Abstract
AIM: Minimal deviation carcinoma of the uterine cervix, otherwise known as extremely well-differentiated adenocarcinoma (EWDA), is characterized by its benign microscopic appearance in contrast to its aggressive behavior. In order to elucidate the clinicopathological features and biological behavior of the gastric counterpart of EWDA, we, using immunohistochemistry, analyzed nine lesions for the phenotypic expression, proliferative activity, and the expression of oncogene-associated products.
METHODS: Clinicopathological features, including pre-operative biopsy diagnosis, were reviewed. Using immunohitstochemistry, Ki-67 labeling index and expression of p53 and c-erbB-2 protein in the gastric lesions were detected.
RESULT: Locations in the middle or upper third of the stomach and polypoid macroscopic features are characteristic of EWDA of the stomach. Although 4 of the 9 lesions showed only focal lymphatic or venous invasion, lymph node metastasis was not present and none of the patients died of the lesions (mean follow-up period, 56 mo). All 9 cases of EWDA could be classified into gastric phenotype (5 lesions) and intestinal phenotype (4 lesions). The former resembled gastric foveolar epithelium, mucous neck cells or pyloric glands, but their papillary structures were frequently elongated and the tumor cells and their nuclei were slightly larger and more hyperchromatic compared to normal epithelium. The latter resembled intestinal metaplasia with minimal nulcear atypia and irregular glands; two of these lesions demonstrated complete intestinal phenotype, while two demonstrated incomplete intestinal phenotype. Ki-67 labeling index was low and none of the cases revealed over-expression of p53 and c-erbB-2 protein.
CONCLUSION: Unlike minimal deviation carcinoma of the cervix, these findings suggest that EWDA of the stomach is a lesion of low-grade malignancy. This favorable biological behavior is supported by the data of a low Ki-67 labeling index and a lack of p53 or c-erbB-2 protein over-expression. Because of its resemblance to normal gastric mucosa or mucosa with intestinal metaplasia, EWDA is often misdiagnosed. To prevent the misdiagnosis of such lesions, the clinical and pathologic characteristics should be taken into consideration.
Keywords: Stomach neoplasms, Extremely well-differentiated adenocarcinoma, Ki-67, p53, c-erbB-2
INTRODUCTION
Silverberg and Hurt proposed the term “minimal deviation carcinoma” for extremely well-differentiated adenocarcinoma (EWDA) of the uterine cervix[1], which has a benign microscopic appearance yet shows an aggressive behavior[1-3]. This carcinoma is characterized by mucinous glands which resemble normal endocervical glands but invade the cervical stroma. Several similar cases of adenocarcinomas which show deceptively benign appearance have been reported in the stomach[4-12]. In those reports, the difficulty with histological diagnosis based on biopsy specimens is well discussed in detail; however, the biological behavior of the lesions still remains unclear.
Based on Lauren classification[13], gastric carcinomas have been classified into two types, intestinal-type and diffuse-type. Following recent advances in mucin histochemistry and immunohistochemistry, it has been clarified that differentiated adenocarcinoma can be classified into two subtypes, these being gastric and intestinal phenotypes[14-20]. With regard to EWDA of the stomach, Endoh et al[11] reported eight cases of EWDA mimicking complete-type intestinal metaplasia, confirmed by phenotypic investigation using immunohistochemical methods. Most reported cases of EWDA of the stomach seem to be intestinal-type carcinomas, resembling complete or incomplete intestinal metaplasia[4-10]. In addition, we have encountered a few reports of cases of EWDA mimicking normal gastric mucosa, where the cases were considered to be lesions of the gastric phenotype. However, in these cases, there was very little objective investigation of the phenotypic expression.
In order to elucidate the characteristics of EWDA including its biological behavior, we describe herein the clinicopathological features of nine cases, including phenotypic expression, proliferative activity and expression of some oncogene-associated products.
MATERIALS AND METHODS
Patients
EWDA is defined as neoplastic lesions composed of highly differentiated neoplastic epithelium which mimicks the normal gastric mucosa or intestinal metaplastic mucosa with mild nuclear atypia, but has the ability to invade the gastric wall. We retrospectively reviewed 3 106 cases from our old consecutive files that had been diagnosed as well-differentiated adenocarcinoma of the stomach, and found three (0.1%) cases of EWDA. Other 6 collected cases of EWDA were added, making a total of 9 cases for this study. One of the reported cases[7] was included in this study. Although we encountered some similar lesions restricted to the mucosa, these were excluded because of difficulty in diagnosing them as malignant.
