Abstract
AIM: To investigate both whether the risk of gastric cancer is associated with the Ile/Val single nucleotide polymorphism (SNP) of human epidermal growth factor receptor-2 (HER-2) transmembrane domain-coding region at codon 655 and the suggested existence of HER-2 expression in gastric cancer cases in a Turkish patient group.
METHODS: Polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) strategy was used to analyze the presence of HER-2 SNP at codon 655. c-erbB-2 expression pattern was analyzed by immunohistochemistry. The results were compared between gastric carcinoma group and chronic gastritis group, as well as between clinicopathological parameters and carcinoma.
RESULTS: Results showed that Ile/Val genotype accounted for 20% within the Turkish gastric carcinoma group, and none in chronic gastritis group, and this genotyping was associated with stage IV gastric cancers (P = 0.04). Positive membranous HER-2 immunoreactivity, on the other hand, accounted for 24% within the Turkish gastric carcinoma group and none from chronic gastritis cases; further, it was correlated with intestinal type carcinomas (P = 0.007), and stage III-IV carcinomas (P = 0.004).
CONCLUSION: These observations imply that the tested HER-2 SNP may participate in the development and progression of gastric cancer. Thus, after confirming these results with large sample groups, HER-2 codon 655 SNP and/or c-erbB-2 overexpression may also be used as a poor prognostic indicator for gastric carcinomas.
Keywords: Gastric carcinoma, HER-2, c-erbB-2, Single nucleotide polymorphism, Immunohistochemistry
INTRODUCTION
Gastric adenocarcinoma is the second leading cause of cancer-specific mortality worldwide and efforts intended for prevention and early detection of gastric carcinoma are effective and yet important[1]. Despite various treatments such as gastrectomy with extensive lymphadenectomy and surgery combined with chemotherapy, the control of gastric cancer at advanced stages is still challenging.
Molecular genetic analyses have indicated that transformation of normal epithelial cells into a cancer is a multi-step process associated with the progressive accumulation of abnormalities in DNA repair genes, tumor suppressor genes, oncogenes, cellular growth factors, surface receptors, and cellular adhesion molecules[2]. Structural and functional alterations of human epidermal growth factor receptor-2 (HER-2) also known as c-ErbB-2/neu proto-oncogene have been reported in different steps of carcinogenesis including initiation, promotion and progression[3]. HER-2 is a member of the epidermal growth factor (EGF) receptor family located at chromosome 17q21 that encodes a 185-kDa transmembrane glycoprotein with tyrosine kinase activity[4-6]. It is localized to the cell membrane and binding of extracellular growth factor ligands to HER-2 leads to the formation of dimers with another molecule of HER-2 or with HER-1 (EGFr), HER-3 or HER-4. This dimerization results in phosphorylation of tyrosine residues on the cytoplasmic domain of the receptor and subsequent activation of intracellular signaling pathways that regulate diverse biologic responses, including proliferation, differentiation, cell motility, and survival[7]. Overexpression of c-erbB-2 has frequently been reported in various cancer types including breast, ovary, lung, salivary gland, colon, prostate and pancreatic and has been suggested to be an indicator of poor prognosis[8,9]. HER-2 DNA amplification is suggested to be the principal mechanism of HER-2/neu protein activation and is nearly always accompanied by its protein overexpression in breast cancer. It has been also reported c-erbB-2 overexpression in 10% to 38% of gastric cancer cases using immunohistochemical, Southern blot, and fluorescent in situ hybridization analyses that suggest differences in c-erbB-2 expression among subtypes of gastric cancer[10-13]. However, controversy continues in terms of correlation between the HER-2 protein expression and gastric cancer prognosis. There have been reports correlating the lymph node metastasis and/or liver metastasis with HER-2 expression[14-17], whereas others showed no correlation between clinicopathologic findings and HER-2 expression[18,19]. In addition, it has also been shown that HER-2 expression is fewer than 10% in diffuse adenocarcinomas[16,17]. The use of trastuzumab, a function blocking monoclonal antibody against the extracellular domain of HER-2, as a treatment option in HER-2-overexpressing advanced breast cancer patients underlines once again the importance of this protein and its downstream signaling cascade(s) in the pathogenesis of above-mentioned cancers.
