Abstract
Herbicides containing glyphosate are widely used in agriculture and private gardens, however, surprisingly little is known on potential side effects on non-target soil organisms. In a greenhouse experiment with white clover we investigated, to what extent a globally-used glyphosate herbicide affects interactions between essential soil organisms such as earthworms and arbuscular mycorrhizal fungi (AMF). We found that herbicides significantly decreased root mycorrhization, soil AMF spore biomass, vesicles and propagules. Herbicide application and earthworms increased soil hyphal biomass and tended to reduce soil water infiltration after a simulated heavy rainfall. Herbicide application in interaction with AMF led to slightly heavier but less active earthworms. Leaching of glyphosate after a simulated rainfall was substantial and altered by earthworms and AMF. These sizeable changes provide impetus for more general attention to side-effects of glyphosate-based herbicides on key soil organisms and their associated ecosystem services.
Earthworms and arbuscular mycorrhizal fungi (AMF) are important components in temperate ecosystems, influencing nutrient cycling and overall ecosystem functioning1,2. Earthworms are considered to be ecosystem engineers because they shred and redistribute organic material in soil, increase soil penetrability for roots, thus improving overall soil fertility3,4. Because of their importance, earthworms have also been used as bioindicators of soil health and quality1,5,6. Mycorrhizal fungi form a symbiosis with over 80% of vascular plant species and are also considered keystone species in temperate ecosystems because of their influence on plant nutrient supply7 and soil aggregation8. In arable soils AMF are the dominant root symbionts that sustain plant growth9. Mycorrhized plants commonly show a higher uptake of phosphorus and nitrogen, as the fungal mycelium has more efficient mechanisms for absorbing mineral nutrients than roots and by extending the root system enabling further exploration of the soil resources5,10,11. In return, host plants provide photoassimilates (predominantly glucose and fructose) that are converted to lipids by the fungus and used for carbon transport and storage9,12. Recently, the analysis of fatty acids as biochemical markers considerably improved our knowledge in AMF distribution and foraging activity in soil13. Thereby, the soil phospholipid fatty acid (PLFA) 16:1ω5 represents viable hyphal biomass, while the neutral lipid fatty acid (NLFA) reflects fungal storage reserves such as spores, vesicles and propagules14,15. Moreover, the ratio of 16:1ω5 NLFA to PLFA indicates fungal phenology such as senescence or active colonization phases12,16.
Despite their important roles in ecosystems, our understanding on ecological interactions between earthworms and AMF is rather limited. The few studies investigating earthworm-AMF interactions suggest that the response patterns are dependent on the species involved; as a result effects range from additive, synergistic, antagonistic or no interactive effects17,18,19,20. Here we examined, whether the interactions between earthworms and AMF are affected by herbicide application. We experimented with two essential players in temperate soil ecosystems: the anecic, vertically burrowing earthworm Lumbricus terrestris (Linnaeus 1758) and the arbuscular mycorrhizal fungi Glomus mosseae (T.H. Nicolson & Gerd.). As a herbicide we used Roundup (RU), the most widely used pesticide worldwide21 containing the active ingredient glyphosate. Glyphosate is a broad-spectrum, post-emergence, non-selective chemical that kills plants by affecting the shikimate-pathway during photosynthesis22. Generally, glyphosate is regarded as environmentally friendly due to its fast biodegradation and strong adsorption to soil particles23. However, there is mounting evidence that many amphibian species24,25,26 and other wildlife27 can be detrimentally affected by glyphosate-based herbicides.
Contrary to the wide use of glyphosate surprisingly little is known on potential side effects on interactions between key soil organisms such as earthworms or AMF. Glyphosate effects on earthworms vary from detrimental28,29,30 to no effects31,32,33, however, to what extent their interaction with other soil organisms is affected by glyphosate has never been investigated. Studies testing glyphosate effects on AMF show an inhibition of AM fungal spore germination and germ tube growth34 or reduced mycorrhiza in soil35, however only at concentrations greater than those recommended for field use. Several other reports show no effect of glyphosate on mycorrhiza when applied at recommended doses36,37,38,39,40. The fate of glyphosate in ecosystems is another aspect which has rarely been investigated41,42. While glyphosate sorbs strongly to soil minerals43, leaching and soil erosion by water or wind can transport glyphosate from land to water environments44. This glyphosate leaching is assumed to be affected by earthworms and/or AMF. Earthworms maintain soil structure and foster macropores, which may influence water infiltration45 and thereby increase glyphosate leaching. On the other hand, mycorrhiza could lead to stronger absorption of glyphosate by binding and enmeshing soil particles into larger aggregates46.
To investigate interrelationships between herbicide application, earthworms and AMF we set up a full-factorial mesocosm greenhouse experiment. We planted the mesocosms with the leguminous forb white clover (Trifolium repens L.), which is frequently used as green manure in agriculture. Three hypotheses were tested. First, herbicide application will increase earthworm activity as an increased amount of dead plant material will be available as food for earthworms. Second, herbicide application will not affect AMF in soil because of the very plant-specific mode of symbiotic interaction. Third, herbicide-stimulated earthworm activity increases the preferential flow of rainwater through burrows and therefore increase leaching of glyphosate; whereas AMF counteract glyphosate leaching as they enhance soil aggregation. Such terrestrial model ecosystems have been proposed as an ideal tool to evaluate the effects of chemicals in soil ecosystems in order to achieve a greater realism in the ecotoxicological evaluation of chemicals to non-target organisms47.
Results
Plants
Trifolium leaves were killed by the herbicide within several hours, whereas stolons remained partly green. Shoot biomass of T. repens at harvest was significantly reduced by earthworms (F1;16 = 5.485, P = 0.032) but not significantly affected by RU application (F1;16 = 2.529, P = 0.131) or AMF (F1;16 = 0.220, P = 0.645; shoot biomass across AMF and RU treatments: −EW 23.724 ± 2.283 g, +EW 18.812 ± 3.169 g). Root biomass of T. repens was unaffected by RU (F1;16 = 0.190, P = 0.668), AMF (F1;16 = 0.682, P = 0.421) or earthworms (F1;16 = 0.082, P = 0.778; root biomass across treatments: 1.775 ± 0.361 g).
Earthworms
Earthworm activity was similar across treatments prior to herbicide application with mean surface cast production of 1.5 ± 0.1 casts day−1 mesocosm−1 and 3.6 ± 0.4 moved toothpicks day−1 mesocosm−1. Earthworm activity measured by toothpicks was marginally significantly lower after herbicide application (F1;10 = 4.490, P = 0.060), however was not influenced by AMF (F1;10 = 0.001, P = 0.977; Figure 1a+1b). Herbicide application reduced earthworm activity (toothpicks) in +AMF mesocosms (F1;4 = 9.042, P = 0.040; Figure 1b) but had no influence on earthworm activity in −AMF mesocosms (Figure 1a). Earthworm activity measured by surface cast production was neither influenced by RU nor AMF (Figure 1).
