Abstract
AIM: To evaluate the anticancer property of the dried latex (DL) of Calotropis procera, a tropical medicinal plant, in the X15-myc transgenic mouse model of hepatocellular carcinoma and to elucidate its mechanism of action in cell culture.
METHODS: The young transgenic mice were orally fed with the aqueous suspension of DL (400 mg/kg for 5 d/wk) for 15 wk and their liver was examined for histopathological changes at 20 wk. Serum levels of vascular endothelial growth factor (VEGF) were also measured in these animals. To characterize the active fraction, DL was extracted with petroleum ether followed by methanol. The methanolic extract was sub-fractionated on a silica gel G column using a combination of non-polar and polar solvents and eleven fractions were obtained. Each fraction was analysed for cytotoxic effect on hepatoma (Huh7) and non-hepatoma (COS-1) cell lines and non-transformed hepatocytes (AML12) using tetrazolium (MTT) assay. Finally, the mechanism of cell death was investigated by measuring the levels of Bcl2, caspase 3 and DNA fragmentation.
RESULTS: DL treatment of mice showed a complete protection against hepatocarcinogenesis. No adverse effect was observed in these animals. The serum VEGF level was significantly lowered in the treated mice as compared to control animals. Cell culture studies revealed that the methanolic extract of DL as well as its fraction 8 induced extensive cell death in both Huh-7 and COS-1 cells while AML12 cells were spared. This was accompanied by extensive fragmentation of DNA in Huh-7 and COS-1 cells. No change in the levels of canonical markers of apoptosis such as Bcl2 and caspase 3 was observed.
CONCLUSION: DL of C. procera has the potential for anti-cancer therapy due to its differentiable targets and non-interference with regular pathway of apoptosis.
Keywords: Calotropis procera, Transgenic mice, Hepatocellular carcinoma, Cytotoxicity, Anticancer agent, Differential killing
INTRODUCTION
The incidence of cancer is increasing worldwide and it is the single most common cause of deaths in both developed and developing countries[1,2]. Irrespective of the cause, the localized malignant tumors are best managed by surgical removal[3,4] while the treatment options for advanced and metastasized tumors include chemotherapy and radiotherapy[5-7]. However, in view of the side effects of drugs used in the chemotherapy of different cancers, traditional herbal medicine and complementary and alternative medicine (CAM) are becoming increasingly popular among cancer patients in the developed countries[8,9]. In the traditional Indian medicinal system, the Ak plant or Calotropis procera (Ait.) R. Br. (Asclepiadaceae) has been used for a variety of disease conditions that includes its use in the treatment of leprosy, ulcers, piles and tumors[10]. The root extract of C. procera has been found to produce a strong cytotoxic effect on COLO 320 tumor cells[11]. Recently, a hemi synthetic derivative of a cardenolide isolated from the root barks of C. procera shows a strong cytotoxic effect on several human cancer lines, a high in vivo tolerance to tumor growth and prolonged survival in the human xenograft models of nude mice[12]. The chloroform-soluble fraction of its roots, ethanolic extract of its flowers and aqueous and organic extracts of its dried latex (DL) also exhibit a strong anti-inflammatory activity in animal models of acute and chronic inflammation[13-15]. Further, recent epidemiological studies have shown that daily intake of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen results in a significant exponential decline in the risk of different clinical cancers[16]. In view of this, we have evaluated the cytotoxic and anti-tumor activities of the latex of C. procera respectively on hepatoma and non-hepatoma cell lines, and in a transgenic mouse model of hepatocellular carcinoma (HCC).
MATERIALS AND METHODS
Collection of latex and its fractionation
C. procera growing in wild was identified by the Raw Materials, Herbarium and Museum Division, National Institute of Science Communication, New Delhi, where a voucher specimen is preserved (Voucher No. PID1739). The latex was collected from the aerial parts of the plant and dried under shade (DL). DL was extracted with petroleum ether (B.P. 40-60 °C) and methanol in a sequential order. The methanol extract (ME) was subjected to silica gel G (mesh 60-120) step column chromatography using a combination of non-polar and polar solvents and eleven fractions were collected[17]. The fractions were evaporated to dryness and tested for cytotoxic properties.
Chemicals and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and Caspase 3 assay kit were procured from Invitrogen (California, USA). Dimethylsulfoxide (DMSO) and (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (Missouri, USA). Silica gel G, petroleum ether (PE) and other chemicals were bought from Merck, India.