The clinicopathological findings were principally based on the General Rules for Gastric Cancer Study as outlined by the Japanese Research Society for Gastric Cancer[21]. Eight specimens were obtained by surgery, and one was endoscopically resected. The resected specimens were fixed in 100 mL/L buffered formalin. The early lesions were cut many times throughout the entire tumors, whereas the advanced lesions were cut only once through their center. The sections were then embedded in paraffin. Then 4-μm thick sections were routinely stained with hematoxylin and eosin stain (H&E). In addition, pre-operative biopsy specimens were also reviewed.
Immunohistochemistry
The monoclonal antibodies against human gastric mucin (45M1, Novocastra, Newcastle-upon-Tyne, UK, diluted 1:50) as a marker for gastric foveolar cells[22,23], MUC6 (Novocastra, Newcastle-upon-Tyne, UK, diluted 1:200) as a marker of gastric mucous neck cells and pyloric glands[24,25], MUC2 (Novocastra, Newcastle-upon-Tyne, UK, diluted 1:200) as a marker for intestinal goblet cells[26-28], CD10 (Novocastra, Newcastle-upon-Tyne, UK, diluted 1:200) as a marker for the small intestinal brush border[29-31], Ki-67 (MIB-1, dilution 1:100; Immunotech, Marseille, France), p53 (PAb 1801, dilution 1:100; Oncogene Research Products, Cambridge, Massachusetts, USA) and c-erbB-2 (dilution 1:200; Nichirei, Tokyo) were used. Immunohistochemical staining was carried out using streptavidin-biotin-peroxidase complex method (Histofine SAB-PO Kit, Nichirei, Tokyo, Japan) following antigen retrieval with microwave heating (citrate buffer, 30 min; phosphate-buffered saline, 10 min, respectively) utilizing an H2800 Microwave Processor (Energy Beam Sciences, Agawan, Massachusetts, USA) at 800 W. Sections were visualized with diaminobenzidine (DAB) and counterstained with methyl-green or hematoxylin. The negative controls consisted of substituting mouse normal serum for the primary antibodies.
Evaluation
The positivities of human gastric mucin (HGM), MUC6, MUC2 and CD10 were estimated as being significantly positive when more than 10% of the area was positive-stained. According to the combination of their expression, phenotypes were classified into four types: gastric type (G-type), incomplete intestinal type (Incomp. I-type), complete intestinal type (Comp. I-type), and unclassified type (Table 1)20).
Table 1.
Phenotypic classification by immunohistochemical stains
| Human gastric mucin or MUC6 | ||||||
| (-) | (+) | |||||
| (+) | C - type | |||||
| D10 | ||||||
| (-) | (+) | |||||
| MUC2 | I - type | |||||
| (-) | ||||||
| U - type | G - type | |||||
C: complete intestinal, I: incomplete intestinal, G: gastric, U: unclassified.
The Ki-67 (MIB-1) labeling index (LI) was defined as a percentage of MIB-1-positive nuclei, and was evaluated in the invasive areas. The MIB-1 LI was determined by counting at least 1 000 nuclei in the selected fields at x400 magnification. p53 immunoreactivity was defined as positive when distinct nuclear staining was recognized in at least 10% of the cells, since most of the previously published studies employed this as the cut-off level. Cases with less than 10% positive cells were regarded as negative. c-erbB-2 was regarded as positive when there was membranous staining in more than 10% of the area of the tumor.
RESULTS
Histologic findings and phenotypic expression
All the EWDA had invaded the submucosa or even deeper; four were restricted to the submucosa, two had invaded the muscularis propria, and three had reached the subserosa beyond the muscularis propria. The EWDAs were classified into gastric phenotype (HGM+ or MUC6+/MUC2-/CD10-) containing 5 cases and intestinal phenotype containing 4 cases. The intestinal phenotype cases were further classified into complete intestinal phenotype (HGM- / MUC6- / MUC2 + / CD10+) and incomplete intestinal phenotype (HGM- / MUC6- / MUC2 + / CD10-), each phenotype contained 2 cases. With regard to MUC6 expression which indicates differentiation to the pyloric glands, MUC6 expression was only detected in 2 of 5 cases of the gastric phenotype.
