gland, liver, and small intestine fixed in 4% formalin. For immunohistochemistry, 3-µm sections of pig tissues were prepared by standard procedures and exposed to the primary affinity-purified rabbit-antirat AQP1 antibody (AB3065, Chemicon International) diluted at 1:1000 for 1 h at room temperature. In some experiments, the primary AQP1 antibody was preincubated with the immunizing peptide before administered to the tissue sections. After washed, the sections were exposed to a horseradish peroxidase-conjugated goat antirabbit IgG (A6154, Sigma) secondary antibody at 1:2000 dilution followed by development with 3,3’-diaminobenzidine (DAB) liquid substrate dropper system (D7679, Sigma). The sections were counterstained with haematoxylin as the last step.
Western blotting
Stably transfected CHO cells were lysed in 10 mM HEPES buffer containing 0.5% SDS, 100 mmol/L DTT and 57.4 µmol/L PMSF (pH 7.5). Red blood cells from pigs, human beings and mice were first burst in hypoosmotic solution, and then the ghosts were centrifuged and lysed in SDS buffer. Protein concentrations in CHO cell and ghost lysates were determined spectrophotometrically. For immunoblotting, 20 µg proteins from the CHO cell lysate or 5 µg proteins from the ghost lysate of red blood cells from pigs, human beings and mice were dissolved in protein sample buffer, heated at 65 °C for 10 min, and then resolved on 12% SDS-PAGE minigels. Proteins were blotted into PVDF membranes, blocked for 1 h, washed with TBS-T (pH 7.4), and incubated for 1 h at room temperature with anti-AQP-1 antibody diluted at 1:1000. After washed, membranes were incubated with HRP-conjugated goat anti-rabbit IgG (1:3000, Sigma), and immunoreactive sites in membranes were revealed by enhanced chemiluminescence (Amersham).
RESULTS
Cloning and analysis of pAQP1 cDNA
The full-length cDNA coding sequence and the predicted amino acid sequence of pAQP1 (Genbenk accession no. AY585335) are shown in Figure 1A. The 813 bp open reading frame encoded a 271-amino acid protein containing the two conserved NPA motifs found in most AQP family members and a cysteine residue located just upstream of the second NPA motif, which could confer mercurial sensitivity in several cloned mammalian AQPs[15,16]. Compared to human and mouse AQP1 coding sequences, an additional segment of GCGGCC at nt 138–143 encoding tow alanines (residues 47 and 48) was found only in the porcine sequence. Figure 1B shows the Kyte-Doolitle hydropathy plot for pAQP1, suggesting six membrane spanning domains similar to those of human and rat AQP1. Figure 2 shows an amino acid sequence alignment of pAQP1 with its homologues from dogs, human beings, and mice. pAQP1 had the greatest amino acid identity to dog AQP1 (94%) and high homology to human AQP1 (93%) and mouse AQP1 (91%). The pAQP1 had a 29 - 52% similarity in amino acid sequences to other mammalian aquaporin members[15,16]. There were two consensus sequences for phosphorylation by protein kinase-C like in human and mouse AQP1. However, pAQP1 contained only one consensus site at residue 42 for N-linked glycosylation compared to two sites in human and mouse AQP1 sequences at residues 42 and 205. In addition, there were two more amino acids in the pig and dog AQP1 sequences at residues 47 and 48 instead of in the human and mouse sequences.
Figure 1.
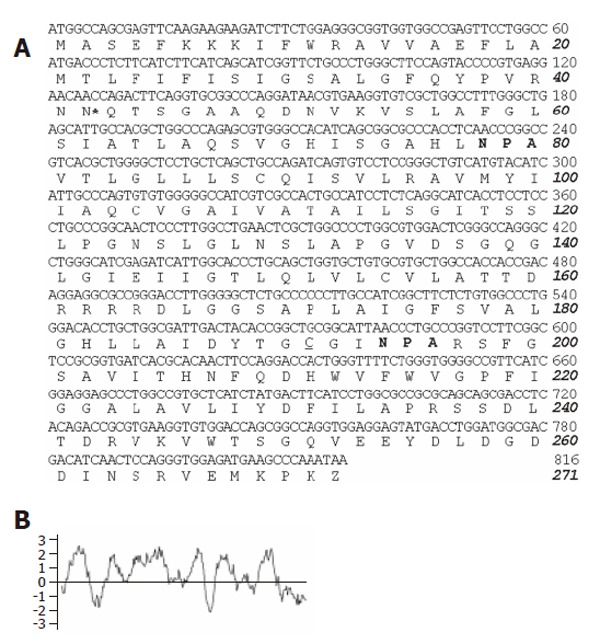
cDNA and deduced amino acid sequences of pig AQP1 water channel. A: Two NPA sequences conserved in water channel proteins of aquaporin family (in bold). *indicates the consensus sequence for N-linked glycosylation and the mercurial-sensitive site cysteine 201 near the second NPA motif is underlined; B: Kytte-Doolittle hydropathy profile of the deduced pAQP1 amino acid sequence.