Presence of a single nucleotide polymorphism (SNP) in the transmembrane coding region of the HER-2 gene at codon 655, encoding either isoleucine (Ile: ATC) or valine (Val: GTC), has been reported in different cancer types[12,13]. Changing the existing isoleucine (Ile: ATC) to valine (Val: GTC) at codon 655, suggests an increased dimerization, autophosphorylation of HER-2 and tyrosine kinase activity, which may cause the transformation of cells[16]. Some of the reports have revealed that the presence of Ile/Val SNP is associated with increased risk of breast cancer development[20]; on the other hand, others have shown that this correlation is controversial[21-24]. One reason for these contradictory results might be the substantial differences in genetic polymorphism in the HER-2 codon 655 between ethnic groups.
In this study, the frequency of HER-2 codon 655 polymorphism, resulting in an A to G transition (Ile655Val) and alterations in c-erbB-2 protein expression have been investigated in Turkish gastric cancer patients in association with the clinicopathologic parameters.
MATERIALS AND METHODS
Materials
The specimens at Pamukkale University Department of Pathology archive have been used and paraffin-embedded tissues of 25 gastric carcinomas (8 with diffuse type, 16 with intestinal type, i.e., 5 with well, 4 with moderately, 7 with poorly differentiated adenocarcinoma, classified according to Lauren classification and 1 with adenosquamous type) were selected as the study group in this study (Table 1). All carcinomas were staged according to UICC criteria, graded according to WHO criteria, and histopathologically typed according to Lauren classification. Paraffin-embedded tissues of 18 chronic gastritis cases selected from the same collection served as the control group.
Table 1.
Clinicopathological details of gastric carcinoma cases
| Patients (n) | ||
| Gender | Male | 15 |
| Female | 10 | |
| Age (yr)( mean ± SD) | 35-80, (58.8 ± 12.8) | |
| Histologic type (Lauren) | Intestinal | 16 |
| Diffuse | 8 | |
| Adenosquamous | 1 | |
| Histologic type (WHO) | Well | 7 |
| Moderatelly | 5 | |
| Poorly | 4 | |
| Serosa invasion | Positive | 19 |
| Negative | 11ND | |
| Nodal involvement | Positive | 14 |
| Negative | 61ND | |
Not Determined: 5 cases were endoscopic biopsy samples so cannot be determined.
DNA extraction and mutation analysis
DNA extraction from paraffin-embedded tissues was performed using the QIAamp DNA Mini Kit (Qiagen, Hilden-Germany) according to the manufacturer’s protocol. SNP in HER-2 transmembrane segment involving codon 655 was analyzed by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) strategy[25]. The 148 bp transmembrane segment of HER-2 was PCR-amplified using the gene specific primers, i.e., forward (HER-2/F) 5’-AGAGCGCCAGCCCTCTGACGTCCAT-3’, and reverse (HER-2/R) 5’-TCCGTTTCCTGCAGCAGTCTCCGCA-3’[25]. All PCR amplifications were performed in a total volume of 50 μL containing 10 μL of extracted DNA, 20 pmol/L of each forward and reverse primer, and 25 μL of HotStarTaq Master Mix [containing 2.5 units of HotStarTaq DNA polymerase, 1 × PCR buffer with 1.5 mmol/L MgCl2, and 200 μmol/L of each dNTP (Qiagen, Hilden-Germany)]. Thermal cycling conditions for the PCR are as follows: initial activation of HotStarTaq DNA polymerase at 95 °C for 15 min, followed by 35 cycles of 94 °C for 0.5 min, 62 °C for 0.5 min, 72 °C for 1 min, and a final extension of 72 °C for 10 min. PCR products were subjected to electrophoresis on a 2% ethidium bromide (Et-Br) stained agarose gel and visualized under UV for the control of their specificity and integrity.