The five lesions classified as gastric phenotype were composed of well-differentiated epithelium mimicking foveolar epithelium, mucous neck cells or pyloric glands with abundant clear cytoplasm and basally situated nuclei. With careful observation, the nuclei were seen to be slightly larger than those of normal gastric mucosa, and to be markedly hyperchromatic. The superficial area tended to resemble the foveolar epithelium while the deep area tended to resemble mucous neck cells or pyloric glands. Two lesions mainly showed remarkable papillary proliferation. The epithelium was lined with a single layer of columnar cells with abundant clear cytoplasm with basally situated nuclei. The glands in this phenotype showed intraluminal papillary projections with or without a fibrous core. In the invasive area, one of five revealed marked desmoplastic reaction, however, other four revealed only slight desmoplastic reaction. One of the cases of gastric-type EWDA is shown in Figure 1.
Figure 1.

Extremely well-differentiated adenocarcinoma of the stomach, gastric-type (case 4). A: Macroscopic view showing a polypoid lesion with an irregular surface; B: cancer invasion of the whole thickness of the gastric wall (low-power view); C: carcinoma mimicking the normal gastric foveolar epithelium with basally located small nuclei (hyperchromatic nuclei) and abundant mucin; D: papillary projections occasionally seen in the carcinomatous glands; E: diffuse positive staining of human gastric mucin in carcinomatous glands; and F: focally positive staining of MUC6 in carcinomatous glands.
The four lesions classified as intestinal phenotype were composed of intestinal-type glands with various amounts of goblet cells and Paneth cells, focally showing an irregular shape. Brush border-like structures were occasionally seen, and were confirmed by CD10 staining in the two cases classified as complete intestinal phenotype. We found difficulty in diagnosing these lesions as neoplastic in the mucosa because their glands were somewhat regular in shape and their cytologic atypia was minimal. However, their glands were of varying sizes and showed irregular branching in the deep portion of the mucosa and the submucosa. The glands in the submucosa or proper muscle layer were surrounded by an acute, chronic inflammatory infiltrate with lymphoid follicles. Occasionally, cystically dilated gland was seen in the submucosa, and mucous which had partially leaked out into the stroma owing to destruction of the glands, was seen in these three lesions. In the invasive area, all the four cases revealed marked desmoplastic reaction. Two cases of intestinal-type EWDA, early and advanced, are shown in Figures 2 and 3.
Figure 2.
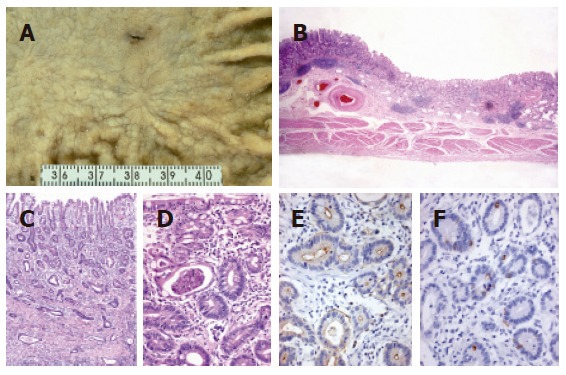
An early lesion of extremely well-differentiated adenocarcinoma of the stomach, complete intestinal-type (case 8). A: Macroscopic view showing a shallow depressed lesion with an irregular margin; B: carcinoma invasion to the submucosal layer (low-power view); C and D: carcinoma mimicking the intestinal metaplasia of complete-type with basally located small nuclei, eosinophilic cytoplasm and scattered goblet cells. Note the irregular arrangement of glands and intraluminal debris; E: CD10 positivity of carcinomatous glands along the luminal surfaces; and F: MUC2 positivity of scattered goblet cells.
Figure 3.

An advanced lesion of extremely well-differentiated adenocarcinoma of the stomach, complete intestinal-type (case 9). A: Macroscopic view showing a polypoid mass with an irregular surface, but unclear margin; B: cancer invasion of the whole thickness of the gastric wall (low-power view); C: carcinomatous gland infiltrating into the submucosa; D: carcinoma mimicking the intestinal metaplasia of complete-type with basally located small nuclei, eosinophilic cytoplasm and scattered goblet cells.
Regarding the background mucosa of the tumors, the surrounding mucosa could not be examined in one gastric phenotypic lesion because the lesion had been endoscopically resected. In another gastric phenotypic lesion, no intestinal metaplasia was seen. As for the other seven cases, various degrees of intestinal metaplasia were seen in the surrounding mucosa of both the gastric and intestinal phenotypes.
