Abstract
AIM: To investigate the influence of a positive proximal margin in total gastrectomy patients with gastric adenocarcinoma of the cardia.
METHODS: Medical records of 191 patients with total gastrectomies for adenocarcinoma of the cardia between 1995 and 2000 were reviewed. The clinicopathologic features associated with a positive margin were determined, and the predictors for survival were analyzed.
RESULTS: The incidence of positive proximal margin was 8.4% (16/191). The positive margins were associated with advanced diseases. The tumor size and the depth of tumor invasion were independent risk factors for a positive margin. The mean survival in the positive margin group was 33.9 mo as compared with 62.4 mo in the negative group (P < 0.001). However, the difference in survival lost significance in subgroup analysis according to stage. Multivariate analysis identified that a positive margin was not an independent prognostic factor for survival.
CONCLUSION: A positive margin is more of an indication of advanced disease in patients with gastric adenocarcinoma of the cardia rather than an independent prognostic factor for survival.
Keywords: Resection margin, Gastric cancer, Total gastrectomy, Outcome
INTRODUCTION
A curative resection is the only treatment offering hope for cure in patients with gastric cancer. This suggests removing the primary lesion with an adequate tumor-free margin[1,2]. Several reports have looked into the detrimental effect of a positive margin on survival in gastric cancer patients[3-5]. However, these studies have included heterogeneous groups with respect to the resection margins. Patients with various tumor locations of gastric cancer or with proximal and distal margins of various types of gastrectomies are grouped together.
Gastric cancer of the cardia has been regarded as a separate neoplasm because of its distinct prognostic and pathological features[6]. A positive proximal resection margin poses a management dilemma for surgeons. To our knowledge, there are few reports on the influence of a positive proximal margin in total gastrectomy for adenocarcinoma of the cardia, and there is some controversy as to whether a positive margin is an independent prognostic factor for survival or not.
The aim of this retrospective study was to investigate the risk factors associated with a positive proximal margin in gastric adenocarcinoma of the cardia, and to assess the influence of a positive margin on the patient’s long-term survival, as well as its independent impact on prognosis.
MATERIALS AND METHODS
Medical records were reviewed of 202 patients with adenocarcinoma of the cardia undergone surgery between September 1995 and December 2000 at the Department of Surgery, Yonsei University College of Medicine. Two patients with recurrent gastric cancer, 2 with preoperative metastatic findings and 7 with incomplete data were excluded from the analysis. Therefore, 191 patients who underwent total gastrectomy with curative intent for gastric adenocarcinoma of the cardia, were enrolled in the study. One hundred and twenty-nine patients (67.5%) were males and 62 patients were females. The median age was 57 years ranging from 29 to 85 years.
The location of tumor and preoperative staging were determined by esophagoduodenogastroscopy, upper gastrointestinal barium examination and abdominal CT scan. All patients had either a type II or III tumor according to the Siewert classifications[7]. A total gastrect-omy with transhiatal resection of the distal esophagus and esophagojejunostomy were performed and a macroscopically tumor free proximal resection margin was achieved on all patients. Intraoperative frozen section margin evaluation was not routinely carried out except in patients with suspicion of obtaining a positive margin and thus was not analyzed. During the operation, one hundred and twenty-five of these patients (65.4%) underwent splenectomy for the purpose of lymph node dissection or suspicion of direct tumor invasion to the spleen. A D2 lymphadenectomy was regularly performed. In our institute, postoperative fluorouracil-based adjuvant chemotherapy was usually given in advanced cancer patients except for those with T2N0M0 stage.
A resection margin was considered positive if the permanent section examination revealed cancer infiltration at the line of the transection. The length of the resection margin was defined as the distance from the proximal limit of the lesion to the site of the resection. Collected data included patient demographics, clinicopathologic features and survivals. Stage was reported according to the fifth version of the UICC/AJCC guidelines. Survival was measured from the date of operation to death or to the last follow-up date of June 30, 2003. Of the 191 patients, 88 (46.1%) died during the follow-up period.