Treatment of oncomouse
The X15-myc transgenic mice expressing HBx and c-myc genes in the liver and thereby resulting in hepatocellular carcinoma[18], were used for this study following approval of the Institutional Animal Ethics Committee. Animals of matched age (5 wk old)) of either sex were used in this study. The overnight fasted animals were fed with bread soaked in aqueous suspension of the DL at a dose of 400 mg/kg for 15 wk (5 d/wk, n = 6). The control animals were given bread alone (n = 6). At 20 wk, the animals of both groups were sacrificed and their liver was collected in buffered formalin (100 mL/L). Paraffin blocks of samples were made and the tissue sections were stained with hematoxylin and eosin to observe histopathological changes.
Estimation of vascular endothelial growth factor (VEGF)
The serum level of VEGF was measured in the mice using a mouse specific ELISA kit (Oncogene Research Products, Germany).
Cell culture and cytotoxicity assay
The human hepatoma Huh-7 cell line[19] was a kind gift from Dr. A. Siddiqui (University of Colorado, Denver). COS-1 (African green monkey kidney cell line, CRL-1 650) and AML-12 cells (mouse hepatocyte, CRL-2 254) were purchased from the American Type Culture Collection (Virginia, USA). All cultures were grown in DMEM without phenol red (DMEM-PR) supplemented with fetal bovine serum (100 mL/L), L-glutamine, penicillin and streptomycin and maintained at 37°C in a CO2 incubator. The cytotoxic effect of ME and each DL fraction was evaluated in cell cultures where the cells were seeded at a density of 4 × 105 cell/60 mm dish and allowed to settle for 12 h. Then these cells were incubated with different fractions of DL at concentrations ranging from 0.1-10 mg/L for either 24h or 48h. Cell viability was analyzed by MTT colorimetric assay[20]. Briefly, after treatment with the DL fractions, the cells were washed with culture medium and incubated with MTT solution (1 mg/mL in DMEM-PR) for 1h at 37 °C. The medium was decanted, the formazan product was dissolved in 1mL DMSO and the absorbance was read at 560 nm.
DNA fragmentation assay
Extract of the cells treated with DL fraction 8 was prepared in TET buffer (10 mmol/L Tris-HCl, pH 7.4, 5 mmol/L EDTA, 5 mL/L Triton X100) and incubated with proteinase K (40 mg/L) at 37 °C for 1h. After extraction with an equal volume of phenol, followed by phenol and chloroform, DNA was precipitated using ethanol. The DNA pellet was resuspended in H2O and incubated with RNase A (40 mg/L) at 37 °C for 1h before electrophoretic separation in a 15 g/L TBE agarose gel[21].
Bcl-2 and caspase assay
The intracellular level of Bcl-2 was measured by immunoprecipitation using anti-Bcl-2 antibody (Santa Cruz Biotechnology, California, USA). The relative caspase activity was measured using the caspase 3 assay kit (Invitrogen, USA). The cell extract was prepared as per supplier’s protocol and the caspase activity was measured in 50 μL aliquots by incubating with the substrate for 2 h at 37 °C and the absorbance was read at 405 nm.
Statistical analysis
The values are expressed as mean ± SE (n = 6) and the statistical analysis was performed by Student’s t test. P < 0.05 was considered significant.
RESULTS
DL exhibits in vivo chemopreventive effect
The chemopreventive effect of orally administered DL was studied in the X15-myc transgenic mice. Histological examination of the liver of untreated mice at 20 wk showed a marked nuclear atypia, hyperchromatia, necrosis and loss in sinusoidal architecture (Figure 1A). Treatment of mice with DL (400 mg/kg) for a period of 15 wk protected these mice from malignant changes occurring in the liver while sinusoidal architecture and cellular integrity was slightly disrupted as compared to normal and hydropic changes were observed (Figures 1B and 1C). We further measured the levels of VEGF, a marker of angiogenesis, in these mice. Treatment with DL produced a significant decrease in the serum VEGF levels of X15-myc mice from 12.18 ± 0.64 μg/L to 9.75 ± 0.27 μg/L (P = 0.002) while the level of VEGF in normal mice was 7.00 ± 0.55 μg/L.
Figure 1.