Patient characteristics
The clinicopathological findings of the nine patients with EWDA of the stomach are summarized in Table 2. The patients included eight men and one woman with ages ranged from 45 to 81 (average 62) years. There were no patients who were diagnosed as Peutz-Jeghers syndrome. None of the patients died or suffered recurrence during the follow-up periods which ranged from 5 to 136 (average, 56) mo.
Table 2.
Clinicopathological data
| Case | Age | Sex | Loc | Size | Macro | Depth | ly | v | LN | Prognosis | Biopsy |
| Gastric phenotype | |||||||||||
| 1 | 81 | m | M | 5.5 | 0-I | sm | (+) | (-) | NA | 5 mo, alive | benign |
| 2 | 51 | m | U | 2.5 | 0-IIa | sm | (-) | (-) | (-) | 23 mo, alive | NA |
| 3 | 63 | m | M | 8 | 1 | ss | (-) | (-) | (-) | 66 mo, alive | benign |
| 4 | 76 | m | U | 3.5 | 1 | ss | (-) | (+) | (-) | 30 mo, alive | Ca, susp |
| 5 | 57 | m | U | 5 | 1 | ss | (+) | (-) | (-) | 48 mo, alive | NA |
| Intestinal phenotype (incomplete intestinal-type) | |||||||||||
| 6 | 65 | f | M | 1.5 | 0-IIa | sm | (+) | (-) | (-) | 38 mo, alive | Ca, susp |
| 7 | 45 | m | M | 2.4 | 1 | mp | (-) | (-) | (-) | 129 mo, alive | Ca, susp |
| (complete intestinal-type) | |||||||||||
| 8 | 54 | m | M | 2.2 | 0-IIc | sm | (-) | (-) | (-) | 136 mo, alive | Ca, susp |
| 9 | 65 | m | M | 4 | 1 | mp | (+) | (+) | (-) | 30 mo, alive | Ca, susp |
Loc: location (U: upper third, M: middle third), Macro: macroscopic feature, Depth: depth of invasion (sm: submucosa, mp: muscularis propria, ss: subserosa), ly: lymphatic permeation, v: venous invasion, LN: lymph node metastasis, NA: not assessed, Ca, susp: carcinoma, suspected.
Macroscopic findings
The tumors had a maximum diameter of 1.5 to 8 (average, 3.6) cm. Of the nine lesions, three tumors were located in the upper third of the stomach, the remaining six were located in the middle third. No lesions were present in the lower third. Among the four early lesions (restricted to the submucosa), two lesions were of superficial elevated (Type 0-IIa) type while the others were of superficial depressed (Type 0-IIc) type or protruding (type 0-I) type. All the advanced lesions (invading the muscularis mucosa and/or the subserosa) were of polypoid type (Type 1).
Pre-operative biopsy
Pre-operative biopsy specimens could be evaluated only in seven cases because of unavailability of specimens in two cases. Two of the seven cases were diagnosed as benign lesions, and the remaining five were initially suspected as being carcinomas, although there was difficulty in distinguishing whether they were neoplastic or regenerative lesions. Only one lesion could be finally diagnosed as a definite carcinoma through repeated biopsy (Case 9).
Ki-67, p53 and c-erbB-2 expressions
None of the cases of EWDA revealed over-expression of p53 or c-erbB-2. Regarding the proliferating activity, the mean value of Ki-67 LI of the EWDAs was 8.7% (range, 0.5%-23.9%).
DISCUSSION
Gastric carcinomas, based on Lauren classification, have been divided into two histologic types by standard hematoxylin and eosin (H&E) staining, such as “intestinal” and “diffuse” types[13]. It has been considered that intestinal-type carcinoma is almost equivalent to differentiated type carcinoma and that diffuse-type carcinoma is almost equal to gastric or undifferentiated type carcinoma. However, gastric carcinomas are currently classified according to the expression of gastric or intestinal phenotypes, using immunohistochemical or mucin-histochemical methods[14,18]. Accordingly, we divided gastric carcinomas into three phenotypes (complete-intestinal type, incomplete-intestinal type and gastric type) according to the type of intestinal metaplasia, as suggested in previous studies[19,20], using immunohistochemical methods for CD10 (CALLA) which is considered to be expressed by the brush border of the small intestine[29,31], MUC2 which is considered to be expressed by intestinal goblet cells[26,28] and human gastric mucin (HGM) which is considered to be expressed by the gastric foveolar epithelial mucin [22,23]. Some authors have also reported that the phenotypic expression is related to the tumor growth pattern and aggressiveness[32,33], and that this classification is clearly in a good accordance with that of the background mucosa[19]. In this study, the phenotype of EWDA of the stomach was investigated using not only these three antibodies, but also MUC6 as a marker of gastric mucous neck cells and pyloric glands[24,25]. Our nine cases of EWDA could be classified into three phenotypes (complete-intestinal type, incomplete-intestinal type and gastric type).
