Abstract
Inflammatory bowel disease is an important cause of gastrointestinal pathology in children and adolescents. The incidence of pediatric inflammatory bowel disease is increasing; therefore, it is important for the clinician to be aware of the presentation of this disease in the pediatric population. Laboratory tests, radiology studies, and endoscopic procedures are helpful in diagnosing inflammatory bowel disease and differentiating between Crohn’s disease and ulcerative colitis. Once diagnosed, the goal of medical management is to induce remission of disease while minimizing the side effects of the medication. Specific attention needs to be paid to achieving normal growth in this susceptible population. Surgical management is usually indicated for failure of medical management, complication, or malignancy. Algorithms for diagnostic evaluation and treatment of pediatric inflammatory bowel disease are presented. The specific psychosocial issues facing these patients are also discussed in this review as are the future goals of research in the complex problem of pediatric inflammatory bowel disease.
Keywords: Pediatric, Inflammatory bowel, Ulcerative colitis, Crohn’s disease
INCIDENCE AND ETIOLOGY
The incidence of inflammatory bowel disease (IBD), which is most commonly divided into ulcerative colitis (UC) and Crohn’s disease (CD), appears to be more common in countries in the northern hemisphere and in industrialized nations. The true worldwide incidence of pediatric IBD is unknown[1]. The annual incidence rates of 0.2-8.5 per 100 000 for CD and 0.5-4.3 per 100 000 for UC have been reported[2,3]. Some reports indicate that annual incidence of CD is continuing to rise and is approaching or surpassing that of UC[2,4,5]. The overall incidence of IBD increased significantly between 1960 and 1990[2,3]. In patients with IBD, 25%-30% of patients with CD and 20% of patients with UC are younger than 20 years old[4]. Although the incidence in children is lower than that in adults, it is also increasing with most recent estimates reported as 47 per 100 000.
The etiology of IBD is unclear. There are several theories but most agree that IBD is multifactorial. It is believed that a complex interaction of environmental, genetic, and immune factors lead to the development of IBD. The role of each of these components is believed to be different in UC and CD[6]. A combined immunogenetic model that encompasses all three is most likely[7]. Environmental factors including breastfeeding, smoking, diet, occupation, education, climate, and stress have been implicated. The most established association is smoking. Smokers are at a decreased risk for developing UC but at an increased risk for developing CD. The fact that it may be protective in UC has led to investigations of treatment regimens that include transdermal nicotine patches without conclusive results[5,6].
There is a genetic association, but not a clearly defined inheritance pattern. Frequency of IBD in first-degree relatives may be as high as 40%[6]. There is a higher rate of concordance for monozygotic twins with CD > UC compared with dizygotic twins. In UC, there is an increased frequency of HLA-A11 and HLA-A7 and a decreased frequency of HLA-A9 in CD. In Japanese patients with UC there is an increased frequency of HLA-DR2, but this is not seen in other populations[6]. Mutations in the NOD2/CARD15 gene on chromosome 16 have been associated with Crohn’s Disease but not UC[7]. This gene is only expressed in monocytes and plays a role in activating nuclear factor-kappa B (NF-κB) which is a critical mediator of inflammatory responses and leads to the production of proinflammatory cytokines[8]. Patients with NOD2/CARD15 genotype have demonstrated fibrostenosing disease, usually in the ileum, and earlier disease onset[9]. It may be negatively associated with UC-like disease or the perforating type of CD[8]. Another gene, IBD5, located within the 5q31 cytokine cluster, has been associated with perianal disease in Crohn’s patients.
The immune system is also involved, but the inciting event is unclear. An infectious etiology has been considered and essentially all patients with IBD have increased titers against bacteria, viruses, and fungi. Of the many infectious agents that have been considered, including the virus that causes measles, E.coli, and M. paratuberculosis, no association of these has been proven[5,6]. Theories that focus on the involvement of the immune system include persistent bacterial infection, defective mucosal barrier, or an imbalance in the regulation of the immune response[7]. One theory of CD is described as an inappropriate immune response triggered by genetic or environmental factors[8]. There is some evidence that interleukin-15 (IL-15) and the IL-15 receptor (IL-15R) system is involved in inflammatory bowel disease. IL-15 is a cytokine with wide cellular distribution in monocytes, epithelium, endothelium, fibroblasts, and muscle cells. It plays a role in T cell proliferation and activation, the production of inflammatory cytokines in natural killer (NK) cells, and the migration of both. It also plays a role in the proliferation and differentiation of B cells. It is implicated in the pathogenesis and maintenance of several chronic inflammatory diseases with immune activation[10].
CLINICAL PRESENTATION
As with adults, the clinical presentation of IBD depends on the site and extent of mucosal inflammation[5,11]. At the time of presentation, 44%-49% of children with UC have rectosigmoid disease, 36%-41% have left-sided disease, and 14%-37% have pancolitis[2]. In terms of severity of illness, 50%-60% present with mild disease, 30% with moderate disease, and 19% with severe disease[4]. In children with CD, 50%-70% of pediatric patients present with disease involving the terminal ileum with more than half also having involvement of the colon, usually the ascending portion. Ten to 20% of children with CD have isolated colonic disease and 10%-15% have diffuse small bowel disease[2]. Gastroduodenal inflammation may be found in 30%-40% of patients with CD, but isolated gastroduodenal disease is rare, less than 5%[4]. The most common presenting symptoms in UC include weight loss, rectal bleeding, diarrhea, and abdominal pain. (Table 1) CD may have an insidious onset with abdominal pain and weight loss which may contribute to a delay in diagnosis. If chronic diarrhea or rectal bleeding are present, diagnosis may be more expeditious. Non-specific symptoms of anorexia, fatigue, delayed sexual maturation, and decreased linear growth may be present but not immediately noticed and if noticed may not prompt a workup for IBD because of their nonspecific nature.