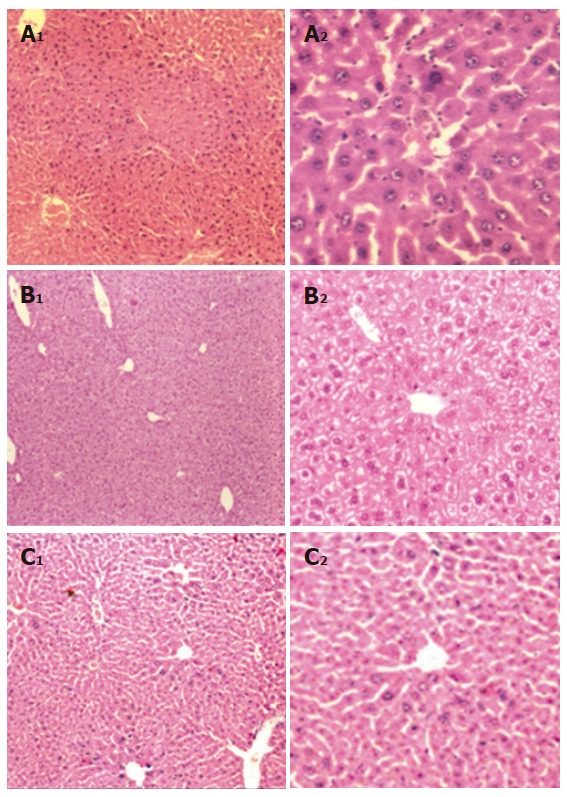
Histological analysis of mouse liver. A: X15-myc control; B: X15-myc mouse treated with DL suspension; C: Normal mouse liver. (A1, B1, C1 = x100 and A2, B2, C2 = x400).
DL is cytotoxic to cancer cells
Since the aqueous extract of DL showed a strong in vivo chemopreventive effect, the molecular basis of its action was investigated in cell culture. The ME of DL was evaluated for cytotoxicity using MTT assay on two different cell lines, viz., Huh-7 and COS-1 cells (Figure 2). ME induced cell death in both cell lines in a concentration dependent manner. Even at low concentration (0.1 mg/L), ME could induce cell death in both the cell lines. The effect was, however, more pronounced (-90% cell death) at higher doses of ME (1 and 10 mg/L).
Figure 2.
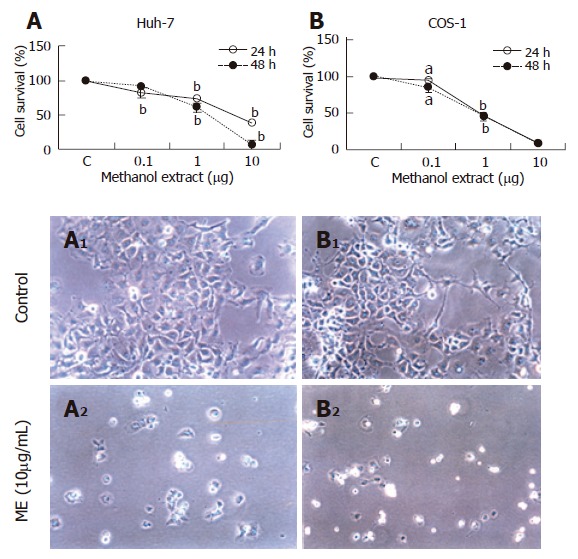
Cytotoxic effect of methanolic extract of DL on cancer cell lines. Huh-7 (A) and COS-1 cells (B) were incubated with different concentrations of ME and analyzed for cell viability at 24h or 48h (n = 6), mean ± SE. aP < 0.001; bP < 0.01; A1 and B1 are control Huh-7 and COS-1 cells; A2 and B2 are ME-treated Huh-7 and COS-1 cells (x200).
A polar fraction of DL contributes to its cytotoxic effect
To identify the active component of DL, ME was subjected to silica gel G column chromatography and eleven fractions were collected[17]. Each fraction was evaluated for its cytotoxic activity on Huh-7 and COS-1 cells at 10 mg/L concentration and cell viability was determined by MTT assay (Figure 3). Out of 11 fractions, only fraction 8 exhibited a potent cytotoxic effect on both Huh-7 and COS-1 cells (~90% cell death) and the effect was comparable to ME (input). A marginal inhibitory effect was also seen with fraction 1 on Huh-7 cells (-24% cell death).
Figure 3.
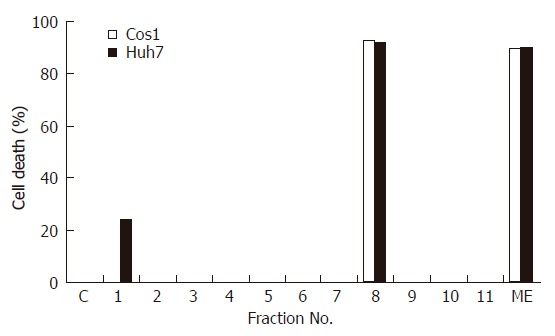
Cytotoxic effect of different fractions of the methanolic extract of DL on cancer cell lines. Huh-7 and COS-1 cells were incubated with either ME or its eleven fractions (all at 10 mg/L). Cell viability was measured at 48 h and results are expressed as % cell death.