For RFLP analysis, 5 μL of each PCR product was digested with BsmAI (New England Biolabs, Ipswich, MA-USA) at 55 °C for 2 h, and enzyme was heat-inactivated at 80 °C for 20 min. BsmAI digestion gave fragments of 116 bp and 32 bp for the Val (GTC) allele and a single 148 bp fragment for the Ile (ATC) allele[11]. Fragments digested with BsmAI were subjected to electrophoresis on a 3% molecular screening grade agarose gel (Roche Diagnostics, GmbH, Mannheim-Germany), stained with Et-Br and visualized under UV light.
Immunohistochemistry
Formalin fixed, paraffin-embedded tumor-rich representative tissues were used for immunohistochemical staining. Reactions were performed with anti-c-erbB-2 polyclonal antibody (DAKO, Glostrup-Denmark) using the streptavidin biotin peroxidase method along with DAB as chromogen by Ventana-NexES IHC automatic immunostainer. Staining patterns were analyzed semiquantitatively and scored in the range of 0 to 3, score 0: Less than 10%; score 1: More than 10% of tumor cells with partial membranous staining; score 2: More than 10% cells with moderate membranous staining; score 3: More than 10% cells with strong membranous staining.
Statistical analysis
The data were analyzed by the Chi-Square test. Differences at P < 0.05 were considered to be significant. Statistical analyses were performed with SSPS software, Version 11.0.
RESULTS
HER-2 PCR-RFLP analysis showed that none of the 18 chronic gastritis cases had the SNP in HER-2 gene at codon 655 (Figure 1A), whereas 4 of 25 gastric carcinoma cases (20%) had the Ile/Val genotype (Figure 1B, Table 2). The difference between these two groups were significant (P = 0.04). All 4 mutation positive cases were stage IV in terms of clinicopathologic parameters (Table 2). However, presence of this SNP did not show any correlation with the age, gender, histological type (according to Lauren), serosal invasion, lymph node metastasis, and distant metastasis.
Figure 1.
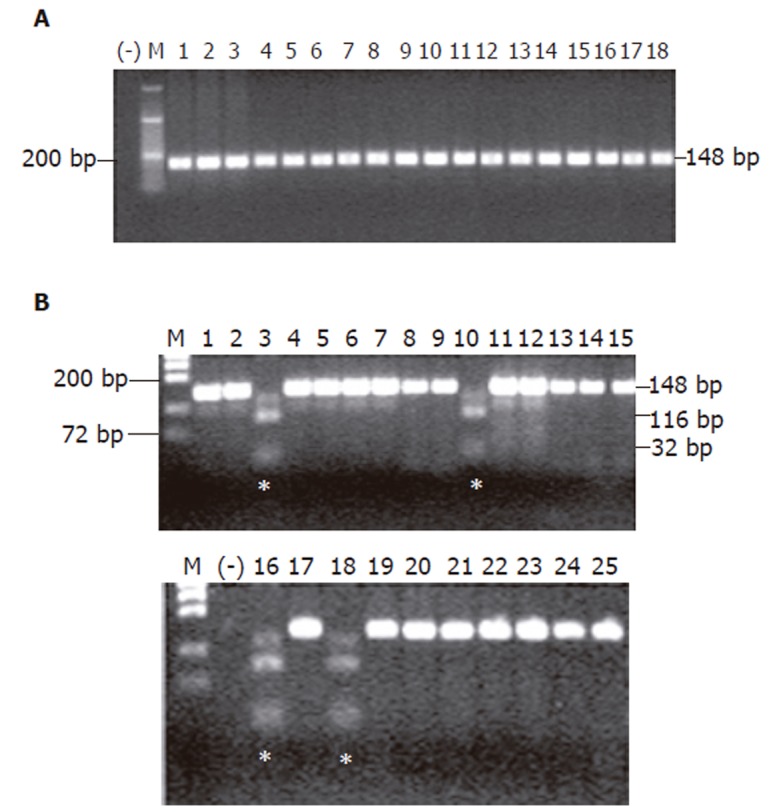
SNP analysis in (A) chronic gastritis group, and (B) gastric carcinoma group. The SNP in codon 655 of HER-2 was detected in patients No 3, 10, 16, and 18 of gastric carcinoma group (*). M, molecular weight marker; (-), negative control for PCR; bp, base pair.