Statistical analyses were performed using SPSS version 10.0 (SPSS, Chicago, IL, USA). Univariate analysis was done using two-tailed χ2 test or unpaired Student’s t-test. The risk factors associated with a positive margin were identified using a logistic regression model. Survival probability was estimated using the Kaplan-Meier method. The survival curves were compared using the log-rank test. The prognostic factors for survival were analyzed using the Cox proportional hazards regression model with forward stepwise method. P < 0.05 was considered statistically significant.
RESULTS
Sixteen out of the 191 patients (8.4%) had a positive margin. The incidence of this event was 26.5% (9/34) in patients with stage IV gastric cancer, while 10.5% (6/57) in stage III and 2.3% (1/43) in stage II. None of the 57 patients with stage I disease had a positive margin. The mean length of the proximal resection margin was 2.5 ± 1.1 cm (range, 0.8-7.0 cm). It was 2.6 ± 1.4 cm (range, 0.8-6.5 cm) in the positive group as compared with 2.5 ± 1.2 cm (range, 0.8-7.0 cm) in the negative margin group (P = 0.703). A positive margin was significantly associated with Borrmann III and IV gross type, large size of the primary tumor, serosal invasion, node involvement and advanced stage disease (Table 1, Table 2). Logistic regression analysis revealed that the size of the primary tumor and the depth of tumor invasion were independent risk factors for a positive margin (Table 3).
Table 1.
Comparison of demographic and pathologic data between patients with positive and negative margins n (%)
| Variable | Positive margins (n = 16) | Negative margins (n = 175) | P value |
| Age (years) | 49.3 ± 12.7 | 57.5 ± 11.2 | 0.006 |
| Sex | 0.161 | ||
| Male | 8 (50.0) | 121 (69.1) | |
| Female | 8 (50.0) | 54 (30.9) | |
| Siewert type | 0.141 | ||
| Type II | 5 (31.3) | 25 (14.3) | |
| Type III | 11 (68.7) | 150 (85.7) | |
| Gross type | 0.001 | ||
| Borrmann I, II | 1 (6.3) | 83 (47.4) | |
| Borrmann III, IV | 15 (93.7) | 92 (52.6) | |
| Tumor size (cm) | 0.001 | ||
| ≤ 5 cm | 2 (12.5) | 98 (56.0) | |
| > 5 cm | 14 (87.5) | 77 (44.0) | |
| Histology | 0.399 | ||
| Differentiated | 3 (18.8) | 56 (32.0) | |
| Undifferentiated | 13 (81.2) | 119 (68.0) | |
| LRM (cm) | 0.985 | ||
| ≤2 | 4 (25) | 42 (24.0) | |
| > 2 and ≤ 4 | 10 (62.5) | 113 (64.6) | |
| > 4 | 2 (12.5) | 20 (11.4) |
LRM: length of resection margin. Age represents mean ± SD.
Table 2.
Comparison of tumor, node, metastasis classification, stage between patients with positive and negative margins n (%)
| Variable | Positive margins (n = 16) | Negative margins (n = 175) | P value |
| Depth of tumor invasion | < 0.001 | ||
| T1 | 0 (0) | 29 (16.6) | |
| T2 | 2 (12.5) | 84 (48.0) | |
| T3 | 11 (68.7) | 55 (31.4) | |
| T4 | 3 (18.8) | 7 (4.0) | |
| Node involvement | < 0.001 | ||
| N0 | 3 (18.8) | 67 (38.3) | |
| N1 | 2 (12.5) | 60 (34.3) | |
| N2 | 2 (12.5) | 26 (14.8) | |
| N3 | 9 (56.2) | 22 (12.6) | |
| Stage | < 0.001 | ||
| I | 0 (0) | 57 (32.6) | |
| II | 1 (6.3) | 42 (24.0) | |
| III | 6 (37.5) | 51 (29.1) | |
| IV | 9 (56.2) | 25 (14.3) |
Table 3.
