Abstract
Poorly differentiated gastric neuroendocrine carcinomas, although rare, deserve particular attention, as they are aggressive and have an extremely poor prognosis. In this report we describe a gastric neuroendocrine carcinoma with rapidly fatal outcome. Immunohistological staining of the resected specimens revealed that the tumor was an endocrine carcinoma. The tumor disclosed intense immunoreactivity to pan-neuroendocrine markers and diffuse somatostatin immunoreactivity. There were no psammoma bodies and no demonstrable association with von Recklinghausen’s neurofibromatosis. In the gastrointestinal tract, neuroendocrine tumors producing predominantly somatostatin have been described only in the duodenum. To the best of our knowledge, the present report is the second case report of a neuroendocrine gastric carcinoma expressing diffusely somatostatin as the only neuroendocrine regulatory peptide.
Keywords: Neuroendocrine carcinoma, Neuroendocrine tumors, Carcinoid, Stomach neoplasms, Somatostatin, Immunohistochemistry, Tumor markers, Enterochromaffin-like cells
INTRODUCTION
Neuroendocrine [NE] carcinoma of the stomach is a rare, highly malignant tumor with an extremely poor prognosis[1-6].
A subset of NE carcinoma is associated with inherited syndromes, such as multiple endocrine neoplasia [MEN1] and von Hippel-Lindau syndrome[7]. However, the vast majority of NE carcinomas occur sporadically[2,8,9,10].
Poorly differentiated NE carcinomas of the stomach are a separated entity in respect to well-differentiated carcinoids. There is little evidence to support the origin of neuroendocrine carcinomas from malignant evolution of well-differentiated tumors. Rather, it is conceivable that they derive from endocrine-committed immature stem cells, as suggested by the studies of mixed exocrine-endocrine tumors[11,12]. Like type III sporadic carcinoids, NE gastric carcinomas develop independently from hypergastrinemia, thus recognizing a substantially different pathogenetic feature with respect to type I and II enterochromaffin-like (ECL) cell tumors[4,9,13].
The first report of a NE carcinoma of the stomach with diffuse somatostatin immunoreactivity was done by Chejfec et al[14]. They reported a neuroendocrine gastric tumor with a rapid fatal outcome, displaying neurosecretory granules by electron microscopy and immunoreactivity to pan-neurosecretory markers, i.e., chromogranin and neuron-specific enolase. The only neuroendocrine regulatory peptide detected in the tumor was somatostatin, identified by immunohistochemistry in the majority of neoplastic cells. In contrast with duodenal somatostatinomas, there were no psammoma bodies and no demonstrable association with von Recklinghausen’s neurofibromatosis. Rindi[4] described two gastric neuroendocrine carcinomas in which immunohistochemical staining revealed that 5% to 30% of tumor cells were positive for somatostatin[4]. Yu et al[3] reported a case of NE gastric carcinoma expressing somatostatin, but they did not describe the intensity of the staining. To the best of our knowledge, the present report is the second case report of NE gastric carcinoma expressing somatostatin in the majority of the neoplastic cells.
MATERIALS AND METHODS
Paraffin-embedded specimens containing a representative sample of the tumor were sliced into 3 μm thick sections and mounted on silanized slides. These underwent immunohistochemical analysis using avidin-biotin immunoperoxidase complex technique.
First, the tissue was deparaffinized and hydrated in alcohol. The next step was antigen recovery in a dampness cooker with a steam solution of 10 mmol/L citrate pH 6.0 at 95-100°C for 35 minutes. Blockage of the endogenous peroxidases was done with a hydrogen-peroxide wash 10V (3%), and following that, non-specific sites were bound with skim milk 2%. Then, the primary antibodies (Dako A/S®, Copenhagen, Denmark) were diluted in this proportion: 1:50 for serotonin, 1:200 for somatostatin, 1:1000 for specific neuronal enolase, 1:800 for synaptophysin, 1:1000 for chromogranin, 1:320 for cytokeratins AE1/AE3, 1:100 for insulin, 1:50 for glucagons and 1:200 for gastrin, in bovine serum albumin (Sigma Diagnostics®, St. Louis, MO). 100 μL of the resulting solution was poured onto the tissue sections and incubated for 18 hours at 4°C.