Figure 1. Earthworm activity measured by surface cast production and moved toothpicks in mesocosms without (a) and with (b) AMF inoculation and without (continuous line) and with herbicide application (dotted line).
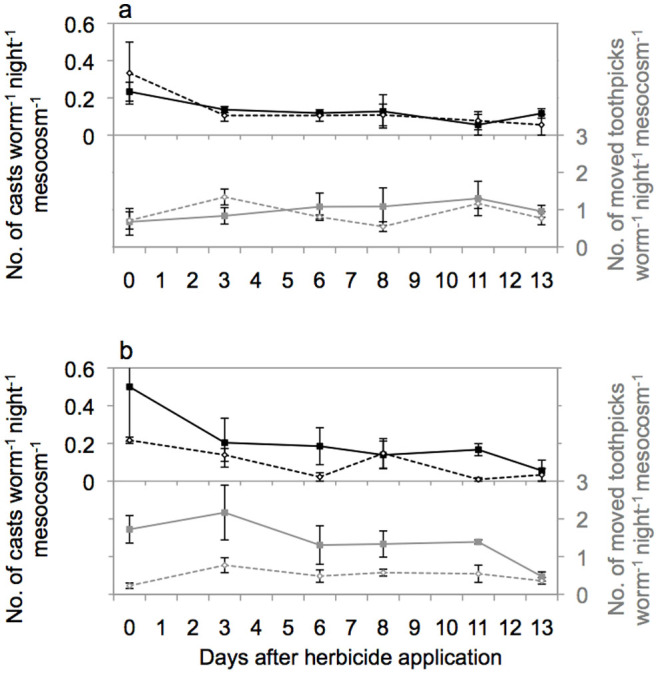
Means ± SE, n = 3.
Earthworm fresh mass at harvest was on average 72% of the initially added fresh mass; neither AMF inoculation (F1;10 = 0.138, P = 0.720) nor RU application (F1;10 = 2.903, P = 0.127) affected recaptured earthworm fresh mass, but a significant AMF × RU interaction occurred (F1;10 = 6.388, P = 0.035). Earthworm mass in the different treatments was: −RU/−AMF 11.0 ± 7.0 g, +RU/−AMF 9.5 ± 5.7 g; −RU/+AMF 5.3 ± 9.1 g, +RU/+AMF 16.4 ± 3.4 g. Earthworm activity (both moved toothpicks and surface castings) was not correlated to earthworm biomass or greenhouse mean air temperature or relative humidity (data not shown).
Mycorrhizae
Thirty-six weeks after AMF inoculation, average mycorrhization rates of Trifolium roots were 26% in +AMF and 15% in −AMF treatments. Across soil layers, herbicide application significantly reduced mycorrhization in +AMF (F1;10 = 7.887, P = 0.023) but had no effect on mycorrhization rates in −AMF mesocosms (Figure 2). The reduction in mycorrhization due to herbicide application was even more pronounced when soil layers were considered separately (Table 1, Figure 2). In −RU/−EW mesocosms mycorrhization was significantly different between layer 0–5 cm and layer 5–10 cm (F1;10 = 14.756, P = 0.018). In −RU + EW mesocosms mycorrhization differed significantly between layer 0–5 cm and layer 10–30 cm (F1;10 = 23.093, P = 0.009). In +RU/−EW mesocosms mycorrhization differed significantly between layer 0–5 cm and layer 10–30 cm (F1;10 = 20.050, P = 0.011). Earthworms had no influence on root AMF colonisation across soil depths (F1;10 = 2.575, P = 0.147; also no RU × EW interaction).
Figure 2. Mycorrhization of T. repens roots in different soil layers without (a) and with (b) earthworms in mesocosms without (white) or with (grey) herbicide application.
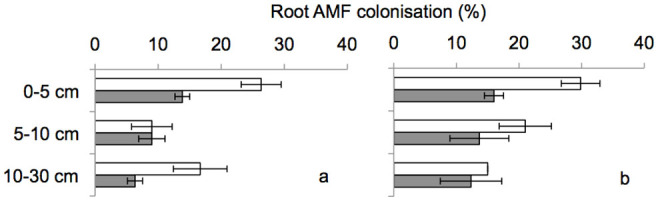
Means ± SE, n = 3.
Table 1. ANOVA results on the effects of herbicide application (RU) and earthworms (EW) on Trifolium repens root AMF colonisation. ANOVA with * for P < 0.05, ** for P < 0.01, *** for P < 0.001.
| Soil layer | Roundup (RU) | Earthworms (EW) | RU × EW | |||
|---|---|---|---|---|---|---|
| F-value | P-value | F-value | P-value | F-value | P-value | |
| 0–5 cm | 29.826 | 0.001** | 1.381 | 0.274 | 0.076 | 0.789 |
| 5–10 cm | 0.994 | 0.348 | 5.133 | 0.053 | 0.994 | 0.348 |
| 10–30 cm | 3.870 | 0.085 | 0.430 | 0.530 | 1.346 | 0.279 |
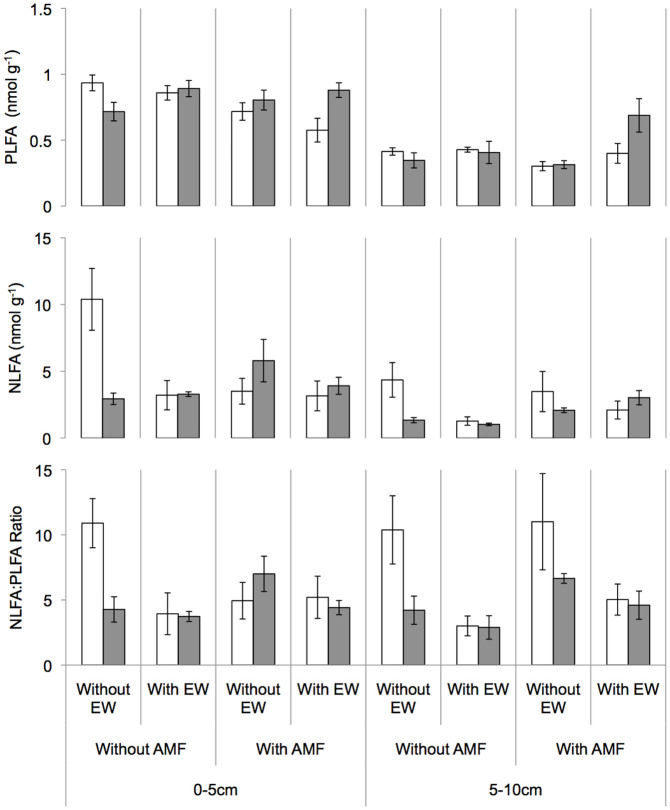
Hyphal biomass in soil assigned by 16:1ω5 PLFAs was not enhanced by AMF inoculation (Table 2, Figure 3). Highest PLFA concentrations of 16:1ω5 in soil were found in the layer 0–5 cm in mesocosms without any manipulation (−EW, −AMF, −RU). A significant herbicide AMF interaction occurred (no interaction between the three treatment factors). We found higher PLFA concentrations of 16:1ω5 in mesocosms with herbicide application, especially in combination with earthworms. AMF spores, vesicles and propagules assigned by 16:1ω5 in soil NLFAs were significantly enhanced by AMF inoculation (Table 2, Figure 3). Most storage reserves were found in mesocosms without any manipulation in layer 0–5 cm. Earthworms reduced the concentration of 16:1ω5 NLFAs and had a strong negative effect on storage structures of AMF assigned by NLFA/PLFA ratio (Table 2, Figure 3). This effect diminished in presence of herbicide, but was still visible. A herbicide-earthworm interaction occurred in layer 5–10 cm: means in NLFA concentration in −RU/−EW was higher than in +RU/+EW mesocosms (Figure 3).
