Abstract
AIM: To investigate the ABH and Lewis antigen expression in erythrocytes, saliva and gastric epithelium, as well as the association between H pylori and the presence of gastric epithelial lesions.
METHODS: The distribution of ABH and Lewis blood group antigens in erythrocytes, saliva and gastric mucosa of H pylori-infected gastric ulcer patients was analyzed. Forty-two patients with gastric ulcer were studied, and fifty healthy individuals were used as control group. The blood group antigens were determined by direct hemagglutination, dot-ELISA and immunohistochemical methods in erythrocytes, saliva and gastric mucosa specimens, respectively. Diagnosis for H pylori infection was performed by conventional optical microscopy and ELISA.
RESULTS: A higher seroprevalence of IgG H pylori specific antibodies was observed in gastric ulcer patients (90%) compared to the control group (60%). We observed a significant increase of phenotypes O, A2 and Lewis b in H pylori-infected patients. The expression of these antigens had progressive alterations in areas of ulcerous lesions and intestinal metaplasia.
CONCLUSION: ABH and Lewis blood group antigens are a good indicator for cellular alterations in the gastric epithelium.
Keywords: Helicobacter pylori, Gastric ulcer, ABH and Lewis blood groups
INTRODUCTION
The presence of H pylori in gastric mucosa is associated with chronic active gastritis and more severe gastric diseases, including chronic atrophic gastritis, peptic ulcers, stomach cancer, and lymphoma[1,2]. However, only a minority of H pylori-infected patients develop gastric diseases. In order to explain this fact, the influences of additional factors such as the genetic predisposition of the host and the genotype of H pylori strains have been analyzed[3]. Biochemical studies[4,5] have discovered a blood group antigen binding adhesin (BabA), which can mediate bacterial adherence to epithelial cells and seems necessary for H pylori pathogenicity by facilitating the subsequent action of the other virulent factors such as VacA and CagA. Borén et al[6,7] demonstrated that the receptors for H pylori on gastric epithelial cells are the H and Leb antigens of the ABH and Lewis (Le) blood group systems. It has been known for decades that individuals of O blood group phenotype have a higher risk of developing duodenal ulcers[8,9] and also a higher incidence of gastric ulcers[8,10]. In ulcer disease patients infected with H pylori little is known about the presence of ABH and Lewis antigens in erythrocytes, saliva and gastric epithelium. However, alterations in these blood group antigen expressions have been extensively described in stomach cancer and precursor lesions[11,12]. This study was to investigate the ABH and Lewis antigen expression in erythrocytes, saliva and gastric epithelium in H pylori-infected patients as well as the association of H pylori status with these blood group phenotypes and the presence of gastric epithelial lesions.
MATERIALS AND METHODS
Patients and control sample
The study included a total of 42 patients with gastric ulcer who were examined by routine upper endoscopy at Ofir Loiola Hospital (Belém, PA, Brazil) between May and December 2000, and comprised 76% males (32/42) and 24% females (10/42). The mean age was 53 years, ranging 28-80 years. Blood and saliva samples and gastric biopsy specimens were collected from each patient. These selected patients did not take non-steroidal anti-inflammatory drugs, H2 receptor antagonists, proton pump inhibitors or anti-microbial drugs for at least 60 d before the samples were obtained. Peripheral blood and saliva samples were collected from 50 patients asymptomatic for gastric diseases. These patients did not receive upper endoscopy. The mean age of these individuals was 49 years, ranging 25 - 80 years. This study was approved by the Ethics Committee at the Tropical Medicine Nucleus of the Pará Federal University and informed consent was obtained from the patients before sample collection.
Histopathological analysis of gastric biopsies
For histological analysis, biopsies from the ulcer lesion border and the adjacent area (perilesion) of each patient were obtained. Paraffin-embelded biopsy specimens were sectioned at 4 µm thickners and stained with haematoxylin - eosin and evaluated using the Sydney classification[13] with regard to the presence of intestinal metaplasia (IM) and the degree of granulocytic and limphocytic infiltration (mild, moderate, severe). The density of H pylori was determined in the sections using a modified Gram staining and graded into absent, mild, moderate and strong, based on the above classification system[13].
Serological detection of specific antibodies against H pylori and CagA
The serum samples were tested for IgG-class antibodies against H pylori by an indirect hemagglutination assay and anti-CagA with a commercial kit based on recombinant Helicobacter antigens p120 EIA. Both tests were performed according to the manufacturer’s instructions (Viva Diagnostika, Hürth, Germany). H pylori infection diagnosis of the control group was performed using only serological methods. However in the ulcer disease patients H pylori status was determined by serological and conventional optical microscopic methods.
Detection of ABO and Lewis blood group antigens
Blood and saliva samples were collected after the endoscopy. In blood the ABO and Lewis phenotypes were determined with a conventional direct hemagglutination technique. The characterization of ABH and Lewis specificities in saliva was tested using the dot-ELISA technique on nitrocellulose[14]. Immunohistochemistry for ABO and Lewis blood group antigen expression in gastric biopsies (ulcer lesion border and perilesion) in the foveolar and fund epithelium was performed using an indirect immunoperoxidase technique[15]. The reaction pattern of these antigens in gastric mucosa was classified as positive (homogeneously with more than 50% of stained cells or heterogeneous with 5 - 50% of stained cells) and negative (without or lower than 5% of stained cells).
Statistical analysis
Statistical tests using Bioestat 3.0 were performed to verify the significance of the differences observed in our study[16]. The chisquaretest (χ2) was used as a global test for any relationship. The Mann-Whitney U test was used to compare unpaired data. Spearman’s rank and contingence correlation tests were used to examine the relationship between density of H pylori, polymorph nuclear activity and chronic inflammation. P<0.05 was considered statistically significant.
RESULTS
Seroprevalence of H pylori infection and CagA strains
Endoscopic diagnosis of patients with gastric ulcer indicated 74% (31/42) ulceration in the antral region and 26% (11/42) in the corpus region. The presence of IgG H pylori specific antibodies was observed in 90% (38/42) of all patients. Approximately 84% (32/38) of these H pylori-infected patients were also CagA seropositive. In the control group, seroprevalence of IgG H pylori specific antibodies was observed in 60% (30/50) of the individuals and 36% (18/50) were also infected with CagA strains. These distributions were significantly lower in H pylori-infected patients than in gastric ulcer patients (P < 0.01).
Distribution of ABO and Lewis phenotypes in erythrocytes and saliva of patients and control group
A comparison of the ABO blood group phenotype distributions in blood and saliva of gastric ulcer patients and control group is showed in Table 1. Regarding the Lewis blood group system in saliva, the Leb antigen was detected in approximately 95% of the patients and in the control group. The distribution frequency of ABO (P > 0.05) and Lewis saliva (P > 0.05) phenotypes observed in gastric ulcer patients had no difference in relation to the control group. We observed a discrepancy in the expression of Lewis antigens in erythrocytes and saliva in some patients (Table 1), which were divided in two groups: concordant (individuals with similar expression of Lewis antigens in blood and saliva) and discordant (individuals with different expressions of Lewis antigens in blood and saliva). A discordant phenotype was observed in 57% (24/42) of the patients. Of these, 83% (19/24) belonged to the negative erythrocyte Lewis phenotype. A difference in the blood and saliva Lewis antigen expression (Table 1) was observed in only 22% (11/50) of the control group.
Table 1.