The slides were washed in sodium phosphate buffer 0.05 M, pH 7.2-7.4 and incubated with the secondary antibody (Large Streptavidin-Avidin-Biotin–Peroxidase System; Dako A/S®, Copenhagen, Denmark). Following this, the slides were developed with chromogene (3-3’-diaminobenzamidine; Sigma Diagnostics®, St. Louis, MO), and counter-stained with Harris’ hematoxylin (Sigma Diagnostics®, St. Louis, MO).
The brownish-color was considered evidence of a positive expression of the respective antibodies in the tumor cells. As a positive and negative control of the reactions, histological cuts of human pancreas were used for serotonin, somatostatin, specific neuronal enolase, chromogranin, insulin and glucagon; human colon for synaptophysin and cytokeratins AE1/AE3; and human stomach for gastrin. Uncolored red blood cells were used as negative internal control.
CASE REPORT
A white man, 73 years old was admitted with a 2-mo history of intense epigastric pain, weight loss of 10 kg, loss of appetite and asthenia. Physical examination revealed only discolored mucosa. Laboratory exams were normal except for a hemoglobin level of 9.8 g%. Gastroduodenoscopy showed gastric lesion in the gastric body, with a Bormann III macroscopic aspect, whilst endoscopic biopsies revealed undifferentiated carcinoma. Pre-operatory tomography showed a mass in the gastric region and hepatomegaly with multiple heterogenous nodules suggesting metastasis. Radiographs of the thorax and colonoscopy was normal. On laparotomy a gastric mass was found measuring 6.5 cm in length, located in the gastric fundus and body invading the body and tail of the pancreas and the spleen. Palliative total gastrectomy, omentectomy, splenectomy and distal pancreatectomy were performed to control the patient’s pain. Histology revealed a neuroendocrine carcinoma which was poorly differentiated, having medium-sized, polygonal cells, with trabecular or acinar, cytoplasm eosinophilic or clear appearance with low pleomorphism (Figure 1). The neoplasia had infiltrated the wall as far as the serosa and there was evidence of vascular and perineural infiltration. The surgical margins were free of any neoplastic infiltration. From a total of 14 perigastric lymph nodes one showed neoplastic involvement. The spleen and pancreas were also infiltrated by neoplasia. Immunohistochemical neoplasia analysis showed immunoexpression for chromogranin, specific neuronal enolase, synaptophysin, cytokeratines AE1/AE3, CAM 5.2 and was strongly positive for somatostatin in the majority of the neoplastic cells (Figure 2). There was no immunoexpression for insulin, glucagon, serotonin, or gastrin. These immunohistochemical investigations of the neoplastic cells confirmed the tumor as a neuroendocrine carcinoma. Post-operatively, abdominal and pelvic tomography showed intra-abdominal collections and the patient was reoperated on the 21st post-operative to drain intracavitary abscesses. An esophageal-jejunal anastomosis fistula appeared and despite parenteral and enteral nutritional support, the patient presented with progressive cachexia and died five months after gastric resection.
Figure 1.
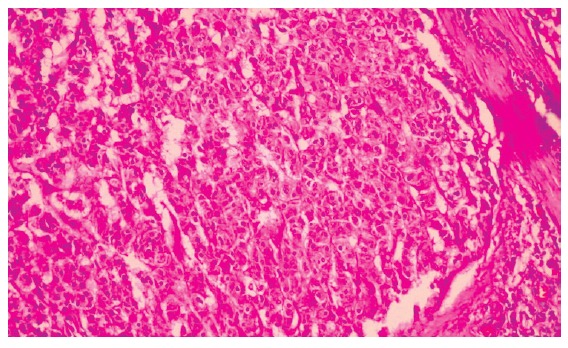
Micrograph of histological section of the neuroendocrine gastric carcinoma showing a proeminent trabecular architecture, organoid pattern with pseudoglandular structure, uniform cells and abundant mitoses. (Hematoxilin & Eosin staining; original magnification, × 200).
Figure 2.