Table 2. ANOVA results for effects of Arbuscular mycorrhizal fungi (AMF), Earthworms (EW), Roundup (RU) and their interactions on PLFA amount of 16:1ω5 and NLFA amount of 16:1ω5 in different soil layers. ANOVA with * for P < 0.05, ** for P < 0.01, *** for P < 0.001.
| PLFA | NLFA | NLFA:PLFA ratio | ||||
|---|---|---|---|---|---|---|
| Parameter | F | P | F | P | F | P |
| Soil depth 0–5 cm | ||||||
| AMF | 4.926 | 0.041* | 0.296 | 0.594 | 0.046 | 0.832 |
| EW | 0.029 | 0.866 | 4.595 | 0.048* | 5.445 | 0.033* |
| RU | 1.154 | 0.299 | 0.064 | 0.803 | 0.520 | 0.481 |
| AMF × EW | 0.751 | 0.399 | 0.425 | 0.524 | 1.207 | 0.288 |
| AMF × RU | 9.069 | 0.008** | 6.353 | 0.023* | 2.730 | 0.118 |
| EW × RU | 5.957 | 0.027* | 2.543 | 0.130 | 0.734 | 0.404 |
| AMF × EW × RU | 0.031 | 0.862 | 3.486 | 0.080 | 4.267 | 0.055 |
| Soil depth 5–10 cm | ||||||
| AMF | 0.011 | 0.918 | 4.485 | 0.050* | 4.165 | 0.058 |
| EW | 8.047 | 0.011* | 4.399 | 0.052 | 13.135 | 0.002** |
| RU | 0.470 | 0.503 | 2.241 | 0.154 | 3.448 | 0.082 |
| AMF × EW | 4.146 | 0.059 | 3.126 | 0.096 | 0.411 | 0.530 |
| AMF × RU | 4.522 | 0.049* | 3.424 | 0.083 | 0.454 | 0.510 |
| EW × RU | 2.050 | 0.171 | 5.449 | 0.033* | 2.346 | 0.145 |
| AMF × EW × RU | 0.948 | 0.345 | 0.038 | 0.849 | 0.522 | 0.480 |
Figure 3. PLFA amount of 16:1ω5, NLFA amount of 16:1ω5 in nmol g−1 DW soil and the ratio of 16:1ω5 NLFA to PLFA in different soil layers without (white) and with (grey) Roundup application, without/with earthworms in mesocosms without/with AMF inoculation.
Mean ± SE, n = 3.
Water infiltration and herbicide leaching
Water infiltration rate was unaffected by earthworms or AMF (Figure 4). Herbicide application showed a trend towards reduced water infiltration (F1;22 = 3.796, P = 0.069; there was no Roundup × AMF interaction).
Figure 4. Water infiltration rate measured in mesocosms without earthworms (a) and with earthworms (b) in response to AMF, without (white) and with (grey) Roundup application.
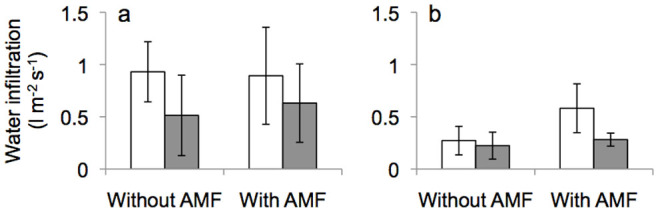
Means ± SE, n = 3.
Concentration of glyphosate or its metabolite AMPA in the leachate was unaffected by earthworms or AMF (Figure 5). However, concentrations of glyphosate were significantly (F1;10 = 7.572, P = 0.025) and of AMPA marginally significantly (F1;10 = 4.515, P = 0.066) interactively affected by earthworms and AMF with increasing earthworm effects in −AMF and decreasing earthworm effects in +AMF mesocosms (Figure 5). In −AMF mesocosms earthworms significantly increased glyphosate leaching (F1;4 = 9.439, P = 0.037).
Figure 5. Glyphosate concentration (a) and its metabolite AMPA (b) in soil leachate.
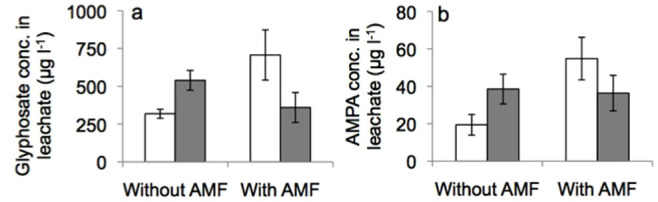
Illustrated are concentrations in mesocosms with herbicide application, without and with AMF inoculation and without (white) and with (grey) earthworms. Means ± SE, n = 3.
Discussion
To our knowledge, this is among the first studies investigating the impact of a glyphosate-based herbicide on ecological interactions between a vertically burrowing earthworm species (Lumbricus terrestris) and symbiotic mycorrhizal fungi. Contrary to our hypothesis, Roundup did not stimulate but rather decrease earthworm activity, especially in mesocosms with AMF amendment. Also in the +AMF mesocosms, earthworm biomass was 50% higher after Roundup application, than in −AMF mesocosms. This suggests that over the short duration of our experiment, Roundup led to heavier earthworms that were less active at the surface, probably because there was abundant food in form of dead roots or AMF in the soil that precluded earthworms from foraging food from the surface. Other studies showed that earthworm biomass was unaffected by glyphosate-based herbicides for endogeic species48, whereas in temperate epigeic species30,49 and tropical earthworms strong mass loss after glyphosate application was found50. Studies investigating effects of Roundup on soil dwelling endogeic earthworm species (Aporrectodea caliginosa) found no alteration of the energy status after acute exposure31. Glyphosate had no effect on growth of A. caliginosa in a pot experiment where the herbicide was mixed with soil51, in contrast, another study showed that glyphosate reduces the growth of A. caliginosa even at a rate lower than recommended by the manufacturer52. Surface dwelling, epigeic earthworms showed no avoidance of Roundup treated leaves (Eisenia andrei32) or response in their depth distribution (E. fetida53) but avoided glyphosate treated soil28,29. Previous studies found no influence of glyphosate on the survival rate in temperate earthworm species Aporrectodea trapezoides, A. rosea, A. caliginosa or A. longa populations30,48, whereas a 50% reduction in mortality was found for the tropical earthworm species Pheretima elongata54. Effects of glyphosate had no influence on reproduction of E. fetida49, whereas others reported a significant reduction of hatched cocoons in glyphosate treated soil for this species29,30.