Logistic regression analysis of risk factors for a positive margin
| Variable | Regression coefficient | Standard error | Odds ratio | 95% CI for odds ratio | P value |
| Serosal invasion | 2.300 | 0.782 | 9.970 | 2.152 - 46.196 | 0.003 |
| Tumor size | 1.875 | 0.787 | 6.524 | 1.395 - 30.512 | 0.017 |
The mean follow-up time and survival time after surgery were 44.3 mo (range, 0.5-93.5 mo) and 59.9 mo (95% confidence interval, 54.6-65.1 mo), respectively. On univariate analysis, the gross type, size of the primary tumor, serosal invasion, lymph node involvement, stage, resection margin status and splenectomy were significantly associated with the long-term survival. The mean survival time in patients with positive margins was 33.9 mo as compared with 62.4 mo in patients with negative margins (P < 0.001, Figure 1A). Since fifteen out of 16 patients (93.8%) with positive margins had stage III and IV gastric cancer in the present study, we then analyzed the subgroup patients stratified by stage, the difference in survival between both groups did not reach significance in stage III and IV gastric cancer (Figure 1B).
Figure 1.
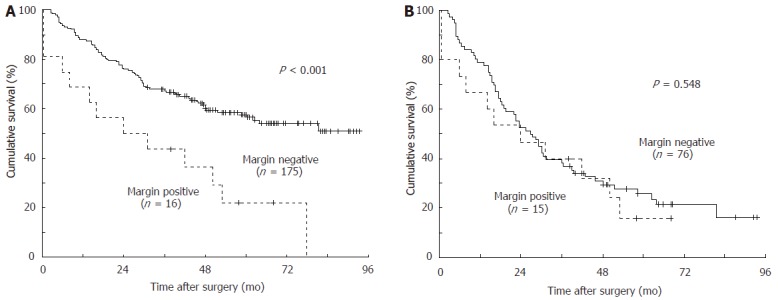
Cumulative survival curves according to the margin status in a total of 191 patients (A) and 91 stage III and IV (B) patients with gastric cancer of the cardia. The mean survival time of patients with positive margins was 33.9 mo compared to 62.4 mo of patients with negative margins in total 191 patients, and 29.2 mo compared to 38.2 mo in 91 patients with stage III and IV diseases
On multivariate analysis, seven variables with a significance level of < 0.05 in univariate analysis were included in the model. Positive margin was not identified to be an independent predictor for survival (Table 4).
Table 4.
Multivariate analysis of prognostic factors for survival
| Variable | Regression coefficient | Standard error | Odds ratio | 95% CI for odds ratio | P value |
| Gross type | -0.174 | 0.258 | 0.840 | 0.507 - 1.392 | 0.500 |
| Tumor size | 0.209 | 0.248 | 1.232 | 0.758 - 2.001 | 0.399 |
| Serosal invasion | -0.125 | 0.264 | 0.883 | 0.526 - 1.481 | 0.637 |
| Node involvement | 0.838 | 0.431 | 2.311 | 0.994 - 5.374 | 0.052 |
| Stage | 1.469 | 0.389 | 4.344 | 2.028 - 9.304 | < 0.001 |
| Margin status | 0.397 | 0.330 | 1.488 | 0.779 - 2.843 | 0.229 |
| Splenectomy | 0.276 | 0.292 | 1.318 | 0.744 - 2.337 | 0.344 |
DISCUSSION
The incidence of positive resection margin has been reported to vary from 5% to 35%[8-10]. In this study, the incidence of this event was 8.4%. However, it was significantly higher in patients with stage III and IV gastric cancer (16.5%) than in patients with stage I and II gastric cancer (1.0%). There are several factors accounting for such a high incidence of positive margin in advanced gastric cancer patients. First, it has been reported that the incidence of positive margin increases with deeper invasion of the tumor into the gastric wall and advanced disease stage[11,12]. Secondly, patients with stage III and IV disease have more Borrmann III and IV gross type of tumors and/or more undifferentiated tumors (data not shown), and these patients may have a higher risk of sub-clinical intramural tumor infiltration to the esophagus[10]. Finally, intraoperative frozen section margin evaluation is not routinely performed in advanced disease patients, which may possibly increase the incidence of a positive margin in those patients[13].
The risk factors for a positive margin in the present study were identified based on the same length of the proximal resection margin between margin positive and negative groups. Logistic regression analysis showed that the risk mainly depended on the depth of cancer invasion and the size of the primary tumor.