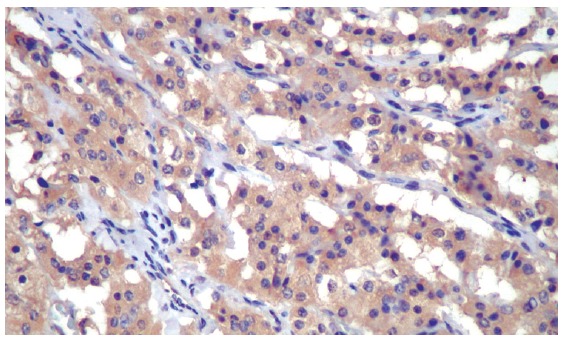
Immunohistochemistry of the neuroendocrine gastric carcinoma showing intense and diffuse somatostatin-positive imunoreactivity in the neoplastic cells (Immunoperoxidase, primary antibody anti-human somatostatin-code A0566-Dako A/S®, Copenhagen, Denmark, original magnification, × 400).
DISCUSSION
Gastric neuroendocrine tumor disease embraces a group of tumors that exhibit a spectrum of histopathologic variations ranging from the clearly benign tumors to those characterized by marked cytological atypia and high mitotic activity, which clinically, behave in a highly malignant fashion. In general, the majority of these tumors are well-differentiated, benign ECL tumors, whose courses are indolent and not life-threatening. A relatively small percentage of well-differentiated ECL tumors may have a low-grade malignant potential and deserve an adequate cancer treatment and follow-up. Poorly differentiated gastric neuroendocrine carcinoma tumors are the most aggressive of the group. They have an extremely poor diagnosis, which justifies an aggressive therapeutic approach[4].
Different endocrine cell types have been identified and characterized in the human gastric mucosa[15]. Besides ECL cell producing histamine, enterochromaffin (EC) cell producing serotonin, and G cell producing gastrin, there is the D cell producing somatostatin. Although in theory any one of these endocrine cell types could undergo neoplastic transformation, only the ECL and gastrin [G] cells are found as the exclusive or dominant tumor cell type in oxyntic and antral neuroendocrine tumors, respectively. Conversely, any one or more of the remaining endocrine cell types can be found as minor subpopulation in gastric neuroendocrine tumors[4]. Somatostatin-producing cells were subsequently identified by immunofluorescence in the stomach and in the first portion of the duodenum[16]. Gastric carcinomas display focal somatostatin immunoreactivity infrequently[17] although it is very frequently observed in metaplastic gastric mucosa associated with peptic ulcer or gastric carcinoma[18]. In this present case, none of the peptides generally associated with gastric neuroendocrine tumors were demonstrable by immunohistochemistry except for somatostatin. The diffuse, strong, and very predominant immunoreactivity for somatostatin noted in the majority of neoplastic cells differed from the focal somatostatin reactivity and the coexpression with other hormones reported in neuroendocrine gastric carcinomas[19].
NE gastric carcinomas are in general not associated with an overt endocrine syndrome, even when hormone-like immunoreactivities were detected in tumor tissue, possibly because of the scarce amount of hormone produced or because inactive prohormones, rather than active molecular species, were produced[12,20,21]. Although pancreatic endocrine tumors have commonly been associated with somatostatinoma syndrome, extrapancreatic somatostatinomas are not usually accompanied by a complete scenario of diabetes mellitus, biliary tract disease, diarrhea, steatorrhea, hypochlorhydria, weight loss, or anemia. These gastrointestinal tumors appear to be hormonally inactive and abdominal pain is the main clinical feature[22]. This also occurred with the patient of the present report.
Neuroendocrine tumors of the upper gastrointestinal tract displaying predominantly somatostatin immunoreactivity have thus far only been described in the duodenum[14].
In this case, neoplastic cells expressed immunoreactivity to pan-neurosecretory markers chromogranin A, synaptophysin, and neuron-specific enolase. As in the case described by Chejfec et al[14], the predominant neuroendocrine regulatory peptide detected in the tumor was somatostatin, identified by immunohistochemistry in the majority of neoplastic cells. There were no psammoma bodies and no demonstrable association with von Recklinghausen’s neurofibromatosis, as is frequently in duodenal somatostatinomas.
NE tumors in non-NE organs are neoplasms that consist of relatively uniform NE cells. These tumors are classified as carcinoid tumors or NE carcinoma based on the combination of NE cell differentiation and cellular atypia. Histologically, NE carcinomas are solid, organoid, trabecular, pseudoglandular, spindle cell, or rosettelike[2]. The tumor of the patient related in this report was composed of malignant neoplasia with cells having a monotome aspect, and exhibiting vascular and perineural invasion as detected in practically all NE carcinomas[21].