Two things are important to note, when evaluating our current results and previous results from the literature. First, we monitored the surface activity of earthworms over a period of only two weeks after Roundup application and therefore no conclusions on long-term effects, consequences for reproduction or changes in belowground activity can be derived from this study. Second, findings on herbicide effects on epigeic species such as E. fetida are important contributions when testing possible mode of actions in ecotoxicological tests, however they are of limited value when aiming to evaluate pesticide effects under field situations as these species preferably live in habitats with an abundant surface litter layer which is not the case in arable agroecosystems where these herbicides are applied.
We found a 40% reduction of mycorrhization after Roundup application in soils amended with the mycorrhizal fungi G. mosseae. This is in contrast to what we hypothesized, based on the allegedly fast biodegradation of the herbicide and the very plant-specific mode of action. We explain this mainly by direct and indirect influences. Roundup could have directly affected active metabolite production in the plant with detrimental effects on root AMF colonisation38. Indirect effects of Roundup on AMF could have affected the intraradical phase of AMF that has been shown to be sensitive to several host plant metabolites which regulate AMF abundance55,56,57. Mycorrhizal infection of maize, soybean and cotton was influenced by glyphosate in pasteurized soil but not in non-pasteurized soil38. Our soil mixture was steam-sterilized, but afterwards amended with a microbial wash from field soil, therefore only differing from field soil by the presence or absence of the inoculated AMF taxa. However, the latter did not enhance AMF hyphal biomass measured by 16:1ω5 PLFA, whereas spores assigned by fungal storage lipids, i.e. 16:1ω5 NLFA, were highest in soils without any manipulations (−AMF, −RU, −EW). These fungal propagules obviously have survived the steam-sterilization procedure. The generally low impact of soil amendment by the mycorrhiza inoculum points to competition with the indigenous soil community hampering the establishment of introduced G. mosseae. However, this cannot be assigned by the used biomarker fatty acid, 16:1ω5, as it is a measure for viable fungal hyphae biomass and storage fat in spores across the genus Glomus15,58,59.
Direct influence of Roundup on AM fungi are generally regarded to be minor as soil fungi are well protected from direct contact with the herbicide. Indeed reports show rather insignificant influence of glyphosate on hyphal growth and germination of spores as well as root AMF colonisation34,37,38,40,57. However, in the present experiment Roundup application affected hyphal (i.e. amount of 16:1ω5 PLFA) and spore (i.e. amount of 16:1ω5 NLFA) biomass in the soil. Spore biomass generally declined with herbicide application, which is in accordance with others who showed reduced spore viability even under the lowest glyphosate rate60. Interestingly, the presence of earthworms resulted in a comparable negative effect on fungal storage structures. Earthworms are reported to influence AMF positively by contribution to the dispersal of spores61,62 yet our results indicate feeding rather than propagating fungal spores63, which is supported by the corresponding changes in the NLFA to PLFA ratio. Spores, hyphae and infected root pieces form the three types of AMF propagules in soil, however their importance varies due to fungal species. In Glomeraceae, the extraradical mycelium is the most important source of inoculum, whereas spores are the main propagules in Gigasporaceae64. Thus, hyphae of Glomus are responsible for rapid colonization of new hosts, and hyphal biomass was positively affected by herbicide application especially in combination with earthworms. This indicates that glyphosate application alters fungal phenology, i.e. fosters fungal foraging over resting structures. Such effects likely are mediated through the modification of host plant physiology57. In sum the performance of AMF was distinctly altered by both Roundup and earthworms, albeit the impact varied with AMF propagule structures. Given the immense importance of AMF for plant nutrition and soil structure9,46, these effects can have ramification for the functioning of ecosystems.
Will effects of herbicide on earthworms and mycorrhiza influence herbicide leaching? We found a tendency that water trickled away more slowly after a simulated heavy rainfall in mesocosms treated with Roundup as compared to those without Roundup application, however water infiltration rate was not influenced by earthworms or AMF. As Roundup had no effect on shoot and root mass of T. repens, we assume that after Roundup application dead plant material soaked up the excessive water and blocked the downflow of water. We hypothesized that earthworms increase glyphosate leaching by a preferential flow of contaminated rainwater through burrows, but also expected AMF to decrease glyphosate leaching by binding and enmeshing soil particles into larger aggregates46,65. Interestingly, earthworms significantly increased glyphosate leaching only in absence of AMF, while in presence of AMF earthworms tended to decrease glyphosate leaching. It remains to be tested whether this is due to glyphosate uptake and accumulation by AMF or whether these AMF hyphae might have been consumed by earthworms thus protecting glyphosate from leaching. Other studies have shown that glyphosate is mostly located in the earthworm mucus31 which is smeared along the walls of earthworm burrows66 and could therefore increase herbicide leaching. Furthermore, a rapid preferential transport for even strongly sorbing pesticides such as glyphosate and pendimethalin was demonstrated44. In contrast, AMF hyphae and other microorganisms could play a role in bonding glyphosate in the burrow walls. Biopore walls represent hot spots for microbial activity and pesticide mineralization67. The drilosphere of the burrows of L. terrestris therefore created an ideal habitat for a diverse microbial community. Many soil bacteria are known to degrade the organophosphonate glyphosate, e.g. dominant rhizosphere colonizer such as Pseudomonas68, bulk soil inhabitants such as Arthrobacter69, or symbiotic groups such as the family Rhizobiaceae70. A higher phosphorus transport via fungal hyphae was reported after glyphosate application for G. mosseae71. These processes in turn decreased glyphosate leaching as burrows transmitted clean water past the herbicide-containing soil matrix72. For the current results this could mean that without earthworms, water from the simulated heavy rainfall seeped through the whole soil matrix and thus absorbing glyphosate from the soil. The hyphae and other soil microorganisms absorbed glyphosate and so decreased glyphosate leaching; because the burrows transmitted clean water past the herbicide-containing soil matrix72.
Taken together, our results show for the first time that Roundup can affect important interactions between earthworms and AMF, two of the most important soil organisms. While the short-term influence of Roundup on earthworms seem rather minor, the detrimental effects on AMF in roots and soil can have wide consequences for crop cultivation. Given AMFs and earthworms eminent role in plant nutrition, a glyphosate-induced decline in AM fungi would require more fertilization with economical and ecological consequences for farmland management. The finding that Roundup affects, together with earthworms and AMF, water infiltration requires more attention especially as climate change models prognosticate heavier rainfalls. Results of this study also highlight the importance of more complex experimental settings that investigate interactions of several species in order to better assess potential effects of pesticides on the environment.