Table 1.
| Symptom | CD (%) | UC (%) |
| Abdominal pain | 62-95 | 33-76 |
| Diarrhea | 52-78 | 67-93 |
| Weight loss | 43-92 | 22-55 |
| Hematochezia | 14-60 | 52-97 |
| Delayed growth | 30-33 | 6 |
| Fever | 11-48 | 4-34 |
| Perianal disease | 25 | 0 |
| Extraintestinal manifestations | 15-25 | 2-16 |
Extraintestinal manifestations are common in both adults and children with IBD, affecting 25%-35% of patients (Table 2)[4]. However, in children, these may precede the onset of gastrointestinal symptoms by years, whereas in adults these symptoms are usually concurrent with exacerbation of disease[4,5]. Arthritis is the most common extraintestinal manifestation occurring in 7%-25% of children with IBD[2,4]. Nephrolithiasis occurs in 5% of children with IBD, erythema nodosum occurs in 3%, and pyoderma granulosum in < 1%. Other extraintestinal manifestations include ocular complications, hepatobiliary complications such as primary sclerosing cholangitis and choledocholithiasis, and pancreatitis[4]. The differential diagnosis for IBD includes common causes of abdominal pain which includes constipation, gastroesophageal reflux, and peptic ulcer disease. In a population of children who are increasingly overweight, cholelithiasis must also be considered. Gynecologic causes of abdominal pain, such as ovarian cysts and torsion, must be considered in females. Infectious causes of abdominal pain including urinary tract infection, mesenteric adenitis, gastroenteritis, and appendicitis should also be included in the differential. Rectal bleeding can be seen with intussusception, infectious colitis, Meckel’s diverticulum, colonic polyposis syndromes, hemorrhoids, and anal fissures. Diarrhea may be seen with gastroenteritis or infectious colitis. It is important to remember, however, that when these symptoms occur together or in the setting of weight loss, anorexia, growth failure, or other extraintestinal manifestations, the diagnosis of IBD must be considered and ruled out.
Table 2.
| System | Manifestation |
| Generalized | Fever Weight loss Malaise Anorexia Fatigue Nausea/vomiting |
| Ocular | Uveitis Episcleritis Iritis Conjunctivitis |
| Oral | Cheilitis Stomatitis Apthae |
| Pulmonary | Pulmonary vasculitis Fibrosing alveolitis |
| Vascular | Vasculitis Thrombosis |
| Hepatobiliary | Primary Sclerosing Cholangitis Hepatitis Cholelithiasis Jaundice |
| Pancreatic | Pancreatitis |
| Gastrointestinal | Abdominal pain Nausea/vomiting Diarrhea Hematochezia |
| Renal/Urinary | Nephrolithiasis Obstructive hydronephrosis Enterovesical fistula UTI Amyloidosis |
| Hematologic | Iron deficiency anemia Anemia of chronic disease Thrombocytosis Vit B12 deficiency Autoimmune hemolytic anemia |
| Endocrine | Decreased growth velocity Delayed sexual maturation |
| Integumentary | Erythema nodosum Pyoderma gangrenosum Perianal disease |
| Musculoskeletal | Osteopenia and osteoporosis Arthritis/Arthralgias Ankylosing spondylitis |
DIAGNOSTIC WORK-UP
History and physical exam are the initial steps in evaluating the patient with complaints suggestive of IBD (Figure 1). Important historical information includes recent infections, antibiotics, and travel history. Family history with specific attention to IBD diagnosis or related symptoms must be obtained. Without a thorough growth history to determine height velocity, growth failure might not otherwise be detected. Physical exam would include height and weight and calculation of the percentiles for each. Weight loss is present in 85% of children with CD and 65% of children with UC[2,4]. The abdominal exam may be normal or may demonstrate nonspecific tenderness. A mass in the right lower quadrant may be indicative of CD. A rectal exam is important to check for fecal blood, to evaluate for fissures or hemorrhoids, and to evaluate the surrounding area for fistulas or other perianal disease frequently associated with CD.
Figure 1.
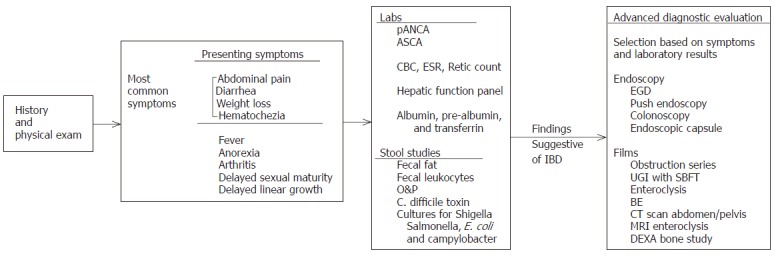
Diagnostic Evaluation for IBD in Pediatric Patients.
Laboratory evaluation is important to assess several aspects of patient status. First, serum markers for IBD should be checked[14]. Anti-Saccharomyces cerevisiae antibody (ASCA) and perinuclear antineutrophil cytoplasmic antibody (pANCA) assays are highly disease-specific for CD and UC, respectively, and can assist clinicians in diagnosing and categorizing patients with IBD[11]. ASCA is associated with CD and if IgG ASCA and IgA ASCA are both detected, this is 100% specific for CD[8,11]. Higher ASCA levels are independently associated with onset at an earlier age and with fibrostenosing disease[8]. Positive titers for pANCAs are associated with UC in 60%-80% of cases[11]. Elevated titers can also be seen in left-sided or pan-colitis in children with CD[8,11]. When both tests are considered together, ASCA and pANCA have a specificity of 95% and positive predictive value of 96% for IBD[11]. A complete blood count (CBC), retic count, and sed rate should be checked to look for anemia and signs of acute or chronic inflammation. Albumin, pre-albumin, and transferrin should be checked as an assessment of nutritional status. Finally, stool studies should be performed to evaluate for evidence of malabsorption or infectious etiology. Cultures should evaluate for C. difficile, Salmonella, Shigella, E.coli (O157:H7), and Campylobacter.