Clinicopathologically, EWDA of the stomach had several characteristic features, in comparison with the previously reported cases of EWDA listed in Table 3[4-6,8-12]. The location and macroscopic features of the tumors are characteristic. Usually, more than 40% of the gastric carcinomas are located in the distal part of the stomach and polypoid type is rare (3.3%) among advanced gastric carcinomas, as reported by our previous study [34]. All EWDA in our study and most (13/15) ones of the previous reports were located in the middle and upper stomach. Macroscopically, the early lesions of EWDA were flatly elevated (0-IIa) or depressed (0-IIc), while all the advanced lesions were polypoid. The same tendency was also seen in the previous reports. This finding implies that the EWDA arises as a flat lesion but latter grows into a polypoid mass due to massive infiltration of carcinoma cells beneath the mucosa.
Table 3.
Previously reported cases of gastric EWDA
| Case | Author | Age | Sex | Macro | Location | Size | Depth | ly | v | n | Prognosis | Phenotype | Biopsy | |
| 1 | Araki | (1984) | 50 | m | 1 | M | 45 | ss | 1 | 0 | 0 | ? | Incomp-I? | benign |
| 2 | Satoh | (1987) | 65 | m | 1 | U | 40 | mp | 0 | 0 | 0 | ? | Comp-I? | benign |
| 3 | Yaosaka | (1989) | 53 | m | 1 | M | 80 | mp | 2 | 0 | 1 | ? | Comp-I? | benign |
| 4 | Matsunaga | (1995) | 42 | m | 0-I | L | 45 | sm | 2 | 0 | 0 | ? | ? | reg. Atypia |
| 5 | Kobayashi | (1999) | 55 | m | 1 | M | 20 | ss | 0 | 0 | 0 | ? | Comp-I | Ca, susp |
| 6 | Endoh | (1999) | 60 | f | 0-IIa+IIc | M | 10 | sm | 0 | 0 | 0 | ? | Comp-I | Ca |
| 7 | Endoh | (1999) | 68 | f | 0-IIc+IIb | M | 20 | sm | 0 | 0 | 0 | ? | Comp-I | Ca |
| 8 | Endoh | (1999) | 70 | m | 0-IIb | M | 27 | sm | 0 | 0 | 0 | ? | Comp-I | NA |
| 9 | Endoh | (1999) | 62 | f | 0-IIc+IIa | M | 15 | sm | 0 | 0 | 0 | ? | Comp-I | NA |
| 10 | Endoh | (1999) | 59 | m | 0-IIa | M | 15 | sm | 0 | 0 | 0 | ? | Comp-I | Ca |
| 11 | Endoh | (1999) | 74 | m | 0-IIa+IIc | L | 18 | sm | 0 | 0 | 0 | ? | Comp-I | NA |
| 12 | Endoh | (1999) | 70 | m | 0-IIa+IIc | M | 25 | sm | 0 | 0 | 0 | ? | Comp-I | benign |
| 13 | Endoh | (1999) | 65 | m | 1 | M | 55 | se | 0 | 0 | 0 | ? | Comp-I | benign |
| 14 | Adachi | (2000) | 54 | f | 0-I | M | 40 | sm | ? | 1 | 0 | ? | I? | benign |
| 15 | Sato | (2004) | 50 | m | 2 | M | 48 | ss | 2 | 1 | 0 | ? | Mixed | benign |
Incomp-I: incomplete intestinal, Comp-I: complete intestinal, I: intestinal, reg. atypia: regenerative atypia (benign), NA: not assessed, Ca: carcinoma, Ca, susp: carcinoma, suspected, Macro: macroscopic type Location (U: upper third, M: middle third, L: lower third) Depth: depth of invastion (sm: submucosa, mp: muscularis propria, ss: subserosa).
The gastric phenotype of gastric EWDA is more likely to be confused with normal gastric mucosa or hyperplastic polyps, whereas the intestinal phenotype of gastric EWDA is more likely to be confused with intestinal metaplastic epithelium. The high degree of differentiation and mild cellular atypia of these lesions result in frequent diagnostic difficulties especially with regard to biopsy specimens prior to surgery. In fact, pre-operative biopsies were negative in eight of 12 cases in previous reports and in two of our current seven cases. These highly differentiated lesions of the stomach have received relatively limited attention. Although EWDA of the stomach is very rare, it is important to take it into consideration when making a differential diagnosis of neoplastic or dysplastic lesions in the stomach.