Distribution of ABO and Lewis phenotypes in erythrocytes and saliva of gastric ulcer patients and controls
| Lewis erythrocytes |
Saliva |
Secretor | Lewis phenotypes Blood/Salive |
ABO Phenotypes |
||||||||||
| A1 |
A2 |
B |
AB |
O |
||||||||||
| Lea | Leb | I | II | I | II | I | II | I | II | I | II | |||
| Le (a+ b-) | + | + | S | Concordant | 1 | 2 | 1 | - | - | 1 | 1 | - | 2 | - |
| Le (a- b+) | + | + | S | Concordant | 1 | 7 | 2 | 2 | 1 | 7 | - | 1 | 12 | 20 |
| Le (a- b-) | - | - | S | Concordant | - | 2 | - | - | - | - | - | - | 2 | - |
| + | + | S | Discordant | 3 | - | 1 | 1 | 4 | - | - | - | 7 | 7 | |
| - | + | S | Discordant | 1 | - | - | - | 1 | - | - | - | 2 | - | |
| Total | 6 | 11 | 4 | 3 | 6 | 8 | 1 | 1 | 25 | 27 | ||||
I = gastric ulcer patients, II = control, S = secretor status
Distribution of ABH and Lewis antigen expression in gastric mucosa regions
The pattern of ABH and Lewis antigen distribution in the foveolar epithelium of the perilesion areas compared with ulcer lesion borders showed a loss of A, H and Leb antigen expression, resulting in a decrease of homogeneous expression and an increase in the heterogeneous pattern. At the same time, an increased expression of Lea antigen was observed in the ulcerous lesion border areas (Figure 1A). The presence of incomplete intestinal metaplasia (IM) was observed in 25 out of 42 ulcer patients. In these patients the same abnormal pattern of ABH and Lewis blood group antigen expression was found to be more intensive, with a significant increase (P < 0.01) in the expression of Lea antigen and a decrease in A, H and Leb antigen expression (Figure 1B).
Figure 1.
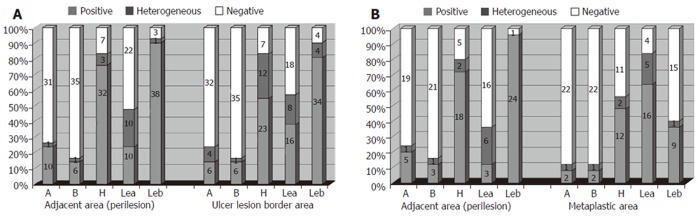
ABO and Lewis antigen distribution pattern in ulcer lesion borders (A) and intestinal metaplastic area (B) of gastric ulcer patients.
Correlation of ABH and Lewis blood group antigen expressions in saliva, erythrocytes and foveolar epithelium of gastric mucosa
In the perilesion area of the foveolar gastric epithelium, the ABH antigen expression was in agreement with that in erythrocytes and saliva. All the patients belonging to the A blood group (10/42) expressed antigens A and B. Six of them also expressed antigen H. Likewise, patients of the B blood group (a total of six) expressed antigen B, antigen H was expressed in four of them. Patients of AB blood group expressed antigens A and B. Antigen H was expressed in all O blood group patients (a total of 25 individuals). Analysis of the Lewis phenotype in the perilesion region showed a similar antigen expression pattern to that in saliva of all patients, including those with discordant Lewis phenotypes in saliva and blood. Only one patient had discordant and concordant expression with the erythrocyte phenotype.
Association of ABH and Lewis blood group antigen distribution with H pylori infection and histopathological findings
The Lewis saliva phenotype was used to associate H pylori status and the development of gastric ulcers, because this expression was similar to that in the foveolar epithelium in gastric biopsies. Furthermore, the presence of ABO and Lewis antigens in the control group was determinated only in saliva and erythrocytes but not in gastric mucosa. In relation to the seroprevalence of H pylori infection, a significantly higher level was observed in a defined combination of O-/A2 Le (a-b+) blood groups than in the set of other blood group phenotypes of the control group (Table 2). However, no significantly different proportions were found in this subdivision, a finding that might be explained by the high prevalence of the infection. A significant positive correlation was found between bacterial density and degree of chronic inflammation (P < 0.05) as well as the polymorph nuclear activity (P < 0.05). The degree of chronic inflammation was found to be positively correlated with polymorph nuclear activity (P< 0.01). Subsequently, no significant correlation was found by contingency analysis for the variability of histological scores in regard to bacterial density between O-/A2 Le (a-b+) individuals and the set of other blood group phenotypes (P > 0.05), lymphocytic (P > 0.05) and granulocytic infiltration degrees (P > 0.05) in biopsies from ulcer patients.