We further investigated the relationship between the dose-response and cytotoxic effect of DL fraction 8 on Huh-7, COS-1 and non-transformed AML12 cells. At concentration ranging from 0.1 to 10 mg/L, it produced a dose-dependent decrease in the survival of Huh-7 and COS-1 cells leaving behind only 20%-30% living cells at 10 mg/L concentration. However, unlike the two cancer cell lines, AML12 cells showed a much better survival (-80%) in the presence of fraction 8 (Figure 4 A-C).
Figure 4.
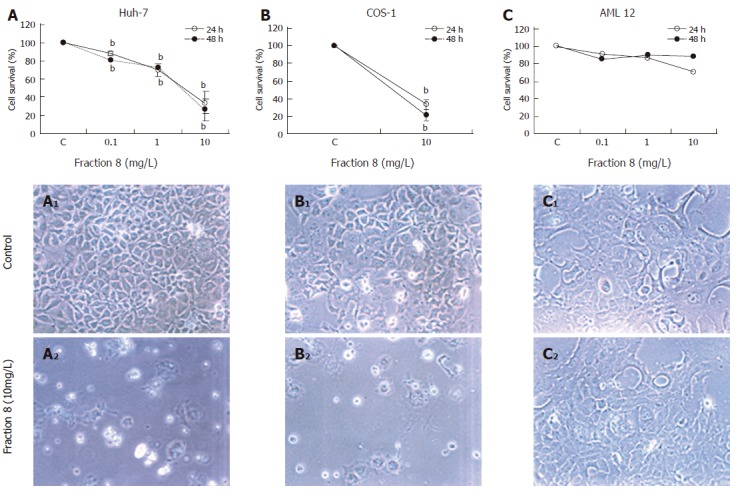
Cytotoxic effect of fraction 8 of the DL methanolic extract on different cell lines. Huh-7 (A), COS-1 (B) and AML12 cells (C) were incubated with different concentrations of fraction 8 and cell viability was measured at 24 and 48 h (mean ± SE, bP < 0.01). A1, B1 and C1 are control Huh-7, COS1 and AML12 cells; A2, B2 and C2 are fraction 8 treated Huh-7, COS-1 and AML12 cells (x200).
Fraction 8 of DL induces DNA fragmentation
To understand the mechanism of DL-induced cell death, the canonical markers of apoptosis like Bcl-2 and caspase 3 were measured in Huh-7 cells after treatment with fraction 8. Surprisingly, no change in the levels of both markers was observed (data not shown). Nonetheless, there was a marked increase in the fragmentation of cellular DNA that was both concentration as well as time dependent thereby suggesting a rise in the cellular nuclease activity (Figure 5).
Figure 5.
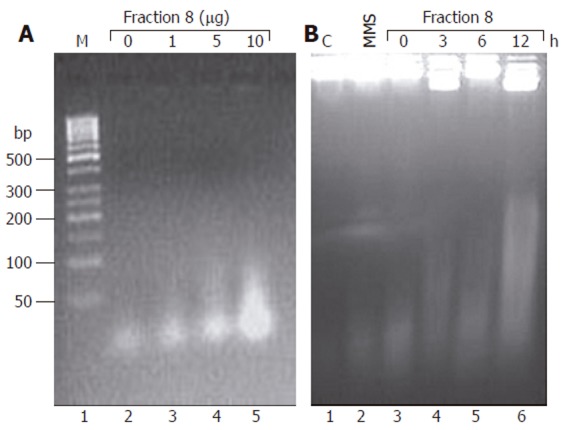
Effect of fraction 8 on DNA fragmentation in Huh-7 cells. Huh-7 cells were treated either with different concentrations (1, 5 and 10 mg/L) of fraction 8 for 3 h (A) or for 3, 6 and 12 h with 10 mg/L of fraction 8 (B). Total DNA was extracted after proteinase K and RNase A treatment and resolved by agarose gel electrophoresis. C: DNA from control cells; M: 100 base pair (bp) ladder; MMS, DNA from cells treated with methyl methanesulfonate (2 g/L) for 15 min.
DISCUSSION
The latex of C. procera has been shown to possess potent anti-inflammatory and antioxidant properties. In this study we have tested the latex for its chemopreventive and in vitro cytotoxic properties. The study was carried out in an mouse model of HCC developed by the integration of a chimeric transgene having hepatitis B virus X and murine c-myc genes exhibiting liver-specific neoplastic changes that are associated with inflammation and angiogenesis[18]. Five days a week oral administration of DL to the X15-myc mouse produced a marked chemopreventive effect as revealed by histological analysis. The mitotic changes produced by transgene expression were inhