Abstract
Understanding and characterization of pain and other sensory symptoms are among the most important issues in the diagnosis and assessment of patient with gastrointestinal disorders. Methods to evoke and assess experimental pain have recently developed into a new area with the possibility for multimodal stimulation (e.g., electrical, mechanical, thermal and chemical stimulation) of different nerves and pain pathways in the human gut. Such methods mimic to a high degree the pain experienced in the clinic. Multimodal pain methods have increased our basic understanding of different peripheral receptors in the gut in health and disease. Together with advanced muscle analysis, the methods have increased our understanding of receptors sensitive to mechanical, chemical and temperature stimuli in diseases, such as systemic sclerosis and diabetes. The methods can also be used to unravel central pain mechanisms, such as those involved in allodynia, hyperalgesia and referred pain. Abnormalities in central pain mechanisms are often seen in patients with chronic gut pain and hence methods relying on multimodal pain stimulation may help to understand the symptoms in these patients. Sex differences have been observed in several diseases of the gut, and differences in central pain processing between males and females have been hypothesized using multimodal pain stimulations. Finally, multimodal methods have recently been used to gain more insight into the effect of drugs against pain in the GI tract. Hence, the multimodal methods undoubtedly represents a major step forward in the future characterization and treatment of patients with various diseases of the gut.
Keywords: Pain, Gut, Experimental, Allodynia, Hyperalgesia, Neurophysiology
INTRODUCTION
Abdominal pain is very common in the general population[1], and pain is the most prevalent symptom in the gastroenterological clinic[2]. Consequently, characterization of gut pain is one of the most important issues in the diagnosis and assessment of organ dysfunction. However, in clinical practice, the different symptoms of the underlying diseases confound the characterization of pain. These confounders may include complaints relating to psychological, cognitive and social aspects of the illness, as well as systemic reactions, such as fever and general malaise[3]. Furthermore, treatment with analgesics often causes sedation and other side effects. This will invariably bias the clinical evaluation of the pain-related symptoms. Hence, the patients tend to interpret other effects of the medication, e.g. an effect on the anxiety and depression relating to the disease, as a relief of pain[4]. Because of these confounding factors experimental pain models are often advantageous. Using these models, the investigator can control the experimentally induced pain (including the nature, localization, intensity, frequency and duration of the stimulus), and provide quantitative measures of the psychophysical, behavioral or the neurophysiological responses[3,5,6].
Experimental models have been used in different animal species. Here the investigators can study the neuronal activity in anesthetized or spinalized animals directly with invasive techniques or with assessment of behavior[7]. However, the neurobiology of the pain system differs between the animal species. This limits to a high degree the interpolation of findings from animal studies to man. Pain is the net effect of complex multidimensional mechanisms including intensity coding, affective, behavioral and cognitive components that involve most parts of the central nervous system. Furthermore, in humans, pain is closely related to linguistic terms and expressions. Thus, it is a complex sensory experience which is difficult to quantify with simple neurophysiological and/or behavioral methods. Therefore, animal experiments can only to some degree reflect the experience of clinical pain in humans and the interest in human experimental pain studies has increased rapidly during the last decade[3,8].
The primary advantages of experimental pain approaches are that the stimulus can be controlled, delivered repeatedly and modulated, and that the responses can be assessed quantitatively with psychophysical and/or neurophysiological methods (Figure 1). Depending on the experimental model, different central mechanisms and conditions mimicking pathological pain can be studied. These are increased sensation to normal physiologic/non-painful and painful stimuli (allodynia and hyperalgesia, respectively). Experimental approaches can be applied in the laboratory for basic studies in healthy subjects and in patient groups, or used for preliminary screening of drug efficacy[9]. The methods can also be used in the clinic to characterize patients with sensory dysfunction and pain in organic and functional diseases[5,10-12]. The methods have been widely used in the skin and muscle[6]. However, due to the difficulties with access to the organs in the gastrointestinal (GI) tract, experimental pain testing is much more difficult than stimulation of the skin. The risk of perforation and other complications also limit the possibilities. Thus, most previous studies have relied on relative simple mechanical or electrical stimuli. These methods are easy to apply, but unless advanced modeling is used they have several limitations[3]. Most importantly, as pain is a multidimensional perception, it is obvious that the reaction to a single stimulus of a given modality can represent only a limited fraction of the entire pain experience. The possibility for combining different methods to stimulate the gut and evoke hyperalgesia will approximate the clinical situation, and give more comprehensive and differentiated information about the nociceptive system[3]. Multimodal models have clearly shown their value in testing of analgesics where a single stimulus has been inadequate assessing effects of specific drugs. Hence, Enggaard et al[13] showed that tricyclic antidepressants, which are valuable in treatment of functional pain disorders of the gut, increased the pain threshold to electrical stimuli, but did not reduce cold pressor pain. More sophisticated methods using a vide battery of tests will therefore be able to select the best test procedures to explore different basic aspects of pain as well as pharmacological modulations[9].
Figure 1.
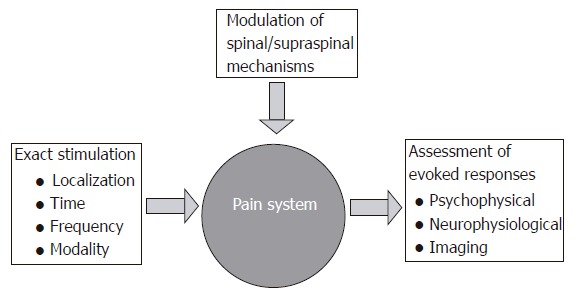
The concept for experimental induction, assessment and modulation of experimental gut pain in man.
In this review, we outline the recent developments into test systems allowing standardized multimodal stimulations of the GI tract and their applications.