The histological features of EWDA with regard to pre-operative biopsy specimens and surgical specimens were retrospectively reviewed. The most useful histological feature in diagnosing the intestinal phenotype of EWDA is the irregularity of the tubules in the deep portion of the mucosa and the submucosa. It is therefore important to obtain biopsy specimens from these areas. Moreover, endoscopic mucosal resection by means of which we can examine the entire thickness of the mucosal layer may be a suitable diagnostic procedure in diagnosing intestinal phenotypic lesions. In the cases of gastric phenotype of EWDA, the neoplastic epithelium resembled gastric surface mucous cells or pyloric glands. Their papillary structure was similar to that of normal foveolar epithelium and hyperplastic polyps, although some of them strikingly elongated. In addition, the individual cells and nuclei were obviously larger and their nuclei were more hyperchromatic than those in normal foveolar epithelium. Since the cellular atypism is minimal, it is important to compare their size and the amount of chromatin with that in the surrounding normal epithelium.
There have been no reports about the biological behavior of EWDA, although a low incidence of lymphovascular invasion and lymph node metastasis has been noted (Table 3). As for our cases, all the patients are currently alive. Three lesions revealed only focal venous or lymphatic invasion, but no lymph node metastasis was seen in our cases of EWDA. These findings suggest a favorable prognosis for EWDA of the stomach unlike the prognosis for minimal deviation adenocarcinoma of the uterine cervix, although it needs to be noted that our series was small with limited follow-up data. With regard to the correlation between phenotypes and clinicopathological features, there was no significant difference between the two except for tumor location. Three of the five EWDAs of gastric phenotype were located in upper third of the stomach, whereas all the EWDAs of intestinal phenotype were located in the middle third.
The proliferative compartment in normal gastric mucosa is known to be restricted to the middle layer of the mucosa. Several reports have indicated the relationship between a high Ki-67 LI and poor prognosis in cases of gastric carcinoma[35,36]. In our present study, the Ki-67 LI was lower (average, 8.7%) compared with the previously reported data(from 41.8% to 47.1%)[35-38]. A low Ki-67 LI (13%) in the submucosal invasive area has also been reported by Endoh et al[11], and this finding reflects the slow growth and reduced aggressiveness of EWDA of the stomach.
The reported prevalences of abnormal expression of p53 and c-erbB-2 protein have been shown to range from 47% to 60%[39-42] and from 5.7% to 33.0%[43-46], respectively. It has been reported that the over-expression of p53[37-39] and c-erbB-2 protein[43] is a marker of poor prognosis in gastric carcinoma. Fortunately, none of the lesions of EWDA showed over-expression of p53 or c-erbB-2 in our study. It seems reasonable to regard these lesions as having a low-grade malignancy, but an ability to invade downward into the gastric wall, a finding which is supported by the data of low Ki-67 LI and no over-expression of p53 or c-erbB-2. In addition, these immunoreactivities of p53 and c-erbB-2 seemed to be useless for the diagnosis of EWDA.
In the practical diagnosis of a stomach biopsy, it is important to bear in mind the existence of extremely well-differentiated adenocarcinoma (EWDA) of both intestinal and gastric types.
ACKNOWLEDGMENTS
We thank Miss Katherine Miller (Royal English Language Centre, Fukuoka, Japan) for proof-reading of the manuscript.