Methods
Experimental setup
We conducted a full-factorial mesocosm experiment manipulating the three factors Earthworms (two levels: earthworm addition, +EW vs. no earthworms, −EW), AMF (two levels: AMF inoculation, +AMF vs. no AMF inoculation, −AMF) and Herbicide application (two levels: Roundup application, +RU vs. no Roundup application, −RU; more details on the individual treatments below). The experiment was conducted in December 2011 in a greenhouse of the University of Natural Resources and Life Sciences Vienna (BOKU), Austria. During the course of the experiment mean daytime air temperature inside the greenhouse was 20.1 ± 3.2°C at a mean relative humidity of 55.2 ± 5.4%; mean nighttime air temperature was 15.3 ± 2.6°C at a mean relative humidity of 68.3 ± 6.7%; to ensure optimal light conditions, three 1000-W Radium lamps (type HRI-T100W/D, WE-EF Leuchten, Bispingen, Germany) were installed in 1.5 m distance above the experimental units (14 hours light, 10 hours night).
We used 24 plastic pots (volume: 20 l, diameter: 31 cm, height: 30 cm; further called mesocosms) which were lined out with two layers of garden fleece at the bottom and extended at the upper rim with a 10 cm high barrier of transparent plastic to prevent earthworms from escaping; the fleece and barriers were also installed in mesocosms containing no earthworms to create similar microclimatic conditions among treatments.
Treatments
AMF treatments were prepared in March 2011 by first filling the mesocosms with 12 l steam-sterilized (3 hours at 100°C) field soil (Haplic Chernozem, silt loam) mixed with quartz sand (grain size 1.4–2.2 mm) in a ratio of 40:60 vol/vol. Characteristics of this soil mixture: Corg = 24.1 g kg−1, Ntot = 0.98 g kg−1, K = 111.2 mg kg−1, P = 58.42 mg kg−1, pH = 7.63. The upper 6 l of the +AMF treatments were filled with the same substrate mixture and amended with 25 g l−1 inoculum of Glomus mosseae (T.H. Nicolson & Gerd.; synonymously Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler) obtained from a commercial supplier (Symbio-m Ltd., Lanskroun, Czech Republic). The −AMF controls were filled with the same amount of steam-sterilized and thus inactive AMF inoculum. We successfully used this substrate mixture in other experiments involving the same earthworm and AMF taxa45,73,74. Then 400 ml of microbial wash was added to each mesocosm to inoculate the steam-sterilized soil with microorganisms present in field soil75. This microbial wash contained 300 ml soil suspension (3500 g fresh soil dispensed in 7200 ml distilled H2O filtered through a sieve-cascade from 2000 μm to 25 μm mesh size) and 100 ml AMF suspension (466 g AMF-inoculum dispensed in 2400 ml distilled H2O filtered through the same sieve-cascade).
In April 2011 mesocosms were planted with white clover (Trifolium repens L.). Therefore, T. repens was first propagated from seeds in steam-sterilized potting soil, then 18 seedlings (average height about 10 mm, seedlings consisted of two cotyledons and two real leaves) were transplanted into each mesocosm in a regular hexagonal pattern with an equidistance to each other of 5 cm (240 seedlings m−2). This seed material is commonly used by farmers in mixtures for green manuring and was obtained from the BOKU Department of Crop Sciences. No fertilizers were applied during the course of the experiment.
In December 2011 we added 4 adult individuals of vertically burrowing Lumbricus terrestris L. to the +EW mesocosms (16.6 ± 2.1 g mesocosm−1, equivalent to 220.6 g m−2). Earthworm densities were roughly oriented on the average earthworm biomass in temperate grasslands ranging between 52–305 g m−2 where 50–75% of the biomass consists of anecic species1. Earthworms were purchased from a local fishing bait shop. To acquaint earthworms with experimental conditions, we cultivated them in plastic boxes (climate chamber at 15°C) filled with steam-sterilized field soil and ground oat flakes as food before they were introduced to the mesocosms. Before earthworms were randomly added to the +EW mesocosms, they were washed free of attached soil, dried off on filter paper and weighed. All earthworms buried themselves in the soil within a few minutes. The mesocosms were randomly placed on greenhouse tables and randomly repositioned every second week to avoid treatment interactions with potential microclimatic gradients inside the greenhouse. No additional food was provided for earthworms in the mesocosms as there was abundant dead organic material on the soil surface. An automatic irrigation system added on average 0.5 l tap water day−1 to each mesocosm.
Herbicide was applied five days after earthworm insertion on half of the mesocosms comprising all treatment combinations. We used Roundup Speed (Monsanto Inc., St. Louis, Missouri, USA), a systemic, broad-spectrum herbicide containing 7.2 g l−1 of the active ingredient glyphosate. This herbicide is recommended for use in home and garden areas and was obtained from a garden center in Vienna. Following the instructions for use, we applied the herbicide directly onto the plants from the original bottle with the attached fine mist spray nozzle. We applied the herbicide once on day 5 after earthworm inserting at 4 p.m. without direct sunlight at an air temperature of 25°C. As recommended in the instruction text we sprayed the herbicide so that the plant surface was homogeneously covered and shiny from the herbicide film. This application needed 14 squirts of Roundup Speed with the spray nozzle mesocosm−1 amounting to 177.48 ml m−2.
These treatments were replicated three times in a full-factorial design: two earthworm treatments × two AMF treatments × two RU treatments × three replicates equals totally 24 mesocosms.
Measurements and analyses
Earthworm activity was indirectly assessed during nighttime by 30 toothpicks mesocosm−1 that were vertically inserted (0.5 cm deep) in a consistent pattern. In the following morning the number of toothpicks differing from the original vertical position was considered as a measure of earthworm activity because earthworms crawl over the soil surface when searching for food. Knocked over toothpicks were counted as 1 and inclined toothpicks were counted as 0.5. As another measure of earthworm activity we additionally measured the number of freshly produced casts on the soil surface76. Both activity measurements were done parallel three times before and six times after herbicide application.
Water infiltration and Roundup leaching was measured seven days after the Roundup application by pouring 3 l of distilled water on top of the mesocosms simulating a rain shower of about 40 l m−2 (see also45). The time from pouring the water onto the mesocosms until the last water pool disappeared from the soil surface was recorded and used to calculate the water infiltration rate in l m−2 s−1. We collected 250 ml of the leachate from the saucers at bottom of the mesocosms immediately stored it in a freezer at −20°C before it was analysed for glyphosate and its main metabolite aminomethylphosphonic acid (AMPA) in the laboratories of the BOKU Department of Forest and Soil Sciences using a HPLC-MS/MS method77,78.