The rationale for multimodal stimulations of the gastrointestinal tract
The ideal experimental stimulus to elicit gut pain in man should be natural, minimally invasive, reliable in test-retest experiments and quantifiable[14]. The response to the stimulus should increase with increasing stimulus intensity and preferably the pain should mimic the observations in diseased organs by evoking phenomena, such as allodynia and hyperalgesia[8]. The different methods for pain stimulation of the human GI tract are electrical, mechanical, chemical, thermal and ischemic stimulation; further detailed informations regarding the advantages and shortcomings of these methods are explained elsewhere[3]. The ischemic stimuli are difficult to quantify in man and is normally not used as a direct stimulus. One of the major limitations of the different models is that they may not mimic clinical pain. Hence, they are relative short-lasting without the inflammation and subsequent activation of the many peripheral and central nervous mechanisms that are typically activated during diseases. Therefore, the basic neurobiological mechanisms in clinical pain may be different from those relating to an experimental stimulus[5,10]. For comprehensive experimental studies mimicking the clinical situation, a multimodal testing approach must therefore be used. A test battery where multimodal stimuli are used will increase the probability for activation of a range of relevant nervous mechanisms. Especially, if the stimulation is relatively long-lasting and includes modalities known to evoke peripheral as well as central sensitization, the likelihood that the model will mimic clinical pain is high despite the non-harmful nature of the stimulation.
In the GI tract, technical limitations of the currently available models have until now made a multimodal stimulation approach difficult. Some authors have combined mechanical and electrical stimuli[15,16] or used electrical stimuli combined with sensitization to acid[17]. The Center for Visceral Biomechanics and Pain in our department has recently introduced a multimodal pain model where mechanical, electrical, cold and warmth stimuli were combined. A summary of the findings in healthy subjects and patients is shown in Tables 1 and 2. Table 2 should be interpreted with caution as many data are still unpublished. In the multimodal model, mechanical stimulation is achieved with bag distension. Quantifications of the bag pressure and cross-sectional area are typically done by means of impedance planimetry or ultrasonography. Thermal stimulation is achieved by re-circulating fluid inside the bag with concomitant measurement of temperature. Chemical and electrical stimulation is done using side-holes placed proximal to the bag and by electrodes on the outside of the bag[18]. Sensitization with acid was added to the protocol to evoke allodynia and hyperalgesia together with increased referred pain areas (Figure 2)[19]. The multimodal approach gives the possibility for a differentiated stimulation of receptors in the superficial and deep layers of the gut. The possibility for induction of hyperalgesia and evoking central phenomena such as summation, allodynia and referred pain makes the models clinically relevant with respect to increasing the knowledge about peripheral and central pain mechanisms. The model have mostly been used in the esophagus, but recently also in the duodenum[20]. The pain assessment should ideally also be multimodal and, for example, include quantitative and qualitative sensations, assessment of referred pain and neurophysiological measurements[19].
Table 1.
Multimodal comparison of clinical experimental data obtained in the esophagus from healthy volunteers
| Group | Mechanical stimuli | Heat stimuli | Cold stimuli | Electrical stimuli | Sensitization with acid |
| Basic data | Differences between the sensations and referred pain areas was evoked by the stimulus modalities[18]. Reliability demonstrated[19,60]. The sensation to mechanical stimulations was unaffected by relaxation of the smooth muscle[40,61]. Evidence for low and high threshold high threshold | Reliability demonstrated. Stimulus-response functions obtained[18,19,60]. | Reliability demonstrated. Stimulus-response functions obtained[18,19,60]. | Reliability demonstrated. Stimulus-response unctions obtained[18,19,60]. | Allodynia and hyperalgesia evoked[19,26], although not consistent for mechanical stimuli (see text)[26,41]. Increased referred pain and amplitude of the nociceptive reflex indicating central hyperexcitability[19,26,46]. Acid perfusion sensitizes the oesophagus to heat but not cold, indicating sensitization of peripheral TRPV1 receptors[28,46]. Remote hyperalgesia was seen in the rectum after acid perfusion of the esophagus[20]. Hyperreactivity of contractions in esophagus, but tone was unaffected[26,46]. |
| Gender differences | Males were more sensitive to stimulations, but an increased referred pain area was seen in females, reflecting sex differences incentral pain processing[27,46]. | No differences in sensation, but the referred area was larger in females[27,46]. | As heat stimuli | Males less sensitive to single and repeated stimuli (Staahl et al., unpublished). | In females, the referred pain area increased to heat after acid sensitization, but no changes were seen to mechanical and cold stimulations[46]. |
| Pharmacologic modulation | Oxycodone was better than morphine (and placebo) in attenuating mechanical pain[59]. | Oxycodone was better than morphine (and placebo) in attenuating heat pain[59]. | Not done | Both morphine and oxycodone attenuated the electrically evoked pain, but there were no differences between the opioids[59]. | Not done |
Table 2.
Multimodal comparison of clinical experimental data obtained in the esophagus from patient with different GI diseases
| Patient group | Mechanical stimuli | Heat stimuli | Cold stimuli | Electrical stimuli | Sensitization with acid |
| Non-cardiac chest pain[41] | No differences to single stimuli, but increased pain to repeated stimuli and increased referred pain area, reflecting central hyperexcitability. | Not done | Not done | Not done | Increased sensation to mechanical stimulations after acid in patients only. |
| Esophagitis[31] | Patients were hyposensitive but with larger and more widespread referred pain. The distension induced more reactive contraction. | Patients were Hypersensitive probably via increased activation of TRPV1 receptors. | No differences | Not done | Not done |
| Non-erosive reflux disease (Reddy et al unpublished data) | Patients were hyposensitive to mechanical stimuli. The distensions induced more reactive contractions in the esophagus in the patients and they had larger referred pain areas. Patients with pathological 24-h pH-measurement were more hyposensitive than the patients with normal pH profile. | The patients were hypersensitive to heat with increased referred pain areas to this modality. | No differences between patients and controls | Not done sensitivity score unpublished data) | Patients had a higher sensitivity score to acid perfusion. |
| Diabetes (Frøkjær et al, unpublished data) | Patients had hyposensitivity to distension, but increased referred pain areas, reflecting peripheral neuropathy and central hyperexcitability. Increased stiffness of the gut wall in diabetes. | As mechanical stimulations | Not done | As mechanical stimulations | Not done |
| Chronic pancreatitis (Dinmcevski et al, unpublished data) | No differences in sensation. No differentiated effect on morphine and oxycodone in attenuation of mechanical pain. | No differences in sensation Oxycodone attenuated heat pain better than morphine. | Not done | Larger referred pain area in the patients. Opioids were not better than placebo in attenuating electrical pain | Not done unpublished data) |
Figure 2.