The depth of invasion into the gastric wall is significantly related to a higher rate of esophageal infiltration. Bozzetti et al[14] reported that the risk of proximal infiltration is significantly higher when the tumor infiltrates the serosa or beyond it. Songun et al[14] demonstrated that the depth of tumor invasion is one of the independent prognostic factors for a positive margin in gastric cancer at various tumor locations. Our results are similar to the aforementioned reports. The size of the primary tumor was also identified as an independent predictor for a positive margin, which is in agreement with that reported by Yokoda et al[16], who found that a lesion exceeding 5 cm in maximum diameter is predisposed to a positive margin in gastric cancer.
Although frozen section has a high sensitivity and specificity, it is not routinely used to assess the resection margin[3,17,18]. It is advisable that this technique should be used selectively for patients who may benefit from it since frozen sections are costly and time-consuming[19]. Our study observed that patients with advanced stage disease had a higher risk of obtaining a positive margin. In addition, a positive margin was associated with deeper tumor invasion to the gastric wall and larger tumor size. These findings may provide some evidence for helping the surgeons select patients to perform intraoperative frozen section margin evaluation.
The influence of a positive margin on prognosis of gastric cancer of the cardia remains controversial. Chan et al[20] reviewed 137 total or proximal gastrectomies for gastric cancer with various lesion sites and observed that a positive oesophageal margin is an independent poor prognostic factor for long-term survival. A similar result has been reported by Mariette et al[21], who reviewed 94 adenocarcinomas of the esophagogastric junction, but different surgical approaches were involved in their work. In addition, there are no difference in the clinicopathologic features between the positive margin group and the negative margin group in the study[21]. These aforementioned studies are not in accordance with the results we observed that a positive margin was not associated with the long-term survival in gastric adenocarcinoma of the cardia. This discrepancy, although has to be interpreted with caution, may be explained by the following reasons. First, it was a retrospective study and the analyses were based on relatively few patients with or without positive margins in our single institution. Second, a positive margin is often associated with advanced disease. Gall et al[22] investigated 87 patients who underwent esophagectomy for cancer and found that the positive margin is involved mainly in late stage disease which does not influence the long-term survival in those patients. Kim et al[23] reported that a positive margin loses its predictive value for survival in patients with T3 or T4 lesions and stage III or IV disease. Finally, because the relationship between margin status and treatment failure patterns were not addressed in our study with the limited data, our results may possibly due to the uncertainty in advanced disease, the patients may usually succumb to metastatic disease before the effect of the positive margin is observed[24].
Our results suggest that a positive margin is more of an indication of advanced disease rather than an independent prognostic factor for survival in patients with gastric cancer of the cardia. However, more definitive conclusions would be based on multi-center studies with the results of large-scale analyses.
Footnotes
Supported by the Korean Science and Engineering Fund through the Cancer Metastasis Research Center at Yonsei University
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
References
- 1.Shao LF, Gao ZG, Yang NP, Wei GQ, Wang YD, Cheng CP. Results of surgical treatment in 6,123 cases of carcinoma of the esophagus and gastric cardia. J Surg Oncol. 1989;42:170–174. doi: 10.1002/jso.2930420308. [DOI] [PubMed] [Google Scholar]
- 2.Sons HU, Streicher HJ. Palliative and curative surgical therapy of malignant stenoses of the esophagus and cardia. J Surg Oncol. 1989;40:162–169. doi: 10.1002/jso.2930400306. [DOI] [PubMed] [Google Scholar]
- 3.Hallissey MT, Jewkes AJ, Dunn JA, Ward L, Fielding JW. Resection-line involvement in gastric cancer: a continuing problem. Br J Surg. 1993;80:1418–1420. doi: 10.1002/bjs.1800801121. [DOI] [PubMed] [Google Scholar]