Diagnostic imaging can provide valuable information in a patient whose history or physical exam is concerning for IBD. Plain films are important in the acutely ill patient to look for complications such as obstruction, toxic megacolon, or perforation. In the non-acutely ill patient, contrast studies may be more beneficial in making a diagnosis. A barium enema (BE) can identify strictures and abnormal mucosa. It is useful to evaluate the colon and often the terminal ileum if the contrast refluxes back through the ileocecal valve. An upper gastrointestinal (UGI) study with a small bowel follow through (SBFT) can evaluate the proximal gastrointestinal tract including small bowel which in the past has been difficult to assess. This study may show mucosal lesions, strictures, or fistulas. If a bowel obstruction is present, the transition point may be identified. Standard enteroclysis is considered superior to SBFT but is more difficult to perform and may not be tolerated by some patients. A computed tomography (CT) scan is helpful in the diagnosis and management of patients presenting with acute onset of symptoms or an exacerbation of their disease. CT can assess both intestinal abnormality and extraluminal abnormalities such as abscess formation. Ulcerative colitis is associated with mural thickening < 1.5 cm, no thickening of the small bowel, increased perirectal and presacral fat, target appearance of the rectum, and lymphadenopathy. Findings consistent with CD include mural thickening > 2 cm, involvement of the small bowel, mesenteric fat-stranding, perianal disease, abscesses, fistulas, and adenopathy[15]. MR enteroclysis is a new technique being evaluated as a method to evaluate the small bowel for CD, and initial reports are promising that it is very sensitive. Thickening of the bowel wall, skipped lesions, stenotic areas, and fistulas have all been diagnosed with more sensitivity when compared to conventional SBFT. It has the added benefit of minimal radiation exposure due to the need for fluoroscopy to place a nasoduodenal tube prior to the study[16].
Finally, bone density studies should be considered either to establish a baseline or, if corticosteroid treatment is prolonged, to evaluate bone mineralization which can be altered in these children. It is important to interpret this study using bone age or height age as opposed to chronological age because using chronological age will lead to an overestimation of the extent of bone disease. Endoscopic evaluation is an essential tool in the workup of IBD. UGI endoscopy looks at the esophagus, stomach, and duodenum. It is useful in identifying other possible sources of fecal blood such as gastritis, duodenitis, or peptic ulcer disease. Using a push endoscopy technique, it may be possible extend visualization to 60-120 cm beyond the ligament of Treitz to evaluate for small bowel CD[17]. Biopsies can be taken to establish or confirm the diagnosis. Colonoscopy is a standard part of the workup for IBD. The advantage of this procedure over contrast studies is the ability to obtain biopsies which can distinguish between UC and CD involving the colon.
Biopsies taken during endoscopic evaluation help distinguish between CD and UC. There are several pathologic features which help distinguish between the two types of inflammatory bowel disease when the colon is the primary site of involvement. UC always involves the rectum and can extend proximally, whereas CD may involve any portion of the colon including isolated right colon involvement or multiple segments with skip areas in between. The microscopic features of UC include diffuse inflammation confined to the mucosa or submucosa. The microscopic features of CD include transmural inflammation with fissuring ulcers, fistulas, granulomas, and vasculitis[18]. The endoscopic capsule has been shown in several studies to have a higher yield than either push endoscopy or enteroclysis when used to evaluate patients with small bowel CD. One such study evaluated two groups of patients, those with the previous diagnosis of CD and those with no prior diagnosis. In both groups, the endoscopy capsule was more sensitive for small bowel pathology than either of the other two techniques[17]. Problems with this technique include difficulty in swallowing the capsule and failure to pass the capsule.
MEDICAL MANAGEMENT
The main goal of medical management for IBD is to achieve clinical and histiologic remission by suppression of inflammation with the least amount side effects from the medications. (Table 3, Table 4) Good control of the inflammatory process will decrease the likelihood of needing surgical intervention. In addition, successful treatment should provide adequate nutritional support to promote normal growth and should attempt to minimize alteration in the physical and social functioning of the child in order to maintain quality of life[1]. For patients with CD, treatment is based on severity of symptoms, which does not necessarily correlate with mucosal healing. CD is classified as mild to moderate, moderate to severe, and severe to fulminant. Mild to moderate disease is treated initially with either 5-aminosalicylic agents (sulfasalazine or mesalamine) or antibiotic therapy with metronidazole and ciprofloxacin[8,12,19]. Both regimens have been reasonably successful in inducing remission in mild disease in adults and children.
Table 3.
| Class | Generic (Trade) | Use | Side Effects |
| Aminosalicylates | Mesalamine (Asacol, Pentasa) Sulfasalazine (Azulfadine) Olsalazine (Dipentum) Balsalazide (Colazal) | May be used to induce remission in mild CD or UC May be used to maintain remission in moderate CD or UC | Headache Nausea Anorexia Leukopenia Diarrhea |
| Corticosteroids | Prednisone Budesonide | Used for induction of remission in CD and UC, not effective in maintenance of remission | Increased risk of infection Hypertension Weight gain Acne Hirsuitism |
| Immunomodulators | Azathioprine (Imuran, Azasan) 6-mercaptopurine (Purinethol) | Used for maintenance of remission in moderate to severe CD or UC | Nausea/vomiting Diarrhea Pancreatitis Hepatitis Myelosuppression |
| Methotrexate (Rheumatrex, Trexall) | May be used to maintain remission in moderate to severe CD or UC that does not respond to azathioprine or 6-mercaptopurine | Myelosuppression Oral ulcers Hepatitis | |
| Cyclosporine (Neoral, Sandimmune) | Has been used to induce and maintain remission in refractory cases of UC | Renal toxicity Hypertension Gingival hyperplasia Hirsuitism | |
| Biologic Agents | Infliximab (Remicade) | May be used for refractory CD or steroid-dependent patients to induce and maintain remission | Infusion reaction (chest pain, Hyper- or hypotension, And shortness of breath) Nausea Fever/Chills Hives Fatigue |
Table 4.