Table 2.
Distribution of ABO and Lewis blood group phenotypes in relation to serological diagnosis of H pylori infection in ulcer patients and controls
| Blood group |
H pylori infection |
|||||
| Patientsa | Controlsb | |||||
| phenotypes |
N |
Positive |
Negative |
N |
Positive |
Negative |
| A2 Le(a-b+) ou O Le(a-b+) | 27 | 26 | 1 | 30 | 25 | 5 |
| A1/AB/B/Le(a-b+) ou O Le(a-b-) | 15 | 12 | 3 | 20 | 5 | 15 |
| Total | 42 | 38 | 4 | 50 | 30 | 20 |
P > 0.05 vs bacterial density, lymphocytic and granulocytic infiltration degree;
P < 0.01 vs polymorph nuclear activity.
DISCUSSION
The sero-prevalence of IgG H pylori specific antibodies is 90% in patients with gastric ulcer, much higher than in asymptomatic individuals (60%). Additionally, the cagA seropositive phenotype shows a significant association with gastric ulcer patients. The frequencies obtained in patients agree with the reported results in previous studies[17], in which a high rate of infection was found in symptomatic adults. The same occurs with the rate obtained in asymptomatic adults. This fact also corroborates the studies of seroprevalence in developing countries, which describe a 60% infection rate in the adult populations[18,19]. The Lewis blood group antigens in saliva and blood of patients and the control group demonstrated a high frequency of discordant Lewis phenotype. Among these individuals, most of them were grouped in the non-genuine negative Lewis phenotype according to the Ørntoft classification[20], where Lewis antigens are present in saliva but not in the erythrocytes, which is different from the genuine Lewis phenotype, in which the Lewis antigens are present neither in saliva nor in erythrocytes. The expression of ABH and Lewis blood group antigens in the foveolar epithelium in perilesion areas was similar to that observed in saliva, with no differences in relation to the normal synthesis of these antigens. This can be explained by the fact that circulating Lewis antigens in the serum are only acquired by red cell membranes[21]. In some physiological conditions and diseases, such as neoplasia, a reduction in the synthesis of these antigens can occur, so that the quantities of these antigens in the blood are not sufficient to be detectable by serological methods, however the salivary phenotypes do not alter[21]. Probably, the increased degree of inflammation in the gastric mucosa due to infection with H pylori, affects the metabolism of glycoconjugates, leading to a decrease in the quantity of Lewis antigens circulating in the plasma. The ABO blood group phenotype frequencies in ulcer patients and the control group were not different. This finding is in contradiction to many studies, which have described the increased prevalence of ulcers among O group individuals[9,10].
An interesting finding of our study is the observation that all A2 subgroup individuals were H pylori positive, which has been described in another study regarding gastritis patients[18]. The reason for this finding is not clear. Theoretically, the qualitative structural difference between A1 and A2 subgroups is based on glycosphingolipids expressed in erythrocytes, so that A1 transferase is more efficient for converting specific carrier types (H type 3 chains or H type 4 chains) into a determinant than A2 transferase, resulting in a higher level of H epitope in A2 subgroup, almost like that in O blood group[20]. Therefore, we believe that analogue fucosylated structures might exist as a mucosal gastric component relating to the different A subgroups, since glycosphingolipids present in gastric mucosa can function as receptors for the bacterium[22]. Besides, complex gangliosides (i.e. repetitive N-acetyllactosamine units, fucose branches and di- or multivalency) have been demonstrated in the binding assays as preferred structural requirements for mediating H pylori adherence to gastrointestinal epithelial cells[23].