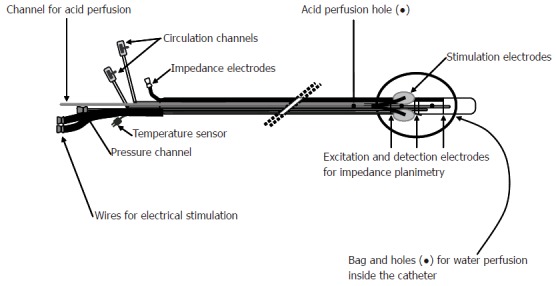
Schematic illustration of the probe used for multi-modal (electrical, mechanical, cold and warmth stimuli) of the esophagus.
Multimodal stimulation and peripheral receptors
The multimodal approach has given valuable information about the receptors in the gut wall. Theoretically, the thermal stimulation activates preferentially the receptors in the mucosa, the electrical stimulation penetrates into deeper layers of the gut and the mechanical stimulations affect predominantly receptors in the muscle layers (Figure 3).
Figure 3.
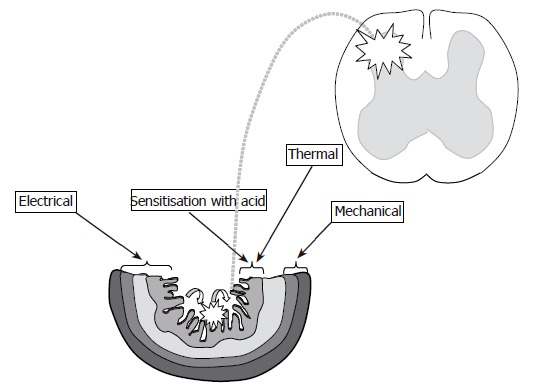
Schematic illustration of the proposed gut layers which are preferentially affected with: (1) Thermal stimuli (mucosa - dark grey and submucosa - light grey); (2) Mechanical stimuli (circular muscle layer - hatched grey and longitudinal muscle layer - prickled grey); (3) Electrical stimuli (all layers depending on stimulus intensity). Perfusion of the esophagus with acid (curved arrows) gives the possibility to evoke peripheral and (mainly) central sensitization (illustrated with stars).
It is generally believed that most visceral afferents are polymodal and respond to a wide range of stimuli including thermal stimuli[21]. However, sub-populations of these receptors exist and recently, we combined controlled distension with statistical modeling to demonstrate the existence of low and high threshold mechano-receptors in the human esophagus[22]. Most data on the sensation to thermal stimuli of the human viscera relate to few and relatively old studies[23-25], although some new studies have recently been published[18,19,26-29]. These studies point towards the existence of sensory pathways for thermal stimuli in the human GI tract. The thermal energy spreads from the superficial layers into the deeper layers of the gut depending on the temperature and conductance of the tissue[4]. However, as thermal stimuli are rather short-lasting in man mainly receptors in the mucosa are thought to be activated. Chemical stimulation with acid or capsaicin also activates receptors in the mucosa. Pedersen et al[28] used a multimodal approach to combine acid and heat stimuli of the esophagus. In this study, sensitization with acid resulted in a significant increase in the sensation to heat stimuli. The TRPV1 receptor is a polymodal detector of potential harmful stimuli, including noxious heat and protons[30]. Hence, it was suggested that TRPV1 receptors (or receptors with the same characteristics) were sensitized with acid and subsequently resulted in increased firing of the afferents to heat stimulation. The role of this receptorsystem in the clinic was addressed in another study where selective hyperalgesia to heat but not cold stimuli was found in patients with reflux and grade B esophagitis[31]. The evidence for receptor-specific activation pattern in the experimental studies was supported in a recent study where the TRPV1 receptor was demonstrated in the human esophagus, and especially the receptor was up-regulated in esophagitis[32]. Thus, the multimodal approach gave valuable quantitative in vivo information about the receptor characteristics and pain mechanisms in healthy subjects as well as in patients with acid-evoked inflammation.
Multimodal stimulation and primary afferents
Electrical stimuli bypass the receptors and although all fiber populations (nociceptive as well as fibers mediating physiologic/non-nociceptive sensations) are excited by electrical stimuli, the relative proportion of activation depends on the stimulus intensity. With normal bipolar electrical stimulation, the current is believed first to activate receptors in the mucosa and submucosa, whereas the deeper layers are activated with increasing current[3]. The depth of activation also depends on the stimulation method and frequency[4]. In the gut, electrical stimulation is thought preferentially to activate thinly myelinated (Aδ) fibers[33]. Chemical and thermal stimuli, on the other hand, activate mainly non-myelinated (C) fibers and the terminals of mechanosensitive fibers are mainly localized in the muscle layers or have intraganglionic nerve endings[34,35]. Roughly speaking, the different modalities may therefore activate different fiber populations and, for example, may thermal and mechanical stimulation activate fibers in the mucosa and muscle layers, respectively. However, the difference between the stimulation paradigms may be of minor importance as Hobson et al[36] showed that the evoked brain potentials to mechanical and electrical stimulation of the gut were similar, reflecting that the same pathways were activated. The mechanical stimulation protocol may also be important. Hence, in the human rectum phasic distensions were shown preferentially to stimulate spinal pathways thought to mediate pain, whereas slow tonic stimuli mainly affect parasympathetic nerves[37]. We recommend a slowly ramp distension as it is more physiological and allows the subjects to assess the pain continuously. We also strongly recommend to precondition the tissue by two-three distensions until the stress-strain relationship becomes reproducible[38]. For advanced muscle analysis, we normally use butylscopolamine to relax the smooth muscle, and this does not seem to modify the sensation per se [20,39,40].
