Abstract
AIM: To investigate the effect of Tripterygium hyp-oglaucum Hutch (THH) on the assembly and disassembly process of tubulin and its possible mode of action.
METHODS: In vitro porcine brain tubulin assembly assay was employed to analyze the inhibitory effects of THH at different concentrations (0.05 μg/L, 0.07 μg/L, 0.09 μg/L). Colchicine (0.0025 mmol/L, 0.0050 mmol/L, 0.0075 mmol/L) was used as a positive control.
RESULTS: THH could significantly inhibit the assembly of isolated porcine brain tubulin at all tested concentrations.
CONCLUSION: THH is capable of inducing aneuploidy in mammals via tubulin polymerization inhibition pathway and may pose a genetic risk to human beings.
Keywords: Tripterygium hypoglaucum (level) Hutch, Tubulin assembly-disassembly
INTRODUCTION
Aneuploidy is an important genetic change associated with birth defects, infertility, spontaneous abortions and cancer development [1], which are dependent upon the functioning of a variety of cell organelles and/or a number of division-specific metabolic activities, such as synthesis and functioning of the proteins of the nuclear spindle, attachment and movement of chromosomes on the spindle apparatus. A variety of chemicals are capable of modifying certain functions of spindle apparatus components which may lead to chromosome mal-segregation [2]. The frequency of aneuploidy can be increased by chemical treatment in various experimental cells. If human beings are exposed to aneugens, the risk of developing aneuploidy is increased, which is a matter of concern. Therefore, it is necessary to analyze the aneugenic potential of chemicals in safety assessment since chemically-induced aneuploidy in human beings can not be excluded.
Cochicine and vinblastine are known aneugens which affect tubulin polymerization and depolymerization, and disrupt microtubule formation during cell cycle [3,4]. As tubulin is the main constituent of the spindle, the balance between microtubule assembly and disassembly is critical for the spindle function. In vitro tubulin assembly assay is a useful screening system which could give information about the possible functional mechanism(s) of tested chemicals and their potential to cause aneuploidy.
The study of compounds and functions of plant origin has generated a great interest in the fields of food and medicine. Many of them have chemo-preventive properties which need to be tested for their effects on human beings. THH is a traditional Chinese medicinal herb belonging to the genus Celastraceae. The water extract from THH contains alkaloids, dulcitols, terpenes, lactones and pigments. The dry root can be immediately used in the form of decoction or the unpurified water extract can be compacted to tablets for clinical use. THH has been widely used in the treatment of various human immunological diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and others. It was reported that the use of THH can lead to reversible inhibition of germinal cell development both in human beings and in rats. Previous research showed that THH is a potent aneugen [5]. THH induces a significantly higher number of kinetochore positive micronuclei both in mouse bone marrow and in mouse NIH 3T3 cells [6,7]. Recently, we also found that THH could induce aneuploidy of chromosome 8 both in mouse bone marrow cells and in sperm cells by fluorescence in situ hybridization (FISH) [8]. The aim of the present study was to investigate the effect(s) of THH on tubulin assembly and disassembly and its possible mode of action. The study may help us to understand the potential adverse effects of THH on human health.
MATERIALS AND METHODS
Chemicals
Dithioerythritol, PIPES, and colchicines (COL) were purchased from Sigma. Guanosine-5’-triphosphate (GTP; Sigma) was a gift from Dr. ID Adler, Germany. Glycerol, MgSO4 dry herb of THH were purchased from Shanghai Chemical Company, and Kunming Medicine Company, China, respectively.
All the chemicals and THH were freshly prepared. COL was dissolved in sterile distilled water according to the test concentrations (0.0025 mmol/L, 0.0050 mmol/L, 0.0075 mmol/L), and 100g of mashed THH sample was extracted in 1000mL boiling water for 5 min and cooled down to room temperature for 30 min. The procedure was repeated three times to ensure maximum extraction. The supernatant was filtered and lyophilized. The yield of the crude THH extract was about 4.8%. THH extract was dissolved again in sterile distilled water before use according to the test concentrations (0.05 μg/L, 0.07 μg/L, 0.09 μg/L). The chemical and compound concentrations selected in the present study were selected in accordance to our preliminary mitotic block study in V79 cells and their solubility.
Purification of tubulin
The brains were obtained from 3 pigs within 2 h after sacrifice. Purified tubulin was obtained from porcine brain tissue by two cycles of assembly-disassembly procedure following the method of Williams and Lee [9]. The protein concentrations of the final preparation were determined by the method of Bradford with bovine serum albumin as standard. The resultant tubulin concentration was 3.34-3.75 μg/L. SDS-PAGE was performed to determine the purity of each tubulin preparation. Bovine serum albumin (66.2 Ku) and rabbit actin (43 Ku) were used as standard markers when the average MW of tubulin was 52.5 Ku.