Footnotes
Supported by Grants-in-aid for Cancer Research from the Ministry of Education, Science and Culture, Japan and the Fukuoka Cancer Society, Fukuoka, Japan
S- Editor Guo SY L- Editor Kumar M E- Editor Bi L
References
- 1.Silverberg SG, Hurt WG. Minimal deviation adenocarcinoma ("adenoma malignum") of the cervix: a reappraisal. Am J Obstet Gynecol. 1975;121:971–975. doi: 10.1016/0002-9378(75)90920-5. [DOI] [PubMed] [Google Scholar]
- 2.Gilks CB, Young RH, Aguirre P, DeLellis RA, Scully RE. Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases. Am J Surg Pathol. 1989;13:717–729. doi: 10.1097/00000478-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kaku T, Toyoshima S, Enjoji M. Tuberous sclerosis with pulmonary and lymph node involvement. Relationship to lymphangiomyomatosis. Acta Pathol Jpn. 1983;33:395–401. doi: 10.1111/j.1440-1827.1983.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 4.Araki K, Okajima K, Nakata K, Kurokawa A. A case of gastric cancer with unusual histologic findings. Pathol Clin Med. 1984;2:1366–1371. [Google Scholar]
- 5.Satoh O, Kamata M. Highly differentiated adenocarcinoma of the stomach: Report of a case with difficulty in pathologic diagnosis on biopsy. Stomach and Intestine. 1987;22:211–218. [Google Scholar]
- 6.Yaosaka T, Suga T, Murashima Y. Extremely well differentiated adenocarcinoma of the stomach causing difficulty in preoperative diagnosis, report of a case. Stomach and Intestine. 1989;24:81–87. [Google Scholar]
- 7.Ueyama T, Akahoshi K, Hashimoto H. An extremely well differentiated adeno-carcinoma. A case report. Jpn J Cancer Clin. 1991;37:1104–1108. [Google Scholar]
- 8.Matsunaga M, Makuuchi H, Otami Y. An extremely well differentiated adenocarcinoma of the stomach preoperatively diagnosed as a submucosal tumor, report of a case. Stomach and Intestine. 1995;30:827–832. [Google Scholar]
- 9.Kobayashi M, Honma T, Iwafuchi M. Advanced gastric carcinoma with submucosal growth and low-grade histologic atypia with difficulty in pathological diagnosis by biopsy specimens, report of a case. Stomach and Intestine. 1999;34:1531–1535. [Google Scholar]
- 10.Adachi K, Katsube T, Ishihara S. Highly well-differentiated early gastric cancer causing difficulty in preoperative diagnosis, report of a case. Stomach and Intestine. 2000;35:677–682. [Google Scholar]
- 11.Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H. Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol. 1999;30:826–832. doi: 10.1016/s0046-8177(99)90144-2. [DOI] [PubMed] [Google Scholar]
- 12.Sato R, Ohta T, Murakami M. Extremely well differentiated adenocarcinoma of the stromach mimicking a submucosal tumor, report of a case. Stomach and Intestine. 2004;39:833–840. [Google Scholar]
- 13.LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Tatematsu M, Ichinose M, Miki K, Hasegawa R, Kato T, Ito N. Gastric and intestinal phenotypic expression of human stomach cancers as revealed by pepsinogen immunohistochemistry and mucin histochemistry. Acta Pathol Jpn. 1990;40:494–504. doi: 10.1111/j.1440-1827.1990.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimoda T, Fujisaki J, Kashimura H. Histological type of gastric carcinoma in relationship to the mode of intramucosal spreading of cancer cells. Stomach and Intestine. 1991;26:1125–1134. [Google Scholar]
- 16.Egashira Y. [Mucin histochemical study of differentiated adenocarcinoma of stomach] Nihon Shokakibyo Gakkai Zasshi. 1994;91:839–848. [PubMed] [Google Scholar]
- 17.Sasaki I, Yao T, Nawata H, Tsuneyoshi M. Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumor development. Cancer. 1999;85:1719–1729. [PubMed] [Google Scholar]
- 18.Matsui N, Yao T, Akazawa K, Nawata H, Tsuneyoshi M. Different characteristics of carcinoma in the gastric remnant: histochemical and immunohistochemical studies. Oncol Rep. 2001;8:17–26. [PubMed] [Google Scholar]
- 19.Yao T, Kabashima A, Kouzuki T, Oya M, Tsuneyoshi M. The phenotypes of the gastric carcinoma - Evaluation by a new immunohistochemical method. Stomach and Intestine. 1999;34:477–485. [Google Scholar]
- 20.Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology. 2000;37:513–522. doi: 10.1046/j.1365-2559.2000.01008.x. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 22.Bara J, Loisillier F, Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980;41:209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bara J, Gautier R, Mouradian P, Decaens C, Daher N. Oncofetal mucin M1 epitope family: characterization and expression during colonic carcinogenesis. Int J Cancer. 1991;47:304–310. doi: 10.1002/ijc.2910470222. [DOI] [PubMed] [Google Scholar]