Furthermore, our study regarding ABO and Lewis phenotype and H pylori infection indicated that individuals with O Le (a-b+) and A2 Le (a-b+) phenotypes had a tendency towards a higher rate of H pylori infection. Studies performed by Bóren et al 6, 7] in 1993 and 1994 have suggested that H pylori uses carbohydrate structures with terminal fucose as receptors in the gastric mucosa containing Leb and H blood group specificities. Later on, Ilver et al[5] in 1998 and Gerhard et al[24] in 1999 showed that there is a higher susceptibility to H pylori infection in individuals of O and Leb blood groups, because they have a higher quantity of fucosylated antigens.
In the current study, the association of ABH and Lewis blood group phenotype distributions with the histological scores was not significantly different. But if one considers that Leb is absent only in 2 out of 42 patients, this result is inappropriate for comparison with other histopathological studies[25-27]. By comparing the ABH and Lewis antigen expression between the ulcerous lesion border and the adjacent areas (perilesion), we observed an increase in the heterogeneous expression pattern, demonstrating the loss of A, H and Leb antigen expression and appearance of Lea reactivity in inflammation regions. Colonization of the gastric mucosa by H pylori is a relevant factor that can alter the normal pattern of homogeneous expression.
Some studies have demonstrated that the expression of these blood group antigens is altered in metaplastic areas[17,28]. The main alterations described are the increase of Lea and the decrease of H and Leb antigens, as was also found in this study. One explanation for this observation is the repression of the secretor enzyme activity, leaving more type I precursor chains available to be transformed into Lea by the Lewis enzyme, consequently reducing the expression of the H antigen and its transformation into A and/or B and Leb antigens in these tissues[29].
In conclusion, the rate of H pylori infection seems to be higher among O, A2 and Le(a-b+) phenotype individuals. The pattern of Lewis expression changes in ulcer disease patients with H pylori presence, mainly in intense inflammation and/or intestinal metaplastic areas, showing the increase of Lea and loss of H and Leb antigens in the gastric mucosa. Therefore, it is important to understand how ABH and Lewis antigens are regulated in gastric cancer, as well as the interaction of these histo-blood group antigens with H pylori adhesion, which needs to be further investigated.
Footnotes
Supported by the Secretaria Executiva de Ciência, Tecnologia e Meio Ambiente - SECTAM and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES.
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF
References
- 1.Gerhard M, Rad R, Prinz C, Naumann M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2002;7 Suppl 1:17–23. doi: 10.1046/j.1523-5378.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 3.Mobley HL. Helicobacter pylori factors associated with disease development. Gastroenterology. 1997;113:S21–S28. doi: 10.1016/s0016-5085(97)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Prinz C, Schöniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, Classen M, Rösch T, Schepp W, Gerhard M. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61:1903–1909. [PubMed] [Google Scholar]
- 5.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borén T, Normark S, Falk P. Helicobacter pylori: molecular basis for host recognition and bacterial adherence. Trends Microbiol. 1994;2:221–228. doi: 10.1016/0966-842x(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 7.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 8.Clarke CA, Evans DA, Mcconnell RB, Sheppard PM. Secretion of blood group antigens and peptic ulcer. Br Med J. 1959;1:603–607. doi: 10.1136/bmj.1.5122.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentis A, Blackwell CC, Weir DM, Spiliadis C, Dailianas A, Skandalis N. ABO blood group, secretor status and detection of Helicobacter pylori among patients with gastric or duodenal ulcers. Epidemiol Infect. 1991;106:221–229. doi: 10.1017/s0950268800048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merikas G, Christakopoulos P, Petropoulos E. Distribution of ABO blood groups in patients with ulcer disease. Its relationship to gastroduodenal bleeding. Am J Dig Dis. 1966;11:790–795. doi: 10.1007/BF02233839. [DOI] [PubMed] [Google Scholar]