Based on both cell size and morphologic features, Matsui et al[2] subdivided NE carcinomas into two variants, namely, small and large cell NE carcinoma. Comparing with small cell NE carcinomas, large cell NE carcinomas had a higher mitotic index, larger polygonal cells, a decreased nuclear-cytoplasmic ratio, coarser nuclear chromatin, and more frequently conspicuous nucleoli. Large cell NE carcinoma was an aggressive tumor with a very poor prognosis that approached that small cell NE carcinoma.
There have been many cases of a poorly differentiated adenocarcinoma that show a minor component with histology similar to that of NE carcinoma[1]. It is generally true that NE carcinoma shows the organoid structure of NE tumor despite cellular atypism, but such histological features can be difficult to evaluate in a small biopsy specimen. Thus, it is considered that a diagnosis of NE carcinoma can be made only after histological evaluation of the resected material[23].
In the present patient, the gastric tumor showed a so-called organoid pattern with diffuse immunoreactivity observed for general NE markers. Therefore, the diagnosis of NE carcinoma was considered to be appropriate, although the initial diagnosis based on the biopsy specimen was undifferentiated carcinoma.
The mean age at presentation of these tumors is in the seventh decade, with no significant sex prevalence. NE gastric carcinomas are generally large [mean size 4.2 cm], fungating or annular lesions, found most frequently in the body/fundus. At the time of diagnosis, most of the tumors are already in advanced stage, particularly the lesions with tumor diameter more than 4 cm, and show extensive metastases[2-4,21,24,25]. This clinical picture was also observed in our case.
Although rare, poorly differentiated gastric NE carcinomas deserve particular attention, as they are aggressive and have an extremely poor prognosis. The surgical resection is the most appropriate form of treatment for this type. The usefulness of multi-drug chemotherapy remains to be evaluated in larger clinical studies[2,3,26].
Mean survival rates of 6.5-14.9 mo have been reported with a 1-year survival rate of 58%[3,10,25,26]. As the patient reported in this study, the majority of the patients die due to extensive metastatic disease[1,27].
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Zhang Y
References
- 1.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 2.Matsui K, Jin XM, Kitagawa M, Miwa A. Clinicopathologic features of neuroendocrine carcinomas of the stomach: appraisal of small cell and large cell variants. Arch Pathol Lab Med. 1998;122:1010–1017. [PubMed] [Google Scholar]
- 3.Yu JY, Wang LP, Meng YH, Hu M, Wang JL, Bordi C. Classification of gastric neuroendocrine tumors and its clinicopathologic significance. World J Gastroenterol. 1998;4:158–161. doi: 10.3748/wjg.v4.i2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rindi G. Clinicopathologic aspects of gastric neuroendocrine tumors. Am J Surg Pathol. 1995;19 Suppl 1:S20–S29. [PubMed] [Google Scholar]
- 5.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 6.Han HS, Kim HS, Woo DK, Kim WH, Kim YI. Loss of heterozygosity in gastric neuroendocrine tumor. Anticancer Res. 2000;20:2849–2854. [PubMed] [Google Scholar]
- 7.Hosoya Y, Nagai H, Koinuma K, Yasuda Y, Kaneko Y, Saito K. A case of aggressive neuroendocrine carcinoma of the stomach. Gastric Cancer. 2003;6:55–59. doi: 10.1007/s101200300007. [DOI] [PubMed] [Google Scholar]
- 8.Staren ED, Lott S, Saavedra VM, Jansson DS, Deziel DJ, Saclarides TJ, Manderino GL, Gould VE. Neuroendocrine carcinomas of the stomach: a clinicopathologic evaluation. Surgery. 1992;112:1039–1046; discussion 1046-1047. [PubMed] [Google Scholar]
- 9.Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 10.Xie SD, Wang LB, Song XY, Pan T. Minute gastric carcinoid tumor with regional lymph node metastasis: a case report and review of literature. World J Gastroenterol. 2004;10:2461–2463. doi: 10.3748/wjg.v10.i16.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solcia E, Rindi G, Fiocca R, Villani L, Buffa R, Ambrosiani L, Capella C. Distinct patterns of chronic gastritis associated with carcinoid and cancer and their role in tumorigenesis. Yale J Biol Med. 1992;65:793–804; discussion 827-829. [PMC free article] [PubMed] [Google Scholar]