Tubulin assembly-disassembly assays
Tubulin assembly was assayed photometrically by measuring the increase in the absorption at 350 nm (OD350nm). Six hundred μL PM-4M buffer containg 0.1mol/L PIPES, 2 mmol/L EGTA, 1 mmol/L MgSO4, 4 mol/L glycerol, 2 mmol/L dithioerythritol, pH6.9, as well as 30μL of 50 mmol/L GTP and 1400 μL tubulin solution were mixed thoroughly and poured immediately into a cuvette placed in the temperature-controlled compartment (37°C) of a recording spectrophotometer. The changes of OD350nm were recorded at an interval of 1 min. The absorption remained at the steady state level at the end of the assembly. The temperature of temperature-controlled compartment was decreased to 4°C in 2 min to start the disassembling of tubulin. The OD350nm was again recorded (Figure 1).
Figure 1.
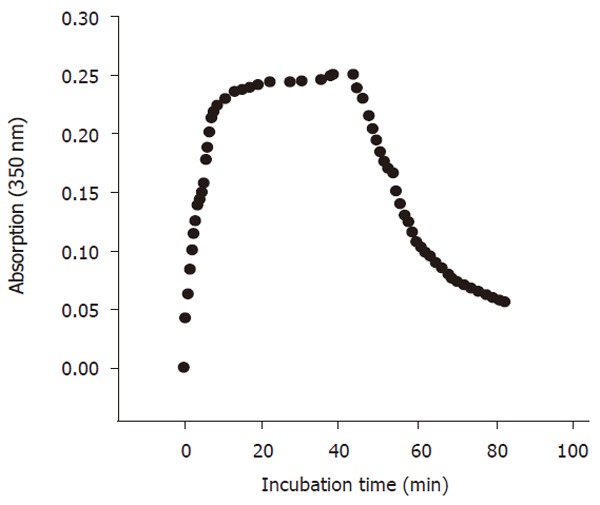
Scheme of in vitro tubulin assay. The tubulin assembly was achieved at the end of the absorption after 20 min incubation and kept a steady state level at 37 °C. The disassembly was carried out at 4 °C and after the assembly (40-min incubation).
Treatment and analysis
In vitro assembly analysis of porcine brain tubulin was carried out by adding 5 μL of THH (0.05 μg/L, 0.07 μg/L, 0.09 μg/L) or positive control (COL, 0.0025 mmol/L, 0.0050 mmol/L, 0.0075 mmol/L) into a ice-both cuvette containing 600μL PM-4M buffer (50, 70, 100 μg/mL), 30 μL of 50 mmol/L GTP and 1400 μL of tubulin solution. The contents were mixed thoroughly and placed immediately in the temperature-controlled compartment of a recording spectrophotometer (37°C). The net OD350nm was recorded at an interval of 1 min. The highest concentration of test compound was selected in accordance with previous laboratory results obtained by in vitro mitotic block assay with V79 cells. The solubility was considered as well.
RESULTS
The results of porcine brain tubulin assembly assay with COL and THH are shown in Figure 2. COL led to a very strong inhibition of the in vitro polymerization of tubulin at all concentrations. THH resulted in a dose-dependent inhibition of polymerization. However, the potency of assembly inhibition by THH was weaker than COL at all concentrations.
Figure 2.
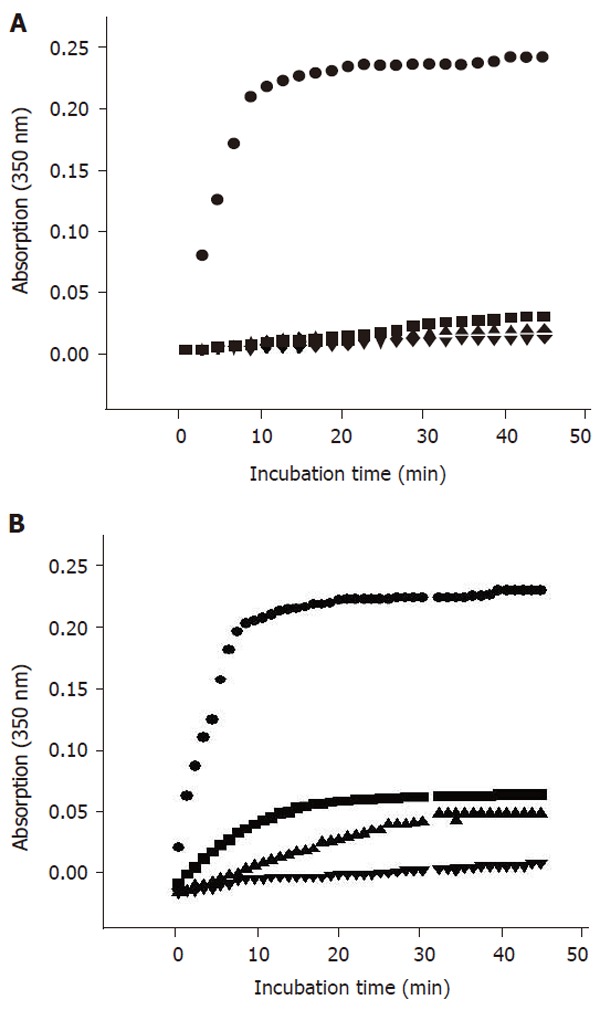
Inhibition of in vitro tubulin assembly by COL at the concentration of ■:0.0025 mmol/L,▲:0.0050 mmol/L,▼:0.0075mmol/L (A) and THH at the concentration of ■:0.05mg/mL,▲:0.07g/mL,▼:0.09mg/mL (B). ●: Solvent control
DISCUSSION
Aneuploidy plays a crucial role in the initiation and progression of tumorigenesis as well as in inherited disorders and fetal wastages. Chromosome segregation is dependent on different cell organelles, which is controlled by a number of metabolic pathways. However, the mechanisms and the cellular targets leading to non-disjunction are largely different from those leading to gene and chromosomal mutations where DNA is the principle target. Aneuploidy-inducing factors include damage to essential elements required for proper chromosome function(s), reduction in chromosomal pairing, induction of chromosome interchange, effect on chromosome condensation, persistence of nucleoli in mitosis or meiosis, increased chromosome stickiness, damage to centrioles or kinetochores, impairment of chromosome alignment, alterations in ion concentrations during mitosis, damage to nuclear membrane, and physical disruption of chromosome segregation [1]. A number of methods have been described for the detection of chemically-induced aneuploidy. However, none of these methods is sufficiently validated for routine screening. Although no conclusive evidence for aneuploidy induction can be obtained from tubulin assembly and disassembly assay, in vitro tubulin assay can be used to pinpoint the aneugenic potential of chemicals by inhibiting the microtubule assembly.