Harvest of the mesocosms started 14 days after herbicide application by cutting the remaining or untreated plants at the soil surface to obtain aboveground plant biomass production. Afterwards, soil was removed from the mesocosms in three separate layers 0–5 cm, 5–10 cm and 10–30 cm. Earthworms present in these soil layers were carefully washed free of soil, placed on moist filter paper, counted, weighed and released to the BOKU garden. Roots present in these soil layers were washed free of attached soil particles under a jet of tap water over a 1-mm sieve and sorted out. Dry mass of shoots and roots was determined after 48 hours oven-drying at 55°C. A portion of roots per layer was collected, cleared with boiling KOH for four minutes and stained for one minute with black Sheaffer ink79. Percentage of root length colonized by AMF considering arbuscules and vesicles (i.e. mycorrhization rate) was determined using the grid-line method by counting at least 100 intersections per sample80.
Lipid extraction from pot soil was carried out by extracting 3–4 g of soil (wet weight) with Bligh & Dyer solvent (chloroform: methanol: citrate buffer as 1:2:0.8, pH 4)81. The obtained lipids were fractionated into neutral lipid (NLFAs), glycolipid and phospholipid fatty acids (PLFAs) on a silica column (HF Bond Elut - SI, Varian Inc.) by elution with chloroform, acetone and methanol, respectively. NLFAs and PLFAs were subjected to an alkaline methanolysis in 0.2 M methanolic KOH, and the fatty acid methyl esters (FAMEs) were extracted with hexane-chloroform. Samples were dissolved in isooctane and stored at −20°C until analysis. FAMEs were analyzed using an Agilent 7890A gas chromatograph equipped with a flame ionization detector (GC-FID) using a HP Ultra 2 capillary column (25 m × 0.2 mm i.d., film thickness 0.33 μm). The oven temperature program started with 170°C and increased by 28°C min−1 to 288°C, followed by 60°C min−1 to 310°C. FAMEs were identified with the Sherlock Pattern Recognition Software (MIDI®) by comparing retention times to a standard mixture, and quantifying based on the internal standard methylnondecanoate (19:0). To verify correct identification (chain length and saturation) a range of samples was additionally analyzed with the Agilent 7890A coupled to a Mass Selective Detector (Agilent 7000 Triplequadrupole) equipped with a HP5MS capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm), operated in splitless mode with helium as carrier gas. Oven temperature program started with 40°C and increased by 46°C min−1 to 200°C, followed by 5°C min−1 to 238°C, 120°C min−1 to 300°C. A mass range of 40–400 m/z was monitored in Scan mode. The fatty acid 16:1ω5 was applied as general marker for AMF, predominantly Glomales, with the PLFA fraction representing hyphal membranes and the NLFA fraction storage lipids58,59.
Statistical analyses
All variables were tested for homogeneity of variances and normality using the tests after Levene and Kolmogorow-Smirnow, respectively. Data on PLFAs and NLFAs were log-transformed to meet the assumptions for parametric tests. We conducted a three way analysis of variance (ANOVA) to test the effects of Earthworms, AMF and Roundup on PLFAs, NLFAs, water infiltration and Roundup leaching. Here analyses for treatment effects on PLFAs and NLFAs were conducted for each soil layer separately. Earthworm activity (moved toothpicks and surface castings) during the course of the experiment was analyzed conducting a repeated measures ANOVA with Roundup and AMF as factors by only including data from mesocosms containing earthworms. Root AMF colonisation was analyzed for each soil layer using a two-way ANOVA considering the factors Earthworms and Roundup; mesocosms without AMF inoculation were not included. We also performed Pearson correlations between earthworm biomass and earthworm activity (moved toothpicks and surface castings) and between earthworm activity and mean air temperature or mean relative humidity. All statistical tests were performed in PASW Statistics 18 (vers. 18.0.0, IBM Corp., Armonk, New York, USA). Values given throughout the text are means ± SE.
Author Contributions
Conceived and designed the experiment: F.H., A.G., J.G.Z. Performed the experiment: F.H., A.G., J.G.Z. Analyzed the data: F.H., J.G.Z., L.R. Contributed reagents/materials/analysis tools: L.R. Wrote the paper: J.G.Z., F.H., L.R., A.G.
Acknowledgments
We are grateful to Alina Schmerbauch, Claudia Lichtenegger, Bernadett Handl, Elsa Ferstl, Thomas Müllner and Norbert Schuller for help in the greenhouse and laboratory, to Peter Liebhard for providing the seed material. Alexander Bruckner supported and Axel Mentler conducted the glyphosate and AMPA analyses. Pia Euteneuer and Karl Refenner from the BOKU Department of Applied Plant Sciences and Plant Biotechnology are acknowledged for providing logistical support.
References
- Edwards C. A. Earthworm Ecology. (CRC/Lewis Press, 1998). [Google Scholar]
- Gianinazzi S. et al. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20, 519–530 (2010). [DOI] [PubMed] [Google Scholar]
- Syers J. K. & Springett J. A. Earthworms and soil fertility. Plant Soil 76, 93–104 (1984). [Google Scholar]
- Lavelle P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. Adv Ecol Res 27, 93–132 (1997). [Google Scholar]
- Gobat J. M., Aragno M. & Matthey W. The Living Soil: Fundamentals of Soil Science and Soil Biology. (Science Publishers, 2004). [Google Scholar]
- Paoletti M. G. The role of earthworms for assessment of sustainability and as bioindicators. Agric Ecosyst Environm 74, 137–155 (1999). [Google Scholar]
- Cameron D. D. Arbuscular mycorrhizal fungi as (agro)ecosystem engineers. Plant Soil 333, 1–5 (2010). [Google Scholar]
- Siddiky M. R. K., Kohler J., Cosme M. & Rillig M. C. Soil biota effects on soil structure: Interactions between arbuscular mycorrhizal fungal mycelium and collembola. Soil Biol. Biochem. 50, 33–39 (2012). [Google Scholar]
- Smith S. E. & Read D. J. Mycorrhizal Symbiosis. 3rd edn, (Academic Press, 2008). [Google Scholar]
- Neumann E. & George E. Colonisation with the arbuscular mycorrhizal fungus Glomus mosseae (Nicol. and Gerd.) enhanced phosphorus uptake from dry soil in Sorghum bicolor (L.). Plant Soil 261, 245–255 (2004). [Google Scholar]
- Govindarajulu M. et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435, 819–823 (2005). [DOI] [PubMed] [Google Scholar]
- Gavito M. E. & Olsson P. A. Allocation of plant carbon to foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 45, 181–187 (2003). [DOI] [PubMed] [Google Scholar]