- 12.Helpap B, Köllermann J. Immunohistochemical analysis of the proliferative activity of neuroendocrine tumors from various organs. Are there indications for a neuroendocrine tumor-carcinoma sequence. Virchows Arch. 2001;438:86–91. doi: 10.1007/s004280000337. [DOI] [PubMed] [Google Scholar]
- 13.Qvigstad G, Qvigstad T, Westre B, Sandvik AK, Brenna E, Waldum HL. Neuroendocrine differentiation in gastric adenocarcinomas associated with severe hypergastrinemia and/or pernicious anemia. APMIS. 2002;110:132–139. doi: 10.1034/j.1600-0463.2002.100302.x. [DOI] [PubMed] [Google Scholar]
- 14.Chejfec G, Kovarick P, Graham G, Eichorst M, Gould VE. Neuroendocrine carcinoma of the stomach with extensive somatostatin immunoreactivity. Ultrastruct Pathol. 1992;16:537–545. doi: 10.3109/01913129209061545. [DOI] [PubMed] [Google Scholar]
- 15.Solcia E, Capella C, Vassallo G, Buffa R. Endocrine cells of the gastric mucosa. Int Rev Cytol. 1975;42:223–286. doi: 10.1016/s0074-7696(08)60982-1. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Saito S. Plasma somatostatin in normal subjects and in various diseases: increased levels in somatostatin-producing tumors. Horm Metab Res. 1982;14:71–76. doi: 10.1055/s-2007-1018927. [DOI] [PubMed] [Google Scholar]
- 17.Bonar SF, Sweeney EC. The prevalence, prognostic significance and hormonal content of endocrine cells in gastric cancer. Histopathology. 1986;10:53–63. doi: 10.1111/j.1365-2559.1986.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 18.Bordi C, Ravazzola M. Endocrine cells in the intestinal metaplasia of gastric mucosa. Am J Pathol. 1979;96:391–398. [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelsohn G, de la Monte S, Dunn JL, Yardley JH. Gastric carcinoid tumors, endocrine cell hyperplasia, and associated intestinal metaplasia. Histologic, histochemical, and immunohistochemical findings. Cancer. 1987;60:1022–1031. doi: 10.1002/1097-0142(19870901)60:5<1022::aid-cncr2820600517>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Waldum HL, Brenna E, Sandvik AK. Relationship of ECL cells and gastric neoplasia. Yale J Biol Med. 1998;71:325–335. [PMC free article] [PubMed] [Google Scholar]
- 21.Solcia E, Rindi G, Larosa S, Capella C. Morphological, molecular, and prognostic aspects of gastric endocrine tumors. Microsc Res Tech. 2000;48:339–348. doi: 10.1002/(SICI)1097-0029(20000315)48:6<339::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Makhlouf HR, Burke AP, Sobin LH. Carcinoid tumors of the ampulla of Vater: a comparison with duodenal carcinoid tumors. Cancer. 1999;85:1241–1249. doi: 10.1002/(sici)1097-0142(19990315)85:6<1241::aid-cncr5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaizaki Y, Fujii T, Kawai T, Saito K, Kurihara K, Fukayama M. Gastric neuroendocrine carcinoma associated with chronic atrophic gastritis type A. J Gastroenterol. 1997;32:643–649. doi: 10.1007/BF02934114. [DOI] [PubMed] [Google Scholar]
- 24.Solcia E, Rindi G, Paolotti D, Luinetti O, Klersy C, Zangrandi A, La Rosa S, Capella C. Natural history, clinicopathologic classification and prognosis of gastric ECL cell tumors. Yale J Biol Med. 1998;71:285–290. [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuji E, Yamaguchi T, Taniguchi H, Sakakura C, Kishimoto M, Urata Y, Tsuchihashi Y, Ashihara T, Takahashi T, Yamagishi H. Malignant endocrine carcinoma of the stomach. Hepatogastroenterology. 2000;47:601–604. [PubMed] [Google Scholar]
- 26.Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Fukui H, Takada M, Chiba T, Kashiwagi R, Sakane M, Tabata F, Kuroda Y, Ueda Y, Kawamata H, Imura J, et al. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours. Gut. 2001;48:853–856. doi: 10.1136/gut.48.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]