Figure 2.

Amino acid sequence alignment of pAQP1 with its dog, human and mouse orthologs. Conserved amino acids are shielded, NPA motifs are boxed and cycteines near the second NPA motifs are underlined. The consensus N-linked glycosylation sites are shown in bold.
Expression and immunolocalization of pAQP1 in digestive organs
Multitissue RT-PCR analysis indicated broad expression of pAQP1 mRNA in pig digestive organs including salivary glands, pancreas, liver and different segments of the gut (Figure 3A). Figure 3B shows Northern blot analysis of total RNA from pig salivary glands, liver, small intestine, and colon respectively using the full length pAQP1 cDNA as a probe. A single transcript of about 3.0 kb was seen in liver ~ small intestine > colon > salivary glands. Figure 4 shows an immunoperoxidase staining of pAQP1 in selected digestive organs. Specific AQP1 labeling was seen both in the endothelia of central lacteals in villi of the small intestine (Figures 4A and 4B) and in the endothelia of microvessels in salivary glands (Figure 4C). In the liver, AQP1 staining was mainly seen both in the epithelium of small bile ducts and endothelium of some small vessels (Figures 4D and 4E). AQP1 labeling was predominant in the apical domain of most stained bile ducts.
Figure 3.
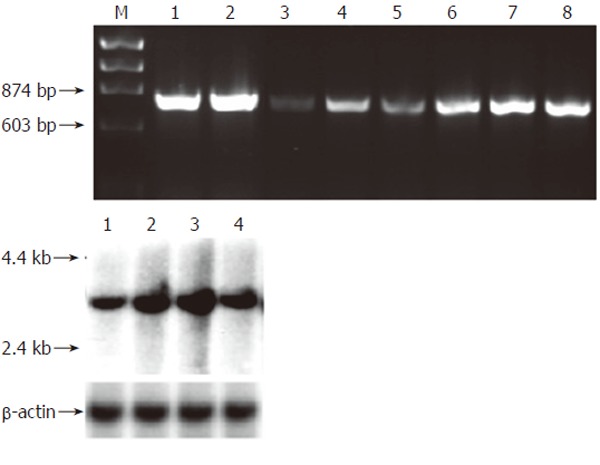
mRNA expression of pAQP1 water channel in pig gastrointestinal organs. A: Expression of pAQP1 transcript by RT-PCR analysis. Lanes 1, Salivary gland; 2, Liver; 3, Pancreas; 4, Esophagus; 5, Stomach; 6, Jejunum; 7, Ileum; 8, Colon; B: Northern blotting of total RNA (20 µg) from salivary gland (lane 1), liver (lane 2), ileum (lane 3) and colon (lane 4) probed with the pAQP1 coding sequence (upper) and the same membrane probed with a 600 bp pig β-actin cDNA sequence (lower).
Figure 4.
Immunolocalization of pAQP1 in pig gastrointestinal tract and exocrine glands. Specific pAQP1 labeling of central lacteals was seen by immunoperoxidase staining of longitudal (A) and traverse (B) sections of the small intestinal villi using an affinity-purified AQP1 antibody. Arrows indicate the endothelium of central lacteals; A salivary gland section showing specific labeling of microvessel endothelium indicated by arrows (C); Specific pAQP1 labeling was seen in the epithelium of intrahepatic bile ducts in longitudal (D) and traverse (E) sections. Arrows indicate heavy pAQP1 staining in the apical domain of bile duct epithelial cells. Arowhead indicates pAQP1 labeling in the endothelium of periductal microvessels; A consecutive section of E showing immunostaining with AQP1 antibody preabsorbed with the immunizing peptide (F).