| CD | UC | |
| Mild | Aminosalicylates Antibiotics | Aminosalicylates |
| Moderate | Corticosteroids→Aminosalicylates or AZA or 6-MP or Methotrexate | Corticosteroids→Aminosalicylates or AZA or 6-MP or Methotrexate |
| Severe | Corticosteroids→AZA or 6-MP or Methotrexate or Infliximab | Corticosteroids→AZA or 6-MP or Methotrexate or Cyclosporine |
| All cases | Nutritional support Monitor growth closely | Nutritional support Monitor growth closely |
For patients with moderate to severe disease, corticosteroids are effective in inducing remission and have been considered the standard for treatment[4,8,12,19]. Prednisone or methylprednisolone remain the first-line therapy in moderately to severely ill children with CD with up to a 90% rate of improvement. The dose is 1-2 mg/kg per day prednisone equivalent (max 4060) for four to six weeks and then taper[4,8,19]. Although corticosteroids act rapidly to alleviate symptoms, they do not heal mucosal lesions and are of no benefit for maintenance therapy[5]. Pitfalls to this treatment include corticosteroid-dependence (1/3-1/2 of patients) and resistance (1/5 of patients) and therefore, weaning as quickly as possible is important to avoid decreased linear growth and minimize potential bone loss[8,19]. Steroids should be avoided in children with profound growth retardation, especially in those who are already Tanner 2 or 3 and who have a limited window of growth remaining[4,8,19]. They should also be avoided in those patients with severe bone loss.
In patients who have achieved remission with corticosteroids, immunomodulators, including azathioprine (AZA) and 6-mercaptopurine (6-MP), a metabolite of AZA, are helpful in maintaining remission[4,8,12,19]. Most pediatric gastroenterologists favor immunomodulators in children with CD as they are better at maintaining remission[8]. They may be initiated at diagnosis, post-op, after a severe attack. They are also used to maintain remission when patients have become steroid-dependent, but they are not effective in inducing remission because of their long onset of action. They have been shown to reduce steroid dependence and resistance from 50% to 20% or less[8]. Adverse effects include hepatotoxicity, myelosuppression, pancreatitis, N/V, and allergic reactions, and these have been associated with an inherited inability to metabolize them. Patients heterozygous or homozygous for an alteration in the gene thiopurine methyltransferase (TPMT) have deficient TPMT activity and are at risk for marked elevations of the active 6-thioguanine nucleotide metabolites and resultant severe leucopenia. Those patients with high TPMT activity can metabolize the drugs to inactive, but potentially hepatotoxic, 6-methylmercaptopurine metabolites. A screening test is available to determine TPMT genotype and activity as well as thiopurine metabolism[8].
Methotrexate (MTX) is another immunomodulator used as an alternative therapy after treatment with the thiopurines (AZA and 6-MP) has proven suboptimal due to inability to maintain remission or patient intolerance to the medication[8]. Methotrexate is also used in patients who do not respond to corticosteroids for induction of remission as it has been shown to induce and maintain remission[4,8,12,19]. Side effects include nausea, vomiting, diarrhea, stomatitis, rash, arthralgias, leucopenia, and hepatotoxicity. The latter is the most treatment- limiting and requires monitoring of serum aminotransferases and biopsy in patients with persistent elevations. There are also concerns of teratogenicity in females of child-bearing age[8].
Finally, biologic therapy in the form of infliximab, a chimeric monoclonal antibody directed against tumor necrosis factor-α, has been used to treat moderate to severe disease in patients who do not respond to 5-ASA, antibiotics, steroids, or immunomodulators. It can be used as an alternative to prednisone to induce remission or as a first-line therapy in CD with severe, perianal, fistulizing disease or intolerance to corticosteroids[8,19]. Most of the literature is on adults but has been used in children with good results. Approximately 30% of patients do not respond to infliximab[5]. It is possible to develop antibodies to infliximab and thus decrease responsiveness. Concomitant therapy with immunomodulators may help maintain responsiveness[8]. It is generally well-tolerated by adults and children[4,8,19]. Side effects include flushing, rash, dyspnea, and headache and are typically seen at the time of infusion. Other side effects include delayed hypersensitivity reactions, including joint pain/stiffness, muscle pain, fever, general malaise, a lupus-like syndrome, and opportunistic infections, including tuberculosis, histoplasmosis, and sepsis. Leukopenia has been seen as well as lymphoproliferative disorders[5].
Patients with UC may be classified as having mild, moderate, or severe disease. In UC, mucosal healing correlates well with clinical remission[4,19]. The accepted treatment of mild to moderate UC is the 5-aminosalicylic acid agents, sulfasalazine and mesalamine. It is important to treat with appropriate dosing as underdosing is the most common cause of treatment failure. In UC, it is also important to remember that topical treatment with enemas is very effective, often more effective than oral therapy[19]. In patients with moderate to severe disease who are refractory to the aminosalicylates, corticosteroid treatment can induce remission but has no role in maintaining remission[4,12,18]. The above side effects are still a concern, and the child should be tapered from corticosteroid therapy as soon as possible[4,19]. In patients with severe UC who fail to respond to corticosteroids, cyclosporine is an option for therapy[12,19]. This drug has a serious side effect profile, including renal insufficiency, hypertension, opportunistic infections, seizures, and electrolyte abnormalities[4,19]. Because of the toxicity of cyclosporine, the patient’s history should be considered to assess whether surgery is a better option.