Table 2.
Summary of HER-2 SNP presence and c-erbB-2 expression in gastric carcinoma cases
| Patient No | Classification according to Lauren | Stage | Ile/Val genotype | Immunohistochemical expression score for c-erbB-2 |
| 1 | D | 4 | - | 0 |
| 2 | D | 4 | - | 0 |
| 3 | I | 4 | + | 1 |
| 4 | D | - | 0 | |
| 5 | D | - | 0 | |
| 6 | D | 4 | - | 0 |
| 7 | D | 3 | - | 0 |
| 8 | D | 4 | - | 0 |
| 9 | I | 4 | - | 0 |
| 10 | I | 4 | + | 3 |
| 11 | D | 3 | - | 0 |
| 12 | I | 4 | - | 2 |
| 13 | I | 2 | - | 1 |
| 14 | I | 4 | - | 0 |
| 15 | I | 4 | - | 1 |
| 16 | I | 4 | + | 0 |
| 17 | I | 1 | - | 1 |
| 18 | I | 4 | + | 3 |
| 19 | I | - | 1 | |
| 20 | I | 4 | - | 2 |
| 21 | I | 2 | - | 2 |
| 22 | I | - | 0 | |
| 23 | I | - | 2 | |
| 24 | I | 3 | - | 0 |
| 25 | Other | 4 | - | 0 |
D: diffuse type; I: intestinal type; (-): negative; (+): positive.
Positive membranous c-erbB-2 immunoreactivity (scores 2-3), on the other hand, was detected in 6 of 25 carcinoma cases (24%), i.e., 4 cases with score 2 (16%) and 2 cases with score 3 (8%) (Figure 2A and B, Table 2), whereas 5 cases showed score 1 staining (20%) and 14 cases showed no staining at all (56%, Table 2). Results from chronic gastritis cases revealed no positive membranous c-erbB-2 immunoreactivity, i.e., 4 cases had score 1 staining (19%) (Figure 2C) and 17 cases had no staining at all (81%). When the results from carcinoma group were compared with clinicopathologic parameters, positive membranous c-erbB-2 immunoreactivity was correlated with intestinal type carcinomas (P = 0.007), and stage III-IV carcinomas (P = 0.004). However, no correlation with the age, gender, serosal invasion, lymph node metastasis, and distant metastasis was observed.
Figure 2.
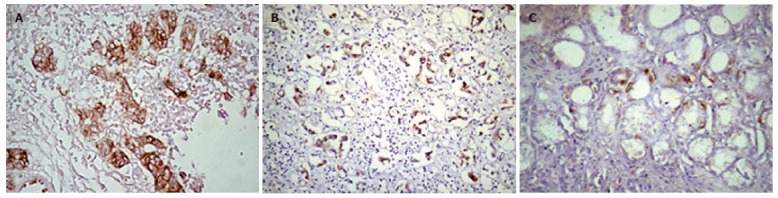
Representative images of HER-2 immunoreactivity in (A) moderately differentiated intestinal type adenocarcinoma (score 3), (B) poorly differentiated intestinal type adenocarcinoma (score 3), and (C) chronic gastritis (score 1).