The multimodal approach has recently been used to compare the response to mechanical stimuli before and after chemical stimulation with acid in patients with non-cardiac chest pain[41]. In these patients, there is a normal sensory response to mechanical stimuli at baseline. However, after acid the evoked hyperalgesia resulted in a marked increase of the sensory response in the patients (Figure 4). Although peripheral sensitization may be important, the findings gave evidence for an amplification of central pain mechanisms manifested as allodynia, hyperalgesia, and increased and widespread referred pain areas to the mechanical stimulations. Mechanical stimulation together with advanced muscle analysis has also been used to explain the symptoms in patients with systemic sclerosis. In these patients, the contraction amplitude was smaller and there was an evidence for a stiffer gut wall in the small intestine[42]. The pain evoked by a controlled strain of the gut was increased and this may explain many of the symptoms reported in the clinic. In patients with diabetes, we also found evidence for increased stiffness in the duodenum using the multimodal approach (Frøkjær et al, unpublished data). This may reflect the increase in collagen deposition seen in these patients and may (together with autonomic neuropathy) explain the motor abnormalities seen in these patients.
Figure 4.
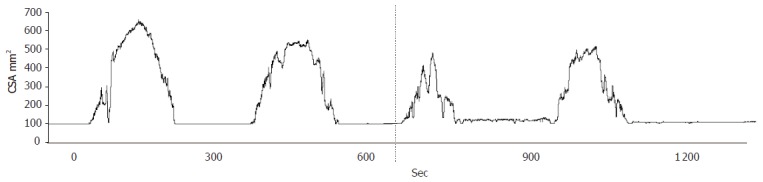
Cross-sectional area (CSA) at moderate pain during two distensions of the esophagus in a patient with non-cardiac chest pain. The distensions were performed before and after perfusion of the distal esophagus with acid (illustrated with the stippled line). A clear reduction in the tolerated mechanical stimulus was seen after acid perfusion.
Multimodal stimulations and central pain mechanisms
In diseases of the GI tract, central sensitization and neuroplastic changes are probably of major importance to understand the sensory response as manifested by pain and hyperalgesia. Central pain mechanisms may be evoked by multiple stimuli (either temporal or spatial summation), resulting in central amplification of the response. The response is comparable to early phase of the frequency-dependent “wind-up” seen in animal experiments. In practice the central integration can be evoked by repeated electrical stimulation above 0.5 Hz[43,44], resulting in increased local and referred pain. Recently, the multimodal probe was used to give repeated mechanical stimulation in patients with non-cardiac chest pain. In this study, the number of stimuli tolerated was significantly lower in the patients compared with healthy controls, reflecting central hyperexcitability as a key to understanding the symptoms in these patients[41].
Sensitization of the esophagus with acid is an another possibility to evoke central (and peripheral) sensitization. Previously, it was shown that acid perfusion of the distal esophagus resulted in an amplified response to electrical, mechanical and thermal stimuli[19,26]. The central changes were documented in an experiment where there was an amplification of the nociceptive reflex[19]. The reflex was evoked by stimulation of the sural nerve, resulting in activity of the biceps muscle of the thigh. The connection from the primary afferents to the motor neurons is a polysynaptic spinal pathway, which can be modulated by other afferent input, spinal neuronal excitability, and activity in descending control systems[5]. The reflex was evoked together with a painful mechanical stimulus of the esophagus. In the experiment, an amplification of the reflex was seen after sensitization of the esophagus with acid, reflecting central changes at the spinal cord level. Evidence for central changes to acid perfusion was also demonstrated by Sarker et al[17] who demonstrated a decreased pain threshold to electrical stimulation of the proximal esophagus after acid perfusion of the distal part. As the proximal esophagus was not affected by the acid, only central changes would explain the findings.
Referred somatic pain to visceral stimuli is regarded as a phenomenon generated by central mechanisms due to visceral nerves terminating in the same area of the spinal cord as somatic afferents[45] (Figure 5). Assessment of the referred pain area to electrical, mechanical and thermal stimulation can be used to determine the central response to these differentiated modalities[19]. The referred pain area to electrical, mechanical, cold and heat pain differs in size and localization reflecting the different peripheral (and hence central) nerves that are activated[18]. The increase in referred pain areas after acid perfusion is also an evidence for central sensitization caused by the chemical stimulation[26,46]. Recently, Pedersen et al[28] showed that the referred pain to heat but not cold stimulation of the esophagus increased after acid perfusion of the organ. This has also been shown in a more recent study[46]. As discussed previously, selective sensitization of the TRPV1 receptors by acid could result in an increased afferent barrage after a heat stimulus, which again was manifested as an increase in the referred pain area. The changes in local and referred pain to mechanical stimulations may, however, be difficult to determine as the acid also evokes secondary contractions that may squeeze the bag and influence the stimulus parameters[26]. On the other hand, an increase in the referred pain after acid perfusion is typically seen to mechanical stimulation of the esophagus in healthy subjects[19,26]. Increased referred pain to mechanical stimulations was also seen in patients with esophagitis and in non-erosive reflux disease, reflecting that facilitation of central pain mechanisms is important in the understanding of these diseases[31]. In patients with diabetes and chronic pancreatitis, we found hypoalgesia to peripheral stimulation, whereas there was a significant increase in the referred pain area. These data were interpreted as a descending inhibition of the afferent input counterbalancing central hyperexcitability (Frøkjær et al, unpublished data). Hence, the multimodal approach may be used explaining the symptoms in these patients, and may be used to evaluate the stage of disease in a more mechanism-based manner. Central changes may also result in allodynia and hyperalgesia to stimulation of other viscera[47]. Such changes are regarded important in the understanding of functional gut disorders where abnormal sensation to physiologic stimuli, such as feces or air in the gut, may contribute to the symptoms (allodynia). Recently, a multimodal approach was used to assess the sensation of the proximal esophagus, duodenum and rectum after sensitization of the distal esophagus with acid[20]. In this study, an increased sensitivity to mechanical stretch in the three gut segments was seen after acid perfusion. This was mainly due to increased sensitivity in the rectum being very remote from the experimentally inflamed esophagus.