The immunomodulators AZA, 6-MP, and MTX have been studied in refractory cases of UC. They are not used to induce remission, but to maintain it[4,12,19]. Their side effects have been discussed above, and monitoring is required. As with the aminosalicylates, underdosing is frequently the cause of failure of therapy.
Aggressive nutritional support can improve growth parameters in pediatric patients with IBD[8]. Special diets have been shown to help alleviate symptoms in CD, but not in UC. However, all children with IBD need appropriate nutritional assessment and support to minimize growth retardation due to malnutrition. During acute exacerbations of IBD, parenteral nutrition may be necessary but has the associated disadvantages of being more invasive and more expensive[3]. In addition, patients receiving parenteral nutrition are at risk of complications from central venous access including infection and bleeding. Enteral nutrition is always preferable if tolerated. Formulas with omega-3 fatty acids may be beneficial[3]. Special attention should be paid to caloric intake and vitamin and mineral supplementation. During active disease, increased metabolic demands may make it difficult to compensate for the added caloric requirements with oral intake alone. IBD patients are also at risk of vitamin and mineral deficiencies, including iron, calcium, vitamin D, vitamin B12, and folic acid. Calcium supplements seem to be the best way to prevent bone disease.
SURGICAL MANAGEMENT
Surgical management of CD is typically required for complications of the disease. Indications include perforation/abscess, obstruction, stricture, fistula, toxic megacolon, or malignancy. Ultimately, greater than 50% of patients will require resection[20-22]. Surgical procedures are based on the location of the disease and the complication. The surgeon must consider the state of health of the patient. Obstruction is the most common cause for surgery in patients with CD. Adhesions are divided carefully, and strictures are either resected or managed with a strictureplasty[21-23]. Given the high rate of recurrence and reoperation, bowel conservation is extremely important to avoid the complications associated with short bowel syndrome and long-term dependence on parenteral nutrition. Strictures less than 10 cm in length can be addressed with a Heineke-Mikulicz strictureplasty. This is performed by incising the bowel lengthwise through the strictured area and closing the defect transversely. Longer strictures, 10-25 cm, may still be managed with strictureplasty using a Finney technique. Again, the bowel is opened in the longitudinal axis through the strictured area and a side-to-side anastamosis is performed. Strictures longer than 25 cm usually require resection. In pediatric patients with severe symptoms despite medical therapy or those with severe growth retardation, surgical resection of the terminal ileum and cecum may be beneficial in providing a disease-free interval of 2-3 years. Primary anastomosis is usually safe in the above mentioned circumstances. However, more severe complications, including perforation, abscess, or toxic megacolon, may require a resection and ostomy formation. Complications after surgery for CD include wound infection, anastomotic leak, anastomotic stricture, fistula, recurrence of disease, small bowel obstruction, and bleeding.
Crohn’s disease is characterized by a chronic relapsing course. Recurrence of symptomatic disease after surgery has been reported as 17% at 1 year, 38% at 3 years, and 60% at 5 years. The disease typically recurs at the site of resection[20]. If defined as the presence of endoscopic abnormality, 1 year recurrence has been reported as high as 75%-93% of patients[20]. Growth improved significantly in pediatric patients after surgery[21,24]. Although there is a high rate of recurrence, any disease-free interval with decreased corticosteroid requirements may allow normal growth and sexual development. Risks and benefits must be clearly defined when determining the need for surgery[24]. There is some evidence to indicate that post-op management of CD patients with 6-MP can decrease rate of recurrence (recurrence defined as endoscopically-evident), but there is no standard of therapy[20]. Infliximab has not been investigated in this setting.
Ulcerative colitis may require surgery for emergent or elective indications. The former includes bleeding, toxic megacolon, and perforation. Indications for non-emergent surgery include failure of medical therapy, severe growth retardation, and dysplasia or malignancy. The rate of colectomy is no higher in children than in adults, but children have a higher rate of progression of disease (65% for children vs 39% for adults)[4]. Unlike in CD, total proctocolectomy for UC is curative. The procedure used depends on the clinical status of the patient but usually involves a total colectomy with or without a protective ileostomy. Creation of a reservoir in the small bowel with a ileoanal anastomosis is the definitive procedure. Outcome parameters of importance include number of bowel movements per day, consistency of the stool, requirement of stool-regulating medicine, whether patients can discriminate between air and stool, emptying of J-pouch, ability to defer defecation, leakage of stool/soiling, and number of episodes of pouchitis[25]. Complications include wound infection, pouchitis, SBO, pelvic sepsis, bleeding, urinary complications, anastoamotic leak or stricture, soiling, mortality, and permanent stoma, which is reported as high as 30%[25].
Whether to perform proctocolectomies as a one-stage or two-stage operation remains controversial. Many studies show that a total colectomy with J-pouch ileoanal anastomosis can be performed safely as a single-stage operation with the right patient selection and careful attention to signs of complications[21,26,27]. The largest patient series is one of 150 patients with a IPAA without a protective ileostomy compared to 92 patients who had IPAA with a protective ileostomy[26]. Results were compared, and a significantly lower complication rate was found in the one-stage procedure. However, the exclusion criteria for the one-stage procedure placed the patients with the highest risk of complication in the two-stage procedure group[26].