- Ngosong C., Gabriel E. & Ruess L. Use of the signature fatty acid 16:1ω5 as a tool to determine the distribution of arbuscular mycorrhizal fungi in soil. J Lipids Article ID 236807, 8 pages (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson P. A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soils. FEMS Microbiol Ecol 29, 303–310 (1999). [Google Scholar]
- Olsson P. A. & Johansen A. Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol Res 104, 429–434 (2000). [Google Scholar]
- Olsson P. A., Jakobsen I. & Wallander H. Foraging and resource allocation strategies of mycorrhizal fungi in a patchy environment. Ecol Studies 157, 93–116 (2002). [Google Scholar]
- Wurst S., Dugassa-Gobena D., Langel R., Bonkowski M. & Scheu S. Combined effects of earthworms and vesicular–arbuscular mycorrhizas on plant and aphid performance. New Phytol. 163, 169–176 (2004). [DOI] [PubMed] [Google Scholar]
- Eisenhauer N. et al. Impacts of earthworms and arbuscular mycorrhizal fungi (Glomus intraradices) on plant performance are not interrelated. Soil Biol. Biochem. 41, 561–567 (2009). [Google Scholar]
- Milleret R., Le Bayon R. C. & Gobat J. M. Root, mycorrhiza and earthworm interactions: their effects on soil structuring processes, plant and soil nutrient concentration and plant biomass. Plant Soil 316, 1–12 (2009). [Google Scholar]
- Zaller J. G., Saccani F. & Frank T. Effects of earthworms and mycorrhizal fungi on the growth of the medicinal herb Calendula officinalis (Asteraceae). Plant Soil Environ. 57, 499–504 (2011). [Google Scholar]
- Woodburn A. T. Glyphosate: production, pricing and use worldwide. Pest Manag Sci 56, 309–312 (2000). [Google Scholar]
- Duke S. O. & Powles S. B. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64, 319–325 (2008). [DOI] [PubMed] [Google Scholar]
- Vereecken H. Mobility and leaching of glyphosate: a review. Pest Manag Sci 61, 1139–1151 (2005). [DOI] [PubMed] [Google Scholar]
- Berger G., Graef F. & Pfeffer H. Glyphosate applications on arable fields considerably coincide with migrating amphibians. Sci. Rep. 3, 2622, 2610.1038/srep02622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl C. A., Schmidt T., Pieper S. & Alscher A. Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci. Rep. 3, 1135, 1110.1038/srep01135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyea R. A. The lethal impact of roundup on aquatic and terrestrial amphibians. Ecol. Appl. 15, 1118–1124 (2005). [Google Scholar]
- Köhler H. R. & Triebskorn R. Wildlife Ecotoxicology of Pesticides: Can We Track Effects to the Population Level and Beyond? Science 341, 759–765, 10.1126/science.1237591 (2013). [DOI] [PubMed] [Google Scholar]
- Verrell P. & Van Buskirk E. As the worm turns: Eisenia fetida avoids soil contaminated by a glyphosate-based herbicide. Bull Environm Contamin Toxicol 72, 219–224 (2004). [DOI] [PubMed] [Google Scholar]
- Casabé N. et al. Ecotoxicological assessment of the effects of glyphosate and chlorpyrifos in an Argentine soya field. J Soils Sediment 8, 1–8 (2007). [Google Scholar]
- Correia F. V. & Moreira J. C. Effects of glyphosate and 2,4-D on earthworms (Eisenia foetida) in laboratory tests. Bull Environm Contamin Toxicol 85, 264–268 (2010). [DOI] [PubMed] [Google Scholar]
- Bon D. et al. In vivo 31P and 1H HR-MAS NMR spectroscopy analysis of the unstarved Aporrectodea caliginosa (Lumbricidae). Biol. Fertil. Soils 43, 191–198 (2006). [Google Scholar]
- Pereira J. L. et al. Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicol 18, 455–463 (2009). [DOI] [PubMed] [Google Scholar]
- Santos M. J. G., Morgado R., Ferreira N. G. C., Soares A. M. V. M. & Loureiro S. Evaluation of the joint effect of glyphosate and dimethoate using a small-scale terrestrial ecosystem. Ecotoxicol Environm Safety 74, 1994–2001 (2011). [DOI] [PubMed] [Google Scholar]
- Malty J. D. S., Siqueira J. O. & Moreira F. M. D. S. Effects of glyphosate on soybean symbiotic microorganisms, in culture media and in greenhouse. Pesquisa Agropecuária Brasileira 41, 285–291 (2006). [Google Scholar]
- Ronco M. G., Ruscitti M. F., Arango M. C. & Beltrano J. Glyphosate and mycorrhization induce changes in plant growth and in root morphology and architecture in pepper plants (Capsicum annuum L.). J Hortic Sci Biotechnol 83, 497–505 (2008). [Google Scholar]
- Mujica M., Fracchia S., Ocampo J. A. & Godeas A. Influence of the herbicides chlorsulfuron and glyphosate on mycorrhizal soybean intercropped with the weeds Brassica campestris or Sorghum halepensis. Symbiosis 27, 73–81 (1999). [Google Scholar]
- Powell J. R. et al. Effect of glyphosate on the tripartite symbiosis formed by Glomus intraradices, Bradyrhizobium japonicum, and genetically modified soybean. Appl. Soil Ecol. 41, 128–136 (2009). [Google Scholar]
- Savin M. C., Purcell L. C., Daigh A. & Manfredini A. Response of mycorrhizal infection to glyphosate applications and P fertilization in glyphosate-tolerant soybean, maize, and cotton. J Plant Nutrition 32, 1702–1717 (2009). [Google Scholar]
- Baumgartner K., Fujiyoshi P., Smith R. & Bettiga L. Weed flora and dormant-season cover crops have no effects on arbuscular mycorrhizae of grapevine. Weed Research 50, 456–466 (2010). [Google Scholar]
- Pasaribu A., Mohamad R. B., Awang Y., Othman R. & Puteh A. Growth and development of symbiotic arbuscular mycorrhizal fungi, Glomus mosseae (Nicol. and Gerd.), in alachlor and glyphosate treated soils. African J Biotechnol 10, 11520–11526 (2011). [Google Scholar]
- Al-Rajab A. J., Amellal S. & Schiavon M. Sorption and leaching of 14C-glyphosate in agricultural soils. Agron Sustain Developm 28, 419–428 (2008). [Google Scholar]
- Laitinen P., Rämö S. & Siimes K. Glyphosate translocation from plants to soil – does this constitute a significant proportion of residues in soil? Plant Soil 300, 51–60 (2007). [Google Scholar]
- Borggaard O. K. & Gimsing A. L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64, 441–456 (2008). [DOI] [PubMed] [Google Scholar]
- Kjær J. et al. Transport modes and pathways of the strongly sorbing pesticides glyphosate and pendimethalin through structured drained soils. Chemosphere 84, 471–479 (2011). [DOI] [PubMed] [Google Scholar]
- Zaller J. G. et al. Earthworm-mycorrhiza interactions can affect the diversity, structure and functioning of establishing model grassland communities. PLOS ONE 6, e29293 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig M. C. & Mummey D. L. Mycorrhizas and soil structure. New Phytol. 171, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- Edwards C. A. Assessing the effects of environmental pollutants on soil organisms, communities, processes and ecosystems. Europ. J. Soil Biol. 38, 225–231 (2002). [Google Scholar]