Figure 5.
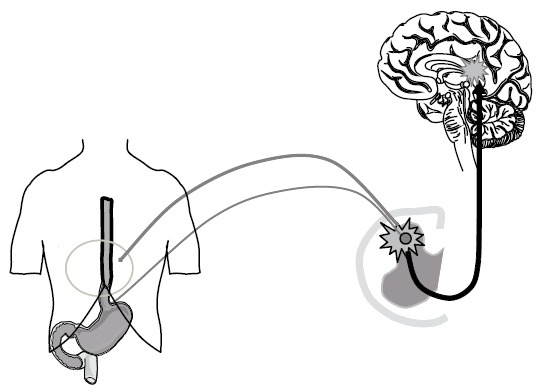
Referred pain in the somatic tissues is believed to be generated by central mechanisms, where visceral and somatic nerves converge on nerves in the same area of the spinal cord or at supraspinal centers. The phenomena also includes unmasking of latent connections and focal central hyperexcitability of the neurons.
Neuroplastic changes at the cortical level may also be shown by multimodal stimulations of the gut. Thus, Sarker et al[48] showed changes in the evoked brain potentials to electrical stimulation of the proximal esophagus after acid perfusion of the distal segment. Recently, we showed that acid perfusion resulted in neuroplastic changes at the cortical level reflected. Reduction in latency and a backward shift of the electrical dipole in the anterior cingulate dipole were observed to electrically evoked pain in the esophagus after acid perfusion of the organ (Sami et al, unpublished data). Such changes were also found when the gut was electrically stimulated in patients with irritable bowel syndrome[49]. The backward shift in the cingulate activation after sensitization with acid in healthy subjects may, therefore, represent the central nervous system change corresponding to the allodynia and hyperalgesia to gut stimuli found in patients with functional disorders of the gut.
Gender differences to multimodal pain stimulations
Women are diagnosed more frequently with chronic visceral pain disorders than men[50]. Extensive evidence indicates that females and males differ in their nociceptive processing, although it seems modality- and tissue-specific. The reason for the female predominance is not known, but sex differences are found in basic GI functions, such as gallbladder emptying and colon transit[51,52]. For most studies using experimentally delivered somatic pain stimuli, females have lower thresholds, less tolerance, and higher pain ratings than males[53]. However, few studies have focused on sex-related differences in visceral pain in man, and these have been contradictory with respect to sex differences[51]. The multimodal probe was recently used to investigate any differences to mechanical and thermal stimuli of the esophagus. The results were somewhat ambiguous, but in general males seemed to be more sensitive to the stimuli[27,46]. However, a greater size of the referred pain areas to the different stimuli was seen in women. After acid perfusion, the males were also more sensitive than females to distensions, but no differences were found in response to the thermal stimuli[46]. In the females, only the referred pain area was increased to heat stimulations after sensitization with acid. The bigger referred pain areas may thus reflect that the central processing of pain to visceral stimuli differs between males and females as previously shown by our group and by Kern et al [54]. Thus, the multimodal stimulations revealed a differentiated response to peripheral and central pain mechanisms, which may explain the sex-related differences seen in several gastrointestinal disorders.
Multimodal stimulations outside the esophagus
Accarino et al[15] used multimodal stimulation (mechanical and electrical) of the jejunum. The verbal response to electrical stimuli and distension was compared, and no differences in the evoked response were found. This led the authors to conclude that the practical differences between the two modalities may probably be of minor importance. Recently, we used thermal and mechanical stimuli of the duodenum in healthy subjects[20] and in patients with diabetes and autonomic neuropathy (Frøkjær et al, unpublished data). The diabetes patients showed hypoalgesia to mechanical and electrical stimuli, whereas no changes were found to heat stimulation compared with controls. Furthermore, the referred pain area in the abdomen was enlarged in the patients (Figure 6). Such data may enhance our knowledge about peripheral and central pain mechanisms in these patients with implications for the treatment.
Figure 6.
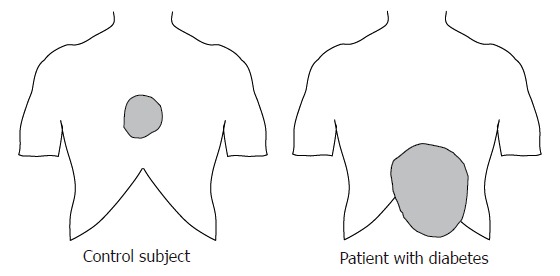
Referred pain area to mechanical distension of the esophagus in a typical healthy subject, and in a patient with diabetes mellitus and autonomic neuropathy. The patient complained of severe nausea and pain in the epigastrium. The referred pain area in the diabetic patient was larger and abnormally localized.