TYPICAL COURSE OF DISEASE
Crohn’s disease is unpredictable and is characterized by recurrent exacerbation of symptoms. The spectrum of disease includes patients that remain in remission, those that have recurrent periods of disease followed by remission, and those that have continuous active disease[5,8]. Serum markers, including ASCA and pANCA, may help stratify patients into subgroups, which help predict natural history[9,28]. ASCA IgG and IgA is found in 100% of CD patients with fibrostenosing disease, whereas pANCA was present in 86% of CD patients with primary colonic involvement. At the time of diagnosis with CD, 40.9% of patients had ileocolonic disease, 28.6% had disease limited to the small bowel and 30.4% had disease involving only the colon and perirectal area[5]. Patients with small bowel disease are more likely to have an obstructive pattern of disease characterized by fibrostenosis. Patients with colonic disease are more likely to have symptoms associated with bleeding and inflammation[5,8]. In general, surgery is for complications of the disease, and three out of four patients will ultimately require surgery. Complications of CD include abscesses, fistulas, strictures, and obstruction from adhesions, and pediatric patients have an even higher risk of developing these complications[5]. They are addressed surgically, with the goal of minimizing loss of length of bowel, since it is not a curative procedure. Removal of the diseased segments is followed by recurrence near the anastomosis. Endoscopic evidence of recurrence is found in up to 93% of patients at 1 year. However, a second operation is only required in 25%-38% of patients at 5 years and 50%-70% of patients at 15 years. Of those requiring a second operation, 37% will require a third one[5].
There is an increased risk of cancer in CD patients, including small bowel adenocarcinoma and colon cancer. The risk is especially high in those patients diagnosed prior to the age of 25 and in patients with a history of pan-colitis. CD patients with small bowel disease are 50-100 times more likely to develop small bowel adenocarcinoma[4]. At the time of diagnosis, most patients with UC have disease distal to the splenic flexure[2,5]. In general, clinical severity at the time of presentation correlates with extent of colonic involvement[2,5,11]. Most patients have a chronic, intermittent course of disease recurrence. Approximately 15%-40% of patients will ultimately require a colectomy, and this correlates with the extent of involvement. Indications for surgery include failure of medical therapy, growth retardation, and complications such as persistent hemorrhage, perforation, toxic megacolon, and the presence of dysplasia or carcinoma[4,5]. Toxic megacolon occurs in 5% of adult patients but is rare in children. It is a surgical emergency associated with risk of perforation, sepsis, electrolyte abnormalities, and hemorrhage. Frank perforation is more common in UC than in CD. It is usually associated with toxic megacolon and serious disease.
The association between UC and colon cancer is well documented[5]. Risk appears to be related to extent of colonic disease, age at diagnosis, and length of time since diagnosis with greater than 10 years being the threshold for identifying an increased risk of cancer[5]. The overall annual incidence of colon cancer in patients with UC of longer than 10 years duration is approximately 1%[5]. Children who develop UC before the age of 14 have a colon cancer incidence rate of 5% at 20 years and 40% at 35 years[4]. It is estimated that the risk of dying from colon cancer is 8% after 10 years from diagnosis[4]. The risk of colon cancer in patients with Crohn’s colitis is 4-20 times that of the general population. Overall, patients with UC do not have a significantly increased mortality rate when compared to the general population. However, those patients with CD do have an increased mortality. Their survival rate is 93.7% of that in the general population at 15 years[5].
PROBLEMS SPECIFIC TO PEDIATRIC PATIENTS
Growth retardation was mentioned briefly earlier in this review but warrants further discussion. It may be present at the time of diagnosis or occur as a result of treatment[3]. Inadequate nutrition, chronic corticosteroid use, and decreased physical activity all play a role[3]. It is important obtain a complete growth history and follow growth parameters closely as growth failure may affect a patient’s treatment plan. Height velocity is the most important parameter to diagnose impaired growth, with more than 85% of patients having impaired growth at the time of diagnosis with IBD[4]. Nutritional insufficiency is due primarily to inadequate intake either from anorexia or food fear related to the association of food intake with pain[4]. Undernutrition delays skeletal and sexual maturation, with delays seen in epiphyseal fusion of long bones and in progression through Tanner stages[4]. Corticosteroids may further complicate growth and maturity. Factors contributing to abnormal bone mineralization over-production of inflammatory cytokines with the resultant increase in bone resorption, malnutrition, sedentary life-style, and corticosteroid use. Corticosteroids inhibit osteoblasts, impair intestinal absorption of calcium, and antagonize growth hormone[3]. Diagnosis of bone disease can be made with radiologic studies. A dual energy X-ray absorptiometry (DEXA) study is particularly useful. However, results should be interpreted using bone age or height age, not chronological age which overestimates the extent of bone disease.
In addition to the multitude of medical problems faced by pediatric IBD patients, there are significant psychosocial stressors for these children. Emotional and behavioral issues are common and stem from the chronic nature of the disease. Lifestyle changes can occur unexpectedly due to disease exacerbation. Anxiety, depression, and antisocial and dependent behavior are all common. Anxiety and depression may be related to concerns about differences from other children and progression of their illness such as relapse of the disease or complications of disease requiring surgery or stoma formation[29]. Difficulty interacting with peers may result from school absenteeism and may be compounded by low self esteem due to delayed growth and development[2,30]. Finally, guilt may be a large psychosocial stressor for the child. The financial strain of a chronic illness, changes in parental work schedules, repeated physician visits, and sibling issues are problems for which a child with IBD may feel responsible. It is also important to remember the educational needs of children with IBD. Due to illness, attendance in school may suffer. If not anticipated and addressed, this can cause the child to fall behind in school. All of the above mentioned problems can be exacerbated if the educational needs of the child with a chronic illness are overlooked[3,29,30].