- 24.Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Byrd JC, Siddiki B, Kim YS. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 25.Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166–170. doi: 10.1002/ijc.1460. [DOI] [PubMed] [Google Scholar]
- 26.Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- 27.Chang SK, Dohrman AF, Basbaum CB, Ho SB, Tsuda T, Toribara NW, Gum JR, Kim YS. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994;107:28–36. doi: 10.1016/0016-5085(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 28.Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 29.Danielsen EM, Vyas JP, Kenny AJ. A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J. 1980;191:645–648. doi: 10.1042/bj1910645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzgar RS, Borowitz MJ, Jones NH, Dowell BL. Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med. 1981;154:1249–1254. doi: 10.1084/jem.154.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G. Expression of the common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol. 1985;38:1002–1006. doi: 10.1136/jcp.38.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koseki K, Takizawa T, Koike M. Subclassification of well differentiated gastric cancer with reference to biological behavior and malignancy, gastric type vs. intestinal type, and papillary carcinoma vs. tubular carcinoma. Stomach and Intestine. 1999;34:507–512. [Google Scholar]
- 33.Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Relationship between biologic behavior and phenotypic expression in intramucosal gastric carcinomas. Hum Pathol. 2002;33:80–86. doi: 10.1053/hupa.2002.30182. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–1037. doi: 10.1002/1097-0142(19920901)70:5<1030::aid-cncr2820700504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Goishi H, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Predictive value of cathepsin D and Ki-67 expression at the deepest penetration site for lymph node metastases in gastric cancer. Oncol Rep. 2000;7:713–718. doi: 10.3892/or.7.4.713. [DOI] [PubMed] [Google Scholar]
- 36.Kikuyama S, Kubota T, Shimizu K, Miyakita M. Ki-67 antigen expression in relation to clinicopathological variables and prognosis in gastric cancer. Oncol Rep. 1998;5:867–870. doi: 10.3892/or.5.4.867. [DOI] [PubMed] [Google Scholar]
- 37.Koide N, Nishio A, Hiraguri M, Shimada K, Shimozawa N, Hanazaki K, Kajikawa S, Adachi W, Amano J. Cell proliferation, apoptosis and angiogenesis in gastric cancer and its hepatic metastases. Hepatogastroenterology. 2002;49:869–873. [PubMed] [Google Scholar]
- 38.Oya M, Yao T, Tsuneyoshi M. Expressions of cell-cycle regulatory gene products in conventional gastric adenomas: possible immunohistochemical markers of malignant transformation. Hum Pathol. 2000;31:279–287. doi: 10.1016/s0046-8177(00)80239-7. [DOI] [PubMed] [Google Scholar]
- 39.Martin HM, Filipe MI, Morris RW, Lane DP, Silvestre F. p53 expression and prognosis in gastric carcinoma. Int J Cancer. 1992;50:859–862. doi: 10.1002/ijc.2910500604. [DOI] [PubMed] [Google Scholar]
- 40.Starzynska T, Bromley M, Ghosh A, Stern PL. Prognostic significance of p53 overexpression in gastric and colorectal carcinoma. Br J Cancer. 1992;66:558–562. doi: 10.1038/bjc.1992.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakeji Y, Korenaga D, Tsujitani S, Baba H, Anai H, Maehara Y, Sugimachi K. Gastric cancer with p53 overexpression has high potential for metastasising to lymph nodes. Br J Cancer. 1993;67:589–593. doi: 10.1038/bjc.1993.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiao YH, Rugge M, Correa P, Lehmann HP, Scheer WD. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994;144:511–517. [PMC free article] [PubMed] [Google Scholar]
- 43.David L, Seruca R, Nesland JM, Soares P, Sansonetty F, Holm R, Børresen AL, Sobrinho-Simões M. c-erbB-2 expression in primary gastric carcinomas and their metastases. Mod Pathol. 1992;5:384–390. [PubMed] [Google Scholar]
- 44.Ooi A, Kobayashi M, Mai M, Nakanishi I. Amplification of c-erbB-2 in gastric cancer: detection in formalin-fixed, paraffin-embedded tissue by fluorescence in situ hybridization. Lab Invest. 1998;78:345–351. [PubMed] [Google Scholar]
- 45.Dursun A, Poyraz A, Celik B, Akyol G. Expression of c-erbB-2 oncoprotein in gastric carcinoma: correlation with histopathologic characteristics and analysis of Ki-67. Pathol Oncol Res. 1999;5:104–106. doi: 10.1053/paor.1999.0171. [DOI] [PubMed] [Google Scholar]
- 46.Endoh Y, Watanabe H, Hitomi J, Nishikura K. Intestinal-type adenocarcinoma in the fundic gland area of the stomach: Its pathological features. Stomach and Intestine. 1994;29:1009–1023. [Google Scholar]