Mechanical stimulation of the rectum is one of the most used methods in experimental visceral research. Electrical and thermal stimulations have also been used in the rectum[3], but combinations of the methods were not done. However, combinations of mechanical and electrical stimulations have been used in assessment of evoked brain potentials[55] and to assess the effect of viscero-visceral hyperalgesia[20]. The stomach and other parts of the digestive system have not yet been studied with multimodal stimulations. Although the complicated anatomy, nervous innervation and function of these organs should be taken into account, multimodal models are obviously highly warranted.
Multimodal stimulations in drug research
Experimental models are widely used in research of the effect of analgesics. The differentiated information of the drug effect can be used as “proof-of concept”, dose-efficacy analysis, and for designing further clinical trials. An approach to mimic the clinical situation is the use of multimodal tests, where different receptor types and mechanisms are activated. The multimodal model has clearly shown its value in somatic pain testing, where a single stimulus has been inadequate to test, for example, pathophysiological changes and effects of specific drugs[9]. Hence, differentiated effects could reflect how the drugs can modify different disease mechanisms. In the esophagus, Sarkar et al[56] recently used a model where the upper esophagus was stimulated following sensitization of the distal segment with acid. The secondary hyperalgesia in the proximal part was reduced with a prostaglandin inhibitor, demonstrating the preferentially central action of prostaglandins in this model. Recently, we used a multimodal (and multi-tissue) approach to test the effect of opioids. Opioids are widely used in treatment of visceral pain despite the many side effects. Opioids preferentially attenuate nociceptive responses produced by central integration (spinally amplified signals) to tonic activation of unmyelinated fibers[4,5,7]. Therefore, evaluation of the anti-nociceptive effects of opioids may be clearer using slow rates of temperature or tonic pressure. In the viscera typically only one modality (pressure) has been used in the testing of analgesics[58]. However, Staahl et al (unpublished data) recently compared the effects of morphine and placebo on the pain thresholds to multimodal stimulation of the esophagus. A clear effect of morphine in attenuating heat, electrical and slow-ramp pressure stimulations was found (Figure 7). Morphine can definitely attenuate GI pain in the clinical situation and the model, therefore, proved its validity. The model was also used to differentiate between morphine and oxycodone, the latter was believed also to affect κ-opioid receptors thought to be predominant on visceral afferents. In equipotent doses, oxycodone was better than morphine in attenuating visceral pain, whereas there were no differences between the drugs on pain evoked in the muscle and skin[59]. The study thus demonstrated a different pharmacological profile of oxycodone compared to morphine, and therefore oxycodone may be a useful alternative to morphine in the treatment of visceral pain syndromes. We recommend that future studies evaluating analgesics in the GI tract should use a multimodal approach to get the necessary insight into visceral pain mechanisms and the effect of drugs in the gut. This will facilitate the design of subsequent clinical (phase III) studies. Hence, a substitution of the current “trial and error design” with a more mechanisms-based approach will reduce the economic and human burden in the development of new drugs targeted against pain in the GI tract.
Figure 7.
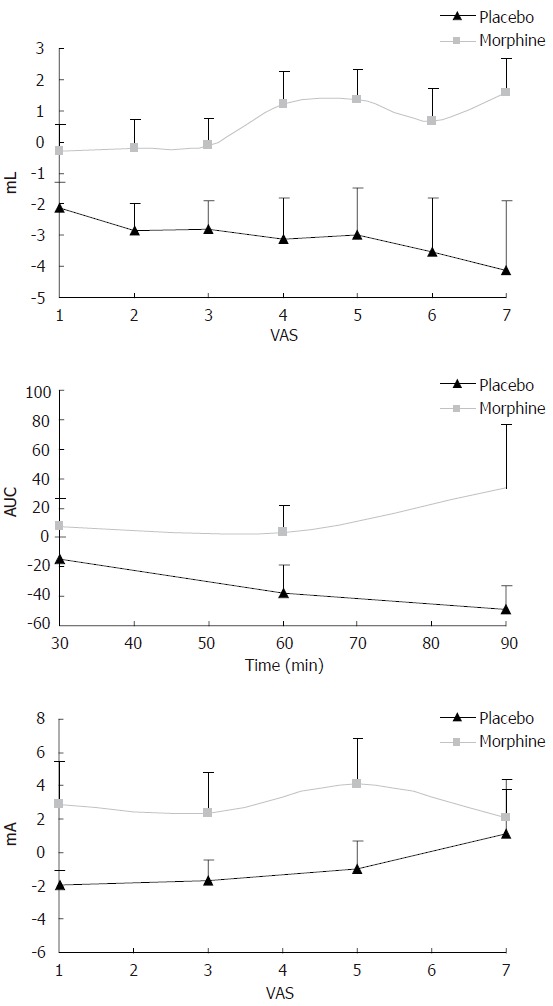
Change in sensory rating compared with baseline recordings for oral morphine and placebo using multimodal stimulation of the esophagus. Top picture: Morphine attenuates mechanically evoked pain better than placebo 60 min after drug intake (P < 0.01). VAS on the X-axis denotes the sensory rating at a visual analogue scale with 5 as the pain threshold. ML at the Y-axis denotes the bag volume. Middle picture: Morphine attenuates heat pain better than placebo (P < 0.05). Here the X-axis illustrates time after drug intake (min) and AUC on the Y-axis denotes the area under the temperature curve used as a proxy for the thermal energy. Bottom picture: Morphine also worked better than placebo (P < 0.05) on electrical stimulation 60 min after drug intake.
CONCLUSION
Multimodal pain stimulation in the human GI tract is a newly developed experimental approach that mimics the clinical pain to a higher degree than previous models. The method has been used to gain more insight into basic peripheral and central pain mechanisms as well as characterizing patients with different diseases of the GI tract. Together with the possibility for pharmacological testing, the models represent a major step forward in the experimental characterization and treatment of patients with gastroenterological diseases.