- Dalby P. R., Baker G. H. & Smith S. E. Glyphosate, 2,4-DB and dimethoate: Effects on earthworm survival and growth. Soil Biol. Biochem. 27, 1661–1662 (1995). [Google Scholar]
- Yasmin S. & D'Souza D. Effect of pesticides on the reproductive output of Eisenia fetida. Bull Environm Contamin Toxicol 79, 529–532 (2007). [DOI] [PubMed] [Google Scholar]
- García-Pérez J. A., Alarcón-Gutiérrez E., Perroni Y. & Barois I. Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl. Soil Ecol., 10.1016/j.apsoil.2013.1009.1006 (2013). [Google Scholar]
- Martin N. A. The effect of herbicides used on asparagus on the growth rate of the earthworm Allolobophora caliginosa. Proc New Zealand Weed Pest Control Conf 35, 328–331 (1982). [Google Scholar]
- Springett J. A. & Gray R. A. J. Effect of repeated low doses of biocides on the earthworm Aporrectodea caliginosa in laboratory culture. Soil Biol. Biochem. 24, 1739–1744 (1992). [Google Scholar]
- Santos M. J. G., Morgado R., Ferreira N. G. C., Soares A. M. V. M. & Loureiro S. Evaluation of the joint effect of glyphosate and dimethoate using a small-scale terrestrial ecosystem. Ecotoxicol Environm Safety 74, 1994–2001 (2011). [DOI] [PubMed] [Google Scholar]
- Morowati M. Histochemical and histopathological study of the intestine of the earthworm (Pheretima elongata) exposed to a field dose of the herbicide glyphosate. Environmentalist 20, 105–111 (2000). [Google Scholar]
- Medina M. J. H. et al. Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Science 164, 993–998 (2003). [Google Scholar]
- Campos-Soriano L., García-Garrido J. M. & Segundo B. S. Activation of basal defense mechanisms of rice plants by Glomus intraradices does not affect the arbuscular mycorrhizal symbiosis. New Phytol. 188, 597–614 (2010). [DOI] [PubMed] [Google Scholar]
- Sheng M., Hamel C. & Fernandez M. Cropping practices modulate the impact of glyphosate on arbuscular mycorrhizal fungi and rhizosphere bacteria in agroecosystems of the semiarid prairie. Can J Microbiol (2012). [DOI] [PubMed] [Google Scholar]
- Olsson P. A., Bääth E., Jakobsen I. & Söderström B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res 99, 623–629 (1995). [Google Scholar]
- Olsson P. A., Larsson L., Bago B., Wallander H. & Arle van I. M. Ergosterol and fatty acids for biomass estimation of mycorrhizal fungi. New Phytol. 159, 1–20 (2003). [DOI] [PubMed] [Google Scholar]
- Druille M., Omacinia M., Golluscio R. A. & Cabello M. N. Arbuscular mycorrhizal fungi are directly and indirectly affected by glyphosate application. Appl. Soil Ecol. 72, 143–149 (2013). [Google Scholar]
- Gange A. C. Translocation of mycorrhizal fungi by earthworms during early succession. Soil Biol. Biochem. 25, 1021–1026 (1993). [Google Scholar]
- Lawrence B., Fisk M. C., Fahey T. J. & Suarez E. R. Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytol. 157, 145–153 (2003). [DOI] [PubMed] [Google Scholar]
- Bonkowski M., Griffiths B. S. & Ritz K. Food preferences of earthworms for soil fungi. Pedobiologia 44, 666–676 (2000). [Google Scholar]
- Schalamuk S. & Cabello M. Arbuscular mycorrhizal fungal propagules from tillage and no-tillage systems: possible effects on Glomeromycota diversity. Mycologia 102, 261–268 (2010). [DOI] [PubMed] [Google Scholar]
- Daynes C. N., Field D. J., Saleeba J. A., Cole M. A. & McGee P. A. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol. Biochem. 57, 683–694 (2013). [Google Scholar]
- Jégou D., Schrader S., Diestel H. & Cluzeau D. Morphological, physical and biochemical characteristics of burrow walls formed by earthworms. Appl. Soil Ecol. 17, 165–174 (2001). [Google Scholar]
- Badawi N., Johnsen A. R., Brandt K. K., Sørensen J. & Aamand J. Hydraulically active biopores stimulate pesticide mineralization in agricultural subsoil. Soil Biol. Biochem. 57, 533–541 (2013). [Google Scholar]
- Jacob G. S. et al. Metabolism of glyphosate in Pseudomonas sp. strain LBr. Appl Environ Microbiol 54, 2953–2958 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipke R., Amrhein N., Jacob G. S., Schaefer J. & Kishore G. M. Metabolism of glyphosate in an Arthrobacter sp. GLP-1. Eur J Biochem 165, 267–273 (1987). [DOI] [PubMed] [Google Scholar]
- Liu C.-M., McLean P. A., Sookdeo C. C. & Cannon F. C. Degradation of the herbicide glyphosate by members of the family rhizobiaceae. Appl Environ Microbiol 57, 1799–1804 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasaribu A. et al. Effect of herbicide on sporulation and infectivity of vesicular mycorrhizal (Glomus mosseae) symbiosis with peanut plant. J Animal Plant Sci 32, 1671–1678 (2013). [Google Scholar]
- Farenhorst A., Topp E., Bowman B. T. & Tomlin A. D. Earthworm burrowing and feeding activity and the potential for atrazine transport by preferential flow. Soil Biol. Biochem. 32, 479–488 (2000). [Google Scholar]
- Zaller J. G., Frank T. & Drapela T. Soil sand content can alter effects of different taxa of mycorrhizal fungi on plant biomass production of grassland species. Europ. J. Soil Biol. 47, 175–181 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaller J. G. et al. Subsurface earthworm casts can be important soil microsites specifically influencing the growth of grassland plants. Biol. Fertil. Soils 49, 1097–1107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide R. T. & Li M. Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytol. 111, 35–44 (1989). [Google Scholar]
- Zaller J. G. & Arnone J. A. Activity of surface-casting earthworms in a calcareous grassland under elevated atmospheric CO2. Oecologia 111, 249–254 (1997). [DOI] [PubMed] [Google Scholar]
- Popp M. et al. Determination of glyphosate and AMPA in surface and waste water using high-performance ion chromatography coupled to inductively coupled plasma dynamic reaction cell mass spectrometry (HPIC-ICP-DRC-MS). Analyt Bioanalyt Chem 391, 695–699 (2008). [DOI] [PubMed] [Google Scholar]
- Todorovic G. et al. Determination of glyphosate and AMPA in three representative agricultural Austrian soils with a HPLC-MS/MS method. Soil Sediments Contamin 22, 332–350 (2013). [Google Scholar]
- Vierheilig H., Coughlan A. P., Wyss U. & Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64, 5004–5007 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M. & Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500 (1980). [Google Scholar]
- Frostegård A., Tunlid A. & Bååth E. Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environm Microbiol 59, 3605–3617 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]