DISCUSSION
The aim of this study was to investigate both whether the risk of gastric cancer is associated with the Ile/Val SNP of HER-2 transmembrane domain-coding region at codon 655 and the suggested existence of c-erbB2 expression in gastric cancer cases in a Turkish patient group. Results showed that Ile/Val genotype accounted for 20% of the Turkish gastric carcinoma group (P = 0.04), and this genotype was associated with stage IV gastric cancers. However, it did not show any correlation with the age, gender, histological type (according to Lauren classification), serosal invasion, lymph node metastasis, and distant metastasis. Positive membranous c-erbB-2 immunoreactivity, on the other hand, accounted for 24% of the Turkish gastric carcinoma group and it was found to be correlated with intestinal type carcinomas (P = 0.007), and stage III-IV carcinomas (P = 0.004). These observations imply that the tested HER-2 SNP may participate in the development and rapid progression of gastric cancer. However, further larger sample studies particularly containing early stage gastric carcinoma patients, are needed to confirm the presence of SNP and/or c-erbB-2 overexpression may also be used as a poor prognostic indicator for gastric carcinomas.
HER-2 encodes a receptor tyrosine kinase functioning as a growth regulatory protein and a cell motility factor[26]. Alterations of HER-2 have been implicated in the carcinogenesis and are frequently observed in a variety of tumors[24,27-29]. The molecular mechanism of the association between the Ile/Val SNP at codon 655 of HER-2 and cancer has not been fully understood; however, it has been suggested that Ile/Val SNP at codon 655 of HER-2 results in increased dimerization, autophosphorylation of HER-2 and tyrosine kinase activity[16], which may cause the transformation of cells. In spite of initial reports on the association of this SNP with the risk of a variety of cancers, there is still an ongoing debate on this topic. To our knowledge, this is the first study in a Turkish gastric carcinoma group investigating the existence of the Ile/Val genotype. Although the study group here is small, the 20% occurrence rate in this Turkish gastric carcinoma patient group is comparable to the reported 21% occurrence rate in a Japanese gastric carcinoma group of 212 patients[25]. It suggests a significantly increased risk of gastric cancer development. The association of this genotyping with stage IV gastric cancers also supports this finding.
Overexpression of c-erbB-2, on the other hand, has been associated with poor prognosis and shorter overall survival[14,30-32] and may be used as a predictive marker for the response to therapy in some cancers[33]. However, a controversy also continues in terms of correlation between the c-erbB-2 protein expression and gastric cancer prognosis. There have been reports correlating the lymph node metastasis and/or liver metastasis with c-erbB-2 expression[14-17], whereas others showed no correlation between the clinicopathologic findings and c-erbB-2 expression[18,19]. Results of this study also correlate with a previous report by Dursun et al[34] in terms of positive membranous c-erbB-2 immunoreactivity within a Turkish gastric carcinoma group. Like suggested previously[34] this study has shown association of positive membranous c-erbB-2 immunoreactivity with intestinal type carcinomas (P = 0.007), and stage III-IV carcinomas (P = 0.004). However, it has to be taken into consideration that both studies have worked with small patient groups leading to a decreased statistical power to detect a positive association of any kind. Thus, further investigation with a desirable study group size is necessary to assess the importance of c-erbB-2 expression in gastric carcinomas for the Turkish population. Nevertheless, results of this study suggest increased c-erbB-2 expression may be considered as a poor prognostic indicator.