Footnotes
Supported by “Det Obelske Familiefond” & “Spar Nord Fonden”
S- Editor Wang J E- Editor Bai SH
References
- 1.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000;45:1166–1171. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 2.Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, Shaheen NJ, Sandler RS. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448–1453. doi: 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115–1130. doi: 10.1080/00365520310004399. [DOI] [PubMed] [Google Scholar]
- 4.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 5.Arendt-Nielsen L. Induction and assessment of experimental pain from human skin, muscle, and viscera. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. Proceedings of the 8th World Congress of Pain: Progress in Pain Research and Management. Seattle: ISAP Press; 1997. pp. 393–425. [Google Scholar]
- 6.Graven-Nielsen T, Segerdahl M, Svensson P, Arendt-Nielsen L. Methods for induction and assessment of pain in humans with clinical and pharmacological examples. In: Kruger L, editor. Methods in Pain Research. Boca Raton: CRC Press; 2001. pp. 264–304. [Google Scholar]
- 7.Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Johnson L, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 484–519. [Google Scholar]
- 8.Curatolo M, Petersen-Felix S, Arendt-Nielsen L. Sensory assessment of regional analgesia in humans: a review of methods and applications. Anesthesiology. 2000;93:1517–1530. doi: 10.1097/00000542-200012000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95:97–111. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 10.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 11.Sanger GJ. Hypersensitivity and hyperreactivity in the irritable bowel syndrome: An opportunity for drug discovery. Dig Dis. 1999;17:90–99. doi: 10.1159/000016910. [DOI] [PubMed] [Google Scholar]
- 12.van der Schaar PJ, Lamers CB, Masclee AA. The role of the barostat in human research and clinical practice. Scand J Gastroenterol Suppl. 1999;230:52–63. doi: 10.1080/003655299750025552. [DOI] [PubMed] [Google Scholar]
- 13.Enggaard TP, Poulsen L, Arendt-Nielsen L, Hansen SH, Bjørnsdottir I, Gram LF, Sindrup SH. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92:277–282. doi: 10.1016/s0304-3959(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 14.Gebhart GF, Meller ST, Euchner-Wamser I, Sengupta JN. Modelling visceral pain. In: Vecchiet L, Albe-Fessard D, Lindblom U, Giamberardino MA, editors. New trends in Referred Pain and Hyperalgesia. Amsterdam: Elsevier; 1993. pp. 129–148. [Google Scholar]
- 15.Accarino AM, Azpiroz F, Malagelada JR. Symptomatic responses to stimulation of sensory pathways in the jejunum. Am J Physiol. 1992;263:G673–G677. doi: 10.1152/ajpgi.1992.263.5.G673. [DOI] [PubMed] [Google Scholar]
- 16.Hollerbach S, Hudoba P, Fitzpatrick D, Hunt R, Upton AR, Tougas G. Cortical evoked responses following esophageal balloon distension and electrical stimulation in healthy volunteers. Dig Dis Sci. 1998;43:2558–2566. doi: 10.1023/a:1026667123187. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356:1154–1159. doi: 10.1016/S0140-6736(00)02758-6. [DOI] [PubMed] [Google Scholar]
- 18.Drewes AM, Schipper KP, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L. Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastrointest Liver Physiol. 2002;283:G95–G103. doi: 10.1152/ajpgi.00496.2001. [DOI] [PubMed] [Google Scholar]
- 19.Drewes AM, Schipper KP, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L. Multi-modal induction and assessment of allodynia and hyperalgesia in the human oesophagus. Eur J Pain. 2003;7:539–549. doi: 10.1016/s1090-3801(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 20.Frøkjaer JB, Andersen SD, Gale J, Arendt-Nielsen L, Gregersen H, Drewes AM. An experimental study of viscero-visceral hyperalgesia using an ultrasound-based multimodal sensory testing approach. Pain. 2005;119:191–200. doi: 10.1016/j.pain.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- 22.Drewes AM, Reddy H, Staahl C, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Lundbye-Christensen S. Statistical modeling of the response characteristics of mechanosensitive stimuli in the human esophagus. J Pain. 2005;6:455–462. doi: 10.1016/j.jpain.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Hertz AF. The sensibility of the alimentary tract in health and disease. Lancet. 1911;1:1051–1056. [Google Scholar]
- 24.Stürup GK. Visceral Pain. London: HK Lewis and Co. Ltd; 1940. [Google Scholar]
- 25.Wolf S, Wolff HG. Pain arising from the stomach and mechanisms underlying gastric symptoms. Assoc Res Nerv Men Dis. 1943;43:289–301. [Google Scholar]
- 26.Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol. 2005;11:4367–4374. doi: 10.3748/wjg.v11.i28.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen J, Reddy H, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Differences between male and female responses to painful thermal and mechanical stimulation of the human esophagus. Dig Dis Sci. 2004;49:1065–1074. doi: 10.1023/b:ddas.0000037789.25734.06. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen J, Reddy H, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Cold and heat pain assessment of the human oesophagus after experimental sensitisation with acid. Pain. 2004;110:393–399. doi: 10.1016/j.pain.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Villanova N, Azpiroz F, Malagelada JR. Perception and gut reflexes induced by stimulation of gastrointestinal thermoreceptors in humans. J Physiol. 1997;502(Pt 1):215–222. doi: 10.1111/j.1469-7793.1997.215bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caterina MJ. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- 31.Drewes AM, Reddy H, Pedersen J, Funch-Jensen P, Gregersen H, Arendt-Nielsen L. Multimodal pain stimulations in patients with grade B oesophagitis. Gut. 2006;55:926–932. doi: 10.1136/gut.2005.067769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Drewes AM, Rössel P, Le Pera D, Arendt-Nielsen L, Valeriani M. Dipolar source modelling of brain potentials evoked by painful electrical stimulation of the human sigmoid colon. Neurosci Lett. 2004;358:45–48. doi: 10.1016/j.neulet.2003.12.101. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta JN, Gebhart GF. Mechanosensitive afferent fibers in the gastrointestinal and lower urinary tracts. In: Gebhart GF, editor. Visceral Pain. Progress in Pain Research and Management. Seattle: IASP Press; 1995. pp. 75–98. [Google Scholar]