THH has been used to treat many immunological diseases. Both human somatic cells and germ cells are exposed to THH itself or its metabolites during long term clinical use. Since its administration leads to reversible inhibition of germinal cell development both in humans and in rats, there is a great need to evaluate the genetic safety of THH. Our research has shown that THH is a mammalian aneugen both in somatic cells and in germinal cells [5-8]. The results in the present study are in accordance with our previous results, suggesting that either THH itself or its components are capable of inducing aneuploidy via interaction with spindle proteins such as tubulin and probably actin or myosin(s) contained in the contractile ring during cell division. THH may pose a genetic risk to human beings. It seems important for Chinese medical professionals to consider a possible genetic risk of THH and cautiously advise patients to abstain from reproduction within 6 months after the treatment with THH. Further studies are neceed to identify the aneugen from THH and to study its possible mode of action.
ACKNOWLEDGMENTS
The authors thank Dr. Lu Yuan, Kunming Institute of Zoology, Chinese Academy of Sciences for his technical support for tubulin isolation.
Footnotes
Supported by the National Natural Science Foundation of China, No, 30560061 and the International Scientific Cooperation Project of Yunnan Province.
S- Editor Guo SY L- Editor Wang XL E- Editor Wu M
References
- 1.Parry EM, Parry JM, Corso C, Doherty A, Haddad F, Hermine TF, Johnson G, Kayani M, Quick E, Warr T, et al. Detection and characterization of mechanisms of action of aneugenic chemicals. Mutagenesis. 2002;17:509–521. doi: 10.1093/mutage/17.6.509. [DOI] [PubMed] [Google Scholar]
- 2.Parry JM. Detecting chemical aneugens: a commentary to 'Aneuploidy: a report of an ECETOC task force'. Mutat Res. 1998;410:117–120. doi: 10.1016/s1383-5742(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 3.Wallin M, Fridén B, Billger M. Studies of the interaction of chemicals with microtubule assembly in vitro can be used as an assay for detection of cytotoxic chemicals and possible inducers of aneuploidy. Mutat Res. 1988;201:303–311. doi: 10.1016/0027-5107(88)90019-x. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Albertini S, Würgler FE. Effects of 10 known or suspected spindle poisons in the in vitro porcine brain tubulin assembly assay. Mutagenesis. 1991;6:65–70. doi: 10.1093/mutage/6.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhuo R, He Z. Aneuploidy induction by water extract from Tripterygium hypoglaucum (Level) Hutch in mouse bone marrow cells. Mutagenesis. 1993;8:395–398. doi: 10.1093/mutage/8.5.395. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Liu SQ, He ZJ, Qiu YJ. Antikinetochore antibody staining to discriminate between two micronucleus origins. Aibian, Jibian, Tubian. 1994;6:60–63. [Google Scholar]
- 7.Jie YM, Jia C. Chromosomal composition of micronuclei in mouse NIH 3T3 cells treated with acrylamide, extract of Tripterygium hypoglaucum (level) hutch, mitomycin C and colchicine, detected by multicolor FISH with centromeric and telomeric DNA probes. Mutagenesis. 2001;16:145–149. doi: 10.1093/mutage/16.2.145. [DOI] [PubMed] [Google Scholar]
- 8.Xu W, Ziqing L, Yinrun D, Xiaoyan W, Jinglun X. Tripterygium hypoglaucum (level) Hutch induces aneuploidy of chromosome 8 in mouse bone marrow cells and sperm. Mutagenesis. 2004;19:379–382. doi: 10.1093/mutage/geh044. [DOI] [PubMed] [Google Scholar]
- 9.Williams RC, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85 Pt B:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]