Assessment of a child’s adaptation to his or her disease is a component of long-term care of children with IBD. Several assessment tools exist with most models focused on evaluating social support, coping skills, effects on activities of daily life, and body image[2,3,30,31]. These broad categories are used to assess quality of life and have been shown to be affected by age, education, and gender[30,31]. Finally, the issue of the transition of care is inevitable for children with IBD. After dealing with a chronic illness for, in some cases, years, medical care is transferred to a different provider. The child has built a relationship with the pediatric gastroenterologist and they must change physicians, perhaps at a time when many other changes are taking place. Preparing adolescents with IBD for this transition takes time and planning. This transition can be facilitated by the pediatric gastroenterologist by gradually increasing the patient’s participation in, and responsibility for, his or her own care. Older adolescents should know their medications and be able to recognize signs of exacerbation of their disease. In addition, they should know the warning signs of an emergency and be able to take appropriate steps to seek immediate treatment. There are several recommendations for the pediatric gastroenterologist to help make this a smooth transition of care. These include: (1) preparing a detailed, up-to-date medical summary describing the course of the disease, therapy, allergies, etc., (2) including copies of relevant test results, and (3) identifying a skilled gastroenterologist who is aware of the special needs of the young adult patient who was diagnosed with IBD as a child[2]. The goal is for everyone involved to view this as a transition of care rather than a transfer of care. Maintaining open communication between the patient and the two physicians is critical for this to be successful.
Future considerations
The North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) has identified areas of interest for future research. These include the role of enteric microbial flora; the study of dietary, nutritional, and environmental factors; the evaluation of new therapies and their effects in early onset disease; and the development of a North American Pediatric IBD database[3]. The Working Group of the Second World Congress of Pediatric Gastroenterology, which includes NASPGHAN, has set goals of future research to include establishing the correlation between genotype and phenotype, the determining whether mucosal response in IBD differs between children and adults, understanding the role of previous exposure to infections, and improving the dissemination of knowledge regarding pediatric IBD[1].
Footnotes
S- Editor Pan BR E- Editor Bai SH
References
- 1.Murch SH, Baldassano R, Buller H, Chin S, Griffiths AM, Hildebrand H, Jasinsky C, Kong T, Moore D, Orsi M. Inflammatory bowel disease: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 Suppl 2:S647–S654. doi: 10.1097/00005176-200406002-00011. [DOI] [PubMed] [Google Scholar]
- 2.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am. 2003;32:967–995, viii. doi: 10.1016/s0889-8553(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004;126:1550–1560. doi: 10.1053/j.gastro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. 1999;28:445–458. doi: 10.1016/s0889-8553(05)70064-9. [DOI] [PubMed] [Google Scholar]
- 5.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281, vii. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 6.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 7.Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 8.Hyams JS, Markowitz JF. Can we alter the natural history of Crohn disease in children. J Pediatr Gastroenterol Nutr. 2005;40:262–272. doi: 10.1097/01.mpg.0000154660.62359.fe. [DOI] [PubMed] [Google Scholar]
- 9.Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, Vasiliauskas EA, Kam LY, Rojany M, Papadakis KA, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679–688. doi: 10.1053/gast.2002.35393. [DOI] [PubMed] [Google Scholar]
- 10.Nishiwaki T, Ina K, Goto H, Watanabe O, Tsuzuki T, Furuta R, Ando T, Hibi K, Kusugami K. Possible involvement of the interleukin-15 and interleukin-15 receptor system in a heightened state of lamina propria B cell activation and differentiation in patients with inflammatory bowel disease. J Gastroenterol. 2005;40:128–136. doi: 10.1007/s00535-004-1510-y. [DOI] [PubMed] [Google Scholar]
- 11.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 12.Fish D, Kugathasan S. Inflammatory bowel disease. Adolesc Med Clin. 2004;15:67–90, ix. doi: 10.1016/j.admecli.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Raj V, Lichtenstein DR. Hepatobiliary manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:491–513. doi: 10.1016/s0889-8553(05)70067-4. [DOI] [PubMed] [Google Scholar]
- 14.Seidman EG. Recent advances in the diagnosis and treatment of pediatric inflammatory bowel disease. Curr Gastroenterol Rep. 2000;2:248–252. doi: 10.1007/s11894-000-0068-y. [DOI] [PubMed] [Google Scholar]
- 15.Scotiniotis I, Rubesin SE, Ginsberg GG. Imaging modalities in inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:391–421, ix. doi: 10.1016/s0889-8553(05)70062-5. [DOI] [PubMed] [Google Scholar]
- 16.Wiarda BM, Kuipers EJ, Houdijk LP, Tuynman HA. MR enteroclysis: imaging technique of choice in diagnosis of small bowel diseases. Dig Dis Sci. 2005;50:1036–1040. doi: 10.1007/s10620-005-2700-z. [DOI] [PubMed] [Google Scholar]
- 17.Chong AK, Taylor A, Miller A, Hennessy O, Connell W, Desmond P. Capsule endoscopy vs. push enteroscopy and enteroclysis in suspected small-bowel Crohn's disease. Gastrointest Endosc. 2005;61:255–261. doi: 10.1016/s0016-5107(04)02571-4. [DOI] [PubMed] [Google Scholar]
- 18.Antonioli DA. Pediatric inflammatory bowel disease. Pediatr Dev Pathol. 2005;8:2–19. doi: 10.1007/s10024-004-0511-4. [DOI] [PubMed] [Google Scholar]
- 19.Friedman S. General principles of medical therapy of inflammatory bowel disease. Gastroenterol Clin North Am. 2004;33:191–208, viii. doi: 10.1016/j.gtc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, Present DH. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723–729. doi: 10.1053/j.gastro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Becker JM. Surgical therapy for ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1999;28:371–390, viii-ix. doi: 10.1016/s0889-8553(05)70061-3. [DOI] [PubMed] [Google Scholar]