- 35.Costa M, Brookes SH, Zagorodnyuk V. How many kinds of visceral afferents. Gut. 2004;53 Suppl 2:ii1–ii4. doi: 10.1136/gut.2003.033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobson AR, Sarkar S, Furlong PL, Thompson DG, Aziz Q. A cortical evoked potential study of afferents mediating human esophageal sensation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G139–G147. doi: 10.1152/ajpgi.2000.279.1.G139. [DOI] [PubMed] [Google Scholar]
- 37.Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 38.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil. 1996;8:277–297. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 39.Barlow JD, Gregersen H, Thompson DG. Identification of the biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2002;282:G683–G689. doi: 10.1152/ajpgi.00134.2001. [DOI] [PubMed] [Google Scholar]
- 40.Drewes AM, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H. Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol. 2003;38:27–35. [PubMed] [Google Scholar]
- 41.Drewes AM, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol. 2006;41:640–649. doi: 10.1080/00365520500442559. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen J, Gao C, Egekvist H, Bjerring P, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain and biomechanical responses to distention of the duodenum in patients with systemic sclerosis. Gastroenterology. 2003;124:1230–1239. doi: 10.1016/s0016-5085(03)00265-8. [DOI] [PubMed] [Google Scholar]
- 43.Arendt-Nielsen L, Drewes AM, Hansen JB, Tage-Jensen U. Gut pain reactions in man: an experimental investigation using short and long duration transmucosal electrical stimulation. Pain. 1997;69:255–262. doi: 10.1016/S0304-3959(96)03244-7. [DOI] [PubMed] [Google Scholar]
- 44.Drewes AM, Petersen P, Qvist P, Nielsen J, Arendt-Nielsen L. An experimental pain model based on electric stimulations of the colon mucosa. Scand J Gastroenterol. 1999;34:765–771. doi: 10.1080/003655299750025688. [DOI] [PubMed] [Google Scholar]
- 45.Arendt-Nielsen L, Laursen RJ, Drewes AM. Referred pain as an indicator for neural plasticity. Prog Brain Res. 2000;129:343–356. doi: 10.1016/s0079-6123(00)29026-2. [DOI] [PubMed] [Google Scholar]
- 46.Reddy H, Arendt-Nielsen L, Staahl C, Pedersen J, Funch-Jensen P, Gregersen H, Drewes AM. Gender differences in pain and biomechanical responses after acid sensitization of the human esophagus. Dig Dis Sci. 2005;50:2050–2058. doi: 10.1007/s10620-005-3006-x. [DOI] [PubMed] [Google Scholar]
- 47.Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur J Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1196–G1202. doi: 10.1152/ajpgi.2001.281.5.G1196. [DOI] [PubMed] [Google Scholar]
- 49.Drewes AM, Rössel P, Le Pera D, Arendt-Nielsen L, Valeriani M. Cortical neuroplastic changes to painful colon stimulation in patients with irritable bowel syndrome. Neurosci Lett. 2005;375:157–161. doi: 10.1016/j.neulet.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 51.Arendt-Nielsen L, Bajaj P, Drewes AM. Visceral pain: gender differences in response to experimental and clinical pain. Eur J Pain. 2004;8:465–472. doi: 10.1016/j.ejpain.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 53.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380; discussion 435-513. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 54.Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1512–G1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 55.Hobday DI, Hobson A, Furlong PL, Thompson DG, Aziz Q. Comparison of cortical potentials evoked by mechanical and electrical stimulation of the rectum. Neurogastroenterol Motil. 2000;12:547–554. doi: 10.1046/j.1365-2982.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar S, Hobson AR, Hughes A, Growcott J, Woolf CJ, Thompson DG, Aziz Q. The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology. 2003;124:18–25. doi: 10.1053/gast.2003.50022. [DOI] [PubMed] [Google Scholar]
- 57.Gracely RH. Pain measurement. Acta Anaesthesiol Scand. 1999;43:897–908. doi: 10.1034/j.1399-6576.1999.430907.x. [DOI] [PubMed] [Google Scholar]
- 58.Kuiken SD, Tytgat GN, Boeckxstaens GE. Review article: drugs interfering with visceral sensitivity for the treatment of functional gastrointestinal disorders--the clinical evidence. Aliment Pharmacol Ther. 2005;21:633–651. doi: 10.1111/j.1365-2036.2005.02392.x. [DOI] [PubMed] [Google Scholar]
- 59.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. Oxycodone shows superior effect in visceral pain compared to morphine in a multi-modal, tissue differentiated experimental pain model. Pain. 2006:In press. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Staahl C, Reddy H, Andersen SD, Arendt-Nielsen L, Drewes AM. Multi-modal and tissue-differentiated experimental pain assessment: reproducibility of a new concept for assessment of analgesics. Basic Clin Pharmacol Toxicol. 2006;98:201–211. doi: 10.1111/j.1742-7843.2006.pto_211.x. [DOI] [PubMed] [Google Scholar]
- 61.Frøkjaer JB, Andersen SD, Lundbye-Christensen S, Funch-Jensen P, Drewes AM, Gregersen H. Sensation and distribution of stress and deformation in the human oesophagus. Neurogastroenterol Motil. 2006;18:104–114. doi: 10.1111/j.1365-2982.2005.00734.x. [DOI] [PubMed] [Google Scholar]