- 4.Shiu MH, Moore E, Sanders M, Huvos A, Freedman B, Goodbold J, Chaiyaphruk S, Wesdorp R, Brennan MF. Influence of the extent of resection on survival after curative treatment of gastric carcinoma. A retrospective multivariate analysis. Arch Surg. 1987;122:1347–1351. doi: 10.1001/archsurg.1987.01400230135024. [DOI] [PubMed] [Google Scholar]
- 5.Jakl RJ, Miholic J, Koller R, Markis E, Wolner E. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. 1995;169:316–319. doi: 10.1016/s0002-9610(99)80166-4. [DOI] [PubMed] [Google Scholar]
- 6.Okamura T, Tsujitani S, Marin P, Haraguchi M, Korenaga D, Baba H, Sugimachi K. Adenocarcinoma in the upper third part of the stomach. Surg Gynecol Obstet. 1987;165:247–250. [PubMed] [Google Scholar]
- 7.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 8.Paulino F, Roselli A. Carcinoma of the stomach. With special reference to total gastrectomy. Curr Probl Surg. 1973:3–72. [PubMed] [Google Scholar]
- 9.Stipa S, Di Giorgio A, Ferri M. Surgical treatment of adenocarcinoma of the cardia. Surgery. 1992;111:386–393. [PubMed] [Google Scholar]
- 10.Bozzetti F, Bignami P, Bertario L, Fissi S, Eboli M. Surgical treatment of gastric cancer invading the oesophagus. Eur J Surg Oncol. 2000;26:810–814. doi: 10.1053/ejso.2000.1009. [DOI] [PubMed] [Google Scholar]
- 11.Tsujitani S, Okuyama T, Orita H, Kakeji Y, Maehara Y, Sugimachi K, Kaibara N. Margins of resection of the esophagus for gastric cancer with esophageal invasion. Hepatogastroenterology. 1995;42:873–877. [PubMed] [Google Scholar]
- 12.Sefton GK, Cooper DJ, Giddings AE, Grech P. Assessment and resection of carcinoma at the gastroesophageal junction. Surg Gynecol Obstet. 1977;144:563–566. [PubMed] [Google Scholar]
- 13.Papachristou DN, Agnanti N, D'Agostino H, Fortner JG. Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg. 1980;139:711–713. doi: 10.1016/0002-9610(80)90369-4. [DOI] [PubMed] [Google Scholar]
- 14.Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, Doci R, Gennari L. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg. 1982;196:685–690. doi: 10.1097/00000658-198212001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Songun I, Bonenkamp JJ, Hermans J, van Krieken JH, van de Velde CJ. Prognostic value of resection-line involvement in patients undergoing curative resections for gastric cancer. Eur J Cancer. 1996;32A:433–437. doi: 10.1016/0959-8049(95)00591-9. [DOI] [PubMed] [Google Scholar]
- 16.Yokota T, Sawai K, Yamaguchi T, Taniguchi H, Shimada S, Yoneyama C, Takahashi T. Resection margin in patients with gastric cancer associated with esophageal invasion: clinicopathological study. J Surg Oncol. 1993;53:60–63. doi: 10.1002/jso.2930530115. [DOI] [PubMed] [Google Scholar]
- 17.Keighley MR, Moore J, Lee JR, Malins D, Thompson H. Peroperative frozen section and cytology to assess proximal invasion in gastro-oesophageal carcinoma. Br J Surg. 1981;68:73–74. doi: 10.1002/bjs.1800680203. [DOI] [PubMed] [Google Scholar]
- 18.Torp SH, Skjørten FJ. The reliability of frozen section diagnosis. Acta Chir Scand. 1990;156:127–130. [PubMed] [Google Scholar]
- 19.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110:1773–1776. doi: 10.1097/00005537-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 20.Chan WH, Wong WK, Khin LW, Chan HS, Soo KC. Significance of a positive oesophageal margin in stomach cancer. Aust N Z J Surg. 2000;70:700–703. doi: 10.1046/j.1440-1622.2000.01937.x. [DOI] [PubMed] [Google Scholar]
- 21.Mariette C, Castel B, Balon JM, Van Seuningen I, Triboulet JP. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003;29:588–593. doi: 10.1016/s0748-7983(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 22.Gall CA, Rieger NA, Wattchow DA. Positive proximal resection margins after resection for carcinoma of the oesophagus and stomach: effect on survival and symptom recurrence. Aust N Z J Surg. 1996;66:734–737. doi: 10.1111/j.1445-2197.1996.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Karpeh MS, Klimstra DS, Leung D, Brennan MF. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3:24–33. doi: 10.1016/s1091-255x(99)80004-3. [DOI] [PubMed] [Google Scholar]
- 24.Goh HS, Wong TH, Ti TK, Rauff A. Outcome of failure in clearing the oesophageal margin in total gastrectomy for adenocarcinoma. Ann Acad Med Singapore. 1989;18:69–71. [PubMed] [Google Scholar]