Functional characterization of pAQP1
Since AQP1 is a erythrocyte water channel, osmotic water permeability of pig red blood cells was analyzed using stopped-flow light scattering and compared with human and mouse red blood cells. Figure 5A shows the time course of osmotic cell shrinking in response to a 100 mmol/L inwardly directed osmotic gradient. Two contiguous time scales were used to plot the full time course of decreasing cell volume (increasing light intensity). The computed osmotic water permeability of pig, human and mouse erythrocytes is summarized in Figure 5B. The pig erythrocyte membrane showed a high osmotic water permeability that was inhibited > 90% by 0.3 mmol/L HgCl2, similar to that of human and mouse erythrocytes. Water channel function of pAQP1 was also analyzed in heterologous expression system. Figure 5C shows the time course of YFP fluorescence of CHO cells stably transfected with pAQP1 cDNA in response to extra-cellular hypotonisity (50% PBS). The t1/2 of fluorescence dynamics for pAQP1-transfected cells was 1.2 s compared to 4.6 s for the same cells incubated with 0.1mmol/L HgCl2 and > 20 s for mock-transfected cells, indicating that a high membrane water permeability was mediated by pAQP1 water channel inhibited by mercurial compounds. Figure 5D shows Western blot analysis of red blood cells and pAQP1-transfected CHO cells using an affinity-purified AQP1 antibody. An unglycosylated 28 ku band and glycosylated proteins at a higher molecular weight were determined both in red blood cells of human beings, pigs, wildtype mice and in pAQP1-transfected CHO cells, but missed in AQP1-knockout mouse erythrocytes. There was a significant decrease of glycosylated protein in pAQP1 compared to human AQP1 and mouse AQP1 in red blood cells. Western blot analysis of pig gastrointestinal tissues was not done because of the difficulty to remove red blood cell contamination.
Figure 5.
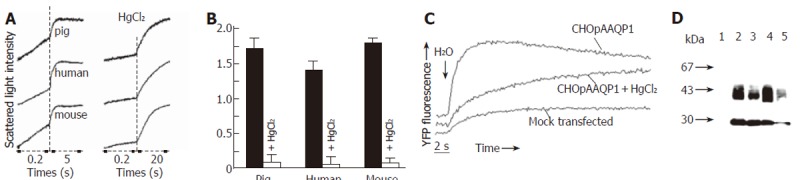
Functional properties of pAQP1 in erythrocytes and stably transfected CHO cells. A: Osmotic water permeability of pig, human and mouse erythrocytes measured by stopped-flow light scattering from the time course of erythrocyte volume in response to a 100 mmol/L inwardly directed sucrose gradient in the absence (left panel) and presence (right panel) of 0.3 mmol/L HgCl2; B: Summary of osmotic water permeability coefficient (Pf) for erythrocytes from pig, human and mouse measured in A (mean ± SE, n = 4); C: Osmotic water permeability of CHO cells stably transfected with pAQP1 cDNA measured by YFP-based fluorescence assay. Water permeability of CHO cells was expressed as half time (t1/2) needed from water injection to the point when the cytoplasmic fluorescence reached the maximum. t1/2 of pAQP1 transfected CHO cells in the absence and presence of 0.1 mmol/L HgCl2 was 1.2 s and 4.6 s separately. t1/2 > 20 s in mock-transfected CHO cells; D: Immunoblot of erythrocytes and transfected CHO cells. Lanes 1 - 4: erythrocytes from AQP1-/- mice, AQP1 +/+ mice, pigs and human beings. Lane 5: CHO cells stably transfected with pAQP1 cDNA.
DISCUSSION
We have successfully cloned pAQP1, the first molecularly identified porcine AQP water channel. The amino acid sequence of pAQP1 has a high identity to dog, human and mouse AQP1 and contains the two highly conserved NPA motifs and the mercurial-sensitive cysteine residue before the second NPA motif in several cloned AQPs in other mammals[16-19]. pAQP1 contains only one consensus sequence compared to two consensus sequences for N-linked glycosylation in human and mouse AQP1[20,21]. Immunoblot analysis showed that glycosylated protein of pAQP1 was decreased compared to human and mouse AQP1 in red blood cells, indicating that the second consensus site at asparageine 205 of human and mouse AQP1 contributes significantly to the N-linked glycosylation of the protein.