In conclusion, this study suggests that the tested SNP in the transmembrane domain-coding region of HER-2 could be associated with development of gastric carcinoma and may serve as a predictor of risk of malignant phenotype of gastric cancer for the Turkish population. Although the association of the HER-2 immunoreactivity with clinicopathologic characteristics, especially with intestinal type carcinomas, is also suggested, this has to be confirmed with a larger sample size.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Zhang Y
References
- 1.Mansfield PF, Yao JC, Crane CH. Holland-Frei Cancer Medicine 6. Hamilton: BC Decker Inc; 2003. pp. 1515–1542. [Google Scholar]
- 2.Weinberg RA. How cancer arises. Sci Am. 1996;275:62–70. doi: 10.1038/scientificamerican0996-62. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 6.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 9.Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Bock JE, Glud E, Nørgaard-Pedersen B, Høgdall CK. Distribution of HER-2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: from the Danish MALOVA Ovarian Cancer Study. Cancer. 2003;98:66–73. doi: 10.1002/cncr.11476. [DOI] [PubMed] [Google Scholar]
- 10.Lee EY, Cibull ML, Strodel WE, Haley JV. Expression of HER-2/neu oncoprotein and epidermal growth factor receptor and prognosis in gastric carcinoma. Arch Pathol Lab Med. 1994;118:235–239. [PubMed] [Google Scholar]
- 11.Slesak B, Harlozinska A, Porebska I, Bojarowski T, Lapinska J, Rzeszutko M, Wojnar A. Expression of epidermal growth factor receptor family proteins (EGFR, c-erbB-2 and c-erbB-3) in gastric cancer and chronic gastritis. Anticancer Res. 1998;18:2727–2732. [PubMed] [Google Scholar]
- 12.Papewalis J, Nikitin AYu MF. G to A polymorphism at amino acid codon 655 of the human erbB-2/HER2 gene. Nucleic Acids Res. 1991;19:5452. doi: 10.1093/nar/19.19.5452-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Habuchi T, Takahashi T, Kamoto T, Zuo T, Mitsumori K, Tsuchiya N, Sato K, Ogawa O, Kato T. No association between HER-2 gene polymorphism at codon 655 and a risk of bladder cancer. Int J Cancer. 2002;97:787–790. doi: 10.1002/ijc.10129. [DOI] [PubMed] [Google Scholar]
- 14.Ougolkov A, Yamashita K, Bilim V, Takahashi Y, Mai M, Minamoto T. Abnormal expression of E-cadherin, beta-catenin, and c-erbB-2 in advanced gastric cancer: its association with liver metastasis. Int J Colorectal Dis. 2003;18:160–166. doi: 10.1007/s00384-002-0427-2. [DOI] [PubMed] [Google Scholar]
- 15.Carter WB, Hoying JB, Boswell C, Williams SK. HER2/neu over-expression induces endothelial cell retraction. Int J Cancer. 2001;91:295–299. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1061>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Mizutani T, Onda M, Tokunaga A, Yamanaka N, Sugisaki Y. Relationship of C-erbB-2 protein expression and gene amplification to invasion and metastasis in human gastric cancer. Cancer. 1993;72:2083–2088. doi: 10.1002/1097-0142(19931001)72:7<2083::AID-CNCR2820720705>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Gürel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, Gülten M, Memik F. The relationship between c-erbB-2 oncogene expression and clinicopathological factors in gastric cancer. J Int Med Res. 1999;27:74–78. doi: 10.1177/030006059902700203. [DOI] [PubMed] [Google Scholar]
- 19.Ohguri T, Sato Y, Koizumi W, Saigenji K, Kameya T. An immunohistochemical study of c-erbB-2 protein in gastric carcinomas and lymph-node metastases: is the c-erbB-2 protein really a prognostic indicator. Int J Cancer. 1993;53:75–79. doi: 10.1002/ijc.2910530115. [DOI] [PubMed] [Google Scholar]