- 11.Murata K, Egami H, Shibata Y, Sakamoto K, Misumi A, Ogawa M. Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am J Clin Pathol. 1992;98:67–75. doi: 10.1093/ajcp/98.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto S, Watanabe T, Tokumaru T, Takagi H, Nakazato H, Lloyd KO. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989;49:745–752. [PubMed] [Google Scholar]
- 13.Misiewicz JJ. The Sydney System: a new classification of gastritis. Introduction. J Gastroenterol Hepatol. 1991;6:207–208. doi: 10.1111/j.1440-1746.1991.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 14.Pflug W, Bässler G, Eberspächer B. ABO and Lewis typing of secretion stains on nitrocellulose membranes using a new dot-blot-ELISA technique. Forensic Sci Int. 1989;43:171–182. doi: 10.1016/0379-0738(89)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.Pedal I, Reichert W, Oliveira Corvelo TC. [Seminal vesicle epithelium of Lewis positive individuals secretes Le(a) in sialyl form] Beitr Gerichtl Med. 1989;47:153–158. [PubMed] [Google Scholar]
- 16.Ayres M, Ayres MJ, Ayres DL, Santos AS. Bioestat 3.0 - Aplicações estatísticas nas áreas das ciências biológicas e médicas. Sociedade Civil Mamirauá MCT - CNPq. 2004. [Google Scholar]
- 17.Genta RM, Gürer IE, Graham DY, Krishnan B, Segura AM, Gutierrez O, Kim JG, Burchette JL. Adherence of Helicobacter pylori to areas of incomplete intestinal metaplasia in the gastric mucosa. Gastroenterology. 1996;111:1206–1211. doi: 10.1053/gast.1996.v111.pm8898634. [DOI] [PubMed] [Google Scholar]
- 18.Aguiar DC, Corvelo TC, Ara jo M, Cruz EM, Daibes S, Assumpção MB. [Expression of ABH and Lewis antigens in chronic gastritis and pre-neoplasic alterations in gastric mucosa] Arq Gastroenterol. 2002;39:222–232. doi: 10.1590/s0004-28032002000400004. [DOI] [PubMed] [Google Scholar]
- 19.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orntoft TF, Holmes EH, Johnson P, Hakomori S, Clausen H. Differential tissue expression of the Lewis blood group antigens: enzymatic, immunohistologic, and immunochemical evidence for Lewis a and b antigen expression in Le(a-b-) individuals. Blood. 1991;77:1389–1396. [PubMed] [Google Scholar]
- 21.Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69:166–182. doi: 10.1111/j.1423-0410.1995.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 22.Teneberg S, Leonardsson I, Karlsson H, Jovall PA, Angstrom J, Danielsson D, Naslund I, Ljungh A, Wadstrom T, Karlsson KA. Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J Biol Chem. 2002;277:19709–19719. doi: 10.1074/jbc.M201113200. [DOI] [PubMed] [Google Scholar]
- 23.Roche N, Angström J, Hurtig M, Larsson T, Borén T, Teneberg S. Helicobacter pylori and complex gangliosides. Infect Immun. 2004;72:1519–1529. doi: 10.1128/IAI.72.3.1519-1529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 25.Heneghan MA, Moran AP, Feeley KM, Egan EL, Goulding J, Connolly CE, McCarthy CF. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998;20:257–266. doi: 10.1111/j.1574-695X.1998.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Alkout AM, Blackwell CC, Weir DM. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J Infect Dis. 2000;181:1364–1369. doi: 10.1086/315375. [DOI] [PubMed] [Google Scholar]
- 27.Sheu BS, Sheu SM, Yang HB, Huang AH, Wu JJ. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut. 2003;52:927–932. doi: 10.1136/gut.52.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikehara Y, Nishihara S, Kudo T, Hiraga T, Morozumi K, Hattori T, Narimatsu H. The aberrant expression of Lewis a antigen in intestinal metaplastic cells of gastric mucosa is caused by augmentation of Lewis enzyme expression. Glycoconj J. 1998;15:799–807. doi: 10.1023/a:1006964016344. [DOI] [PubMed] [Google Scholar]
- 29.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]