Like its human and mouse orthologs, pAQP1 conferred a high osmotic water permeability in a mercurial-sensitive manner both in pig red blood cells and in heterologous expression system, indicating a similar functional water channel in erythrocyte and somatic cell membranes. pAQP1 mRNA and protein were expressed extensively in endothelia and epithelia of pig gastrointestinal system involving digestive glands responsible for secretion of saliva, bile, and pancreatic juice as well as all the intestinal segments responsible for absorption of fluids and nutrients, suggesting that channel-mediated transcellular water pathway plays a role in broad physiological processes of secretion and absorption in various porcine digestive organs. The molecular cloning of pAQP1 makes it possible to investigate its expression and regulation during development and in various physiological and pathophysiological conditions of the pig gastrointestinal system.
ACKNOWLEDGMENTS
The authors thank Ms. Shuqin Pan in China-Japan Union Hospital, Jilin University, for her excellent technical assistance in immunohistochemistry.
Footnotes
Supported by National Natural Science Foundation for Distinguished Young Scholars, No.30325011; National Natural Science Foundation of China, No.30470405; and Distinguished Young Scholar Foundation of Jilin Province, No.20030112.
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF
References
- 1.King LS, Yasui M. Aquaporins and disease: lessons from mice to humans. Trends Endocrinol Metab. 2002;13:355–360. doi: 10.1016/s1043-2760(02)00665-3. [DOI] [PubMed] [Google Scholar]
- 2.Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002;34:192–200. [PubMed] [Google Scholar]
- 3.Koyama Y, Yamamoto T, Tani T, Nihei K, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K, et al. Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol. 1999;276:C621–C627. doi: 10.1152/ajpcell.1999.276.3.C621. [DOI] [PubMed] [Google Scholar]
- 4.Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517(Pt 2):317–326. doi: 10.1111/j.1469-7793.1999.0317t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc. 2004;37:71–80. doi: 10.1007/s00795-004-0246-3. [DOI] [PubMed] [Google Scholar]
- 6.Mobasheri A, Shakibaei M, Marples D. Immunohistochemical localization of aquaporin 10 in the apical membranes of the human ileum: a potential pathway for luminal water and small solute absorption. Histochem Cell Biol. 2004;121:463–471. doi: 10.1007/s00418-004-0657-1. [DOI] [PubMed] [Google Scholar]
- 7.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 8.Ma T, Jayaraman S, Wang KS, Song Y, Yang B, Li J, Bastidas JA, Verkman AS. Defective dietary fat processing in transgenic mice lacking aquaporin-1 water channels. Am J Physiol Cell Physiol. 2001;280:C126–C134. doi: 10.1152/ajpcell.2001.280.1.C126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KS, Ma T, Filiz F, Verkman AS, Bastidas JA. Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am J Physiol Gastrointest Liver Physiol. 2000;279:G463–G470. doi: 10.1152/ajpgi.2000.279.2.G463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang KS, Komar AR, Ma T, Filiz F, McLeroy J, Hoda K, Verkman AS, Bastidas JA. Gastric acid secretion in aquaporin-4 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G448–G453. doi: 10.1152/ajpgi.2000.279.2.G448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 12.Preston GM. Cloning gene family members using PCR with degenerate oligonucleotide primers. Methods Mol Biol. 2003;226:485–498. doi: 10.1385/1-59259-384-4:485. [DOI] [PubMed] [Google Scholar]
- 13.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Ma T, Verkman AS. Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J Biol Chem. 2001;276:624–628. doi: 10.1074/jbc.M008664200. [DOI] [PubMed] [Google Scholar]
- 15.Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- 16.Verkman AS, Shi LB, Frigeri A, Hasegawa H, Farinas J, Mitra A, Skach W, Brown D, Van Hoek AN, Ma T. Structure and function of kidney water channels. Kidney Int. 1995;48:1069–1081. doi: 10.1038/ki.1995.390. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, van Hoek AN, Biwersi J, Verkman AS. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry. 1993;32:2938–2941. doi: 10.1021/bi00063a002. [DOI] [PubMed] [Google Scholar]
- 18.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 19.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 20.Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993;265:F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- 21.Moon C, Williams JB, Preston GM, Copeland NG, Gilbert DJ, Nathans D, Jenkins NA, Agre P. The mouse aquaporin-1 gene. Genomics. 1995;30:354–357. doi: 10.1006/geno.1995.0029. [DOI] [PubMed] [Google Scholar]