- 20.Xie D, Shu XO, Deng Z, Wen WQ, Creek KE, Dai Q, Gao YT, Jin F, Zheng W. Population-based, case-control study of HER2 genetic polymorphism and breast cancer risk. J Natl Cancer Inst. 2000;92:412–417. doi: 10.1093/jnci/92.5.412. [DOI] [PubMed] [Google Scholar]
- 21.Akisik E, Dalay N. Estrogen receptor codon 594 and HER2 codon 655 polymorphisms and breast cancer risk. Exp Mol Pathol. 2004;76:260–263. doi: 10.1016/j.yexmp.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Kamali-Sarvestani E, Talei AR, Merat A. Ile to Val polymorphism at codon 655 of HER-2 gene and breast cancer risk in Iranian women. Cancer Lett. 2004;215:83–87. doi: 10.1016/j.canlet.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Millikan R, Eaton A, Worley K, Biscocho L, Hodgson E, Huang WY, Geradts J, Iacocca M, Cowan D, Conway K, et al. HER2 codon 655 polymorphism and risk of breast cancer in African Americans and whites. Breast Cancer Res Treat. 2003;79:355–364. doi: 10.1023/A:1024068525763. [DOI] [PubMed] [Google Scholar]
- 24.Ameyaw MM, Tayeb M, Thornton N, Folayan G, Tariq M, Mobarek A, Evans DA, Ofori-Adjei D, McLead HL. Ethnic variation in the HER-2 codon 655 genetic polymorphism previously associated with breast cancer. J Hum Genet. 2002;47:172–175. doi: 10.1007/s100380200019. [DOI] [PubMed] [Google Scholar]
- 25.McKean-Cowdin R, Kolonel LN, Press MF, Pike MC, Henderson BE. Germ-line HER-2 variant and breast cancer risk by stage of disease. Cancer Res. 2001;61:8393–8394. [PubMed] [Google Scholar]
- 26.Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE, Schell MJ, Melton LJ 3rd, Liu ET, Conway K. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. J Clin Oncol. 2000;18:267–274. doi: 10.1200/JCO.2000.18.2.267. [DOI] [PubMed] [Google Scholar]
- 27.Tsugawa K, Yonemura Y, Hirono Y, Fushida S, Kaji M, Miwa K, Miyazaki I, Yamamoto H. Amplification of the c-met, c-erbB-2 and epidermal growth factor receptor gene in human gastric cancers: correlation to clinical features. Oncology. 1998;55:475–481. doi: 10.1159/000011898. [DOI] [PubMed] [Google Scholar]
- 28.Yazici H, Altun M, Alatli C, Dogan O, Dalay N. c-erbB-2 gene amplification in nasopharyngeal carcinoma. Cancer Invest. 2000;18:6–10. doi: 10.3109/07357900009023056. [DOI] [PubMed] [Google Scholar]
- 29.Kuraoka K, Matsumura S, Hamai Y, Nakachi K, Imai K, Matsusaki K, Oue N, Ito R, Nakayama H, Yasui W. A single nucleotide polymorphism in the transmembrane domain coding region of HER-2 is associated with development and malignant phenotype of gastric cancer. Int J Cancer. 2003;107:593–596. doi: 10.1002/ijc.11450. [DOI] [PubMed] [Google Scholar]
- 30.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 31.Kameda T, Yasui W, Yoshida K, Tsujino T, Nakayama H, Ito M, Ito H, Tahara E. Expression of ERBB2 in human gastric carcinomas: relationship between p185ERBB2 expression and the gene amplification. Cancer Res. 1990;50:8002–8009. [PubMed] [Google Scholar]
- 32.Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/S0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 33.Paik S, Bryant J, Tan-Chiu E, Yothers G, Park C, Wickerham DL, Wolmark N. HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-15. J Natl Cancer Inst. 2000;92:1991–1998. doi: 10.1093/jnci/92.24.1991. [DOI] [PubMed] [Google Scholar]
- 34.Dursun A, Poyraz A, Celik B, Akyol G. Expression of c-erbB-2 oncoprotein in gastric carcinoma: correlation with histopathologic characteristics and analysis of Ki-67. Pathol Oncol Res. 1999;5:104–106. doi: 10.1053/paor.1999.0171. [DOI] [PubMed] [Google Scholar]