- 22.Schraut WH. The surgical management of Crohn's disease. Gastroenterol Clin North Am. 2002;31:255–263. doi: 10.1016/s0889-8553(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 23.Tekkis PP, Heriot AG, Smith O, Smith JJ, Windsor AC, Nicholls RJ. Long-term outcomes of restorative proctocolectomy for Crohn's disease and indeterminate colitis. Colorectal Dis. 2005;7:218–223. doi: 10.1111/j.1463-1318.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 24.Baldassano RN, Han PD, Jeshion WC, Berlin JA, Piccoli DA, Lautenbach E, Mick R, Lichtenstein GR. Pediatric Crohn's disease: risk factors for postoperative recurrence. Am J Gastroenterol. 2001;96:2169–2176. doi: 10.1111/j.1572-0241.2001.03876.x. [DOI] [PubMed] [Google Scholar]
- 25.Wewer V, Hesselfeldt P, Qvist N, Husby S, Paerregaard A. J-pouch ileoanal anastomosis in children and adolescents with ulcerative colitis: functional outcome, satisfaction and impact on social life. J Pediatr Gastroenterol Nutr. 2005;40:189–193. doi: 10.1097/00005176-200502000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Ikeuchi H, Nakano H, Uchino M, Nakamura M, Noda M, Yanagi H, Yamamura T. Safety of one-stage restorative proctocolectomy for ulcerative colitis. Dis Colon Rectum. 2005;48:1550–1555. doi: 10.1007/s10350-005-0083-z. [DOI] [PubMed] [Google Scholar]
- 27.Blumberg D, Beck DE. Surgery for ulcerative colitis. Gastroenterol Clin North Am. 2002;31:219–235. doi: 10.1016/s0889-8553(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 28.Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day AS, Whitten KE, Bohane TD. Childhood inflammatory bowel disease: parental concerns and expectations. World J Gastroenterol. 2005;11:1028–1031. doi: 10.3748/wjg.v11.i7.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Boer M, Grootenhuis M, Derkx B, Last B. Health-related quality of life and psychosocial functioning of adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:400–406. doi: 10.1097/01.mib.0000164024.10848.0a. [DOI] [PubMed] [Google Scholar]
- 31.Sainsbury A, Heatley RV. Review article: psychosocial factors in the quality of life of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;21:499–508. doi: 10.1111/j.1365-2036.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 32.Cuffari C, Darbari A. Inflammatory bowel disease in the pediatric and adolescent patient. Gastroenterol Clin North Am. 2002;31:275–291. doi: 10.1016/s0889-8553(01)00017-6. [DOI] [PubMed] [Google Scholar]
- 33.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Silva MA, Menezes J, Deslandres C, Seidman EG. Anti-inflammatory role of interleukin-15 in Crohn's disease. Inflamm Bowel Dis. 2005;11:219–230. doi: 10.1097/01.mib.0000160804.52072.6a. [DOI] [PubMed] [Google Scholar]
- 35.Zankel E, Rogler G, Andus T, Reng CM, Schölmerich J, Timmer A. Crohn's disease patient characteristics in a tertiary referral center: comparison with patients from a population-based cohort. Eur J Gastroenterol Hepatol. 2005;17:395–401. doi: 10.1097/00042737-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Levine A, Shamir R, Wine E, Weiss B, Karban A, Shaoul RR, Reif SS, Yakir B, Friedlander M, Kaniel Y, et al. TNF promoter polymorphisms and modulation of growth retardation and disease severity in pediatric Crohn's disease. Am J Gastroenterol. 2005;100:1598–1604. doi: 10.1111/j.1572-0241.2005.41737.x. [DOI] [PubMed] [Google Scholar]
- 37.Mackner LM, Crandall WV. Long-term psychosocial outcomes reported by children and adolescents with inflammatory bowel disease. Am J Gastroenterol. 2005;100:1386–1392. doi: 10.1111/j.1572-0241.2005.41428.x. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JD, Deren JJ, Lichtenstein GR. Cancer risk in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:459–477, x. doi: 10.1016/s0889-8553(05)70065-0. [DOI] [PubMed] [Google Scholar]
- 39.Han PD, Burke A, Baldassano RN, Rombeau JL, Lichtenstein GR. Nutrition and inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:423–443, ix. doi: 10.1016/s0889-8553(05)70063-7. [DOI] [PubMed] [Google Scholar]
- 40.Sharan R, Schoen RE. Cancer in inflammatory bowel disease. An evidence-based analysis and guide for physicians and patients. Gastroenterol Clin North Am. 2002;31:237–254. doi: 10.1016/s0889-8553(01)00014-0. [DOI] [PubMed] [Google Scholar]
- 41.Eidelwein AP, Cuffari C, Abadom V, Oliva-Hemker M. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis. 2005;11:213–218. doi: 10.1097/01.mib.0000160803.44449.a5. [DOI] [PubMed] [Google Scholar]
- 42.Stephens MC, Shepanski MA, Mamula P, Markowitz JE, Brown KA, Baldassano RN. Safety and steroid-sparing experience using infliximab for Crohn's disease at a pediatric inflammatory bowel disease center. Am J Gastroenterol. 2003;98:104–111. doi: 10.1111/j.1572-0241.2003.07161.x. [DOI] [PubMed] [Google Scholar]
- 43.Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, Winter HS. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98:833–838. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 44.Markowitz J, Grancher K, Kohn N, Daum F. Immunomodulatory therapy for pediatric inflammatory bowel disease: changing patterns of use, 1990-2000. Am J Gastroenterol. 2002;97:928–932. doi: 10.1111/j.1572-0241.2002.05611.x. [DOI] [PubMed] [Google Scholar]
- 45.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 46.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 47.Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 48.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 49.Chen QK, Yuan SZ, Wen ZF, Zhong YQ, Li CJ, Wu HS, Mai CR, Xie PY, Lu YM, Yu ZL. Characteristics and therapeutic efficacy of sulfasalazine in patients with mildly and moderately active ulcerative colitis. World J Gastroenterol. 2005;11:2462–2466. doi: 10.3748/wjg.v11.i16.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lomer MC, Grainger SL, Ede R, Catterall AP, Greenfield SM, Cowan RE, Vicary FR, Jenkins AP, Fidler H, Harvey RS, et al. Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn's disease. Eur J Gastroenterol Hepatol. 2005;17:377–384. doi: 10.1097/00042737-200503000-00019. [DOI] [PubMed] [Google Scholar]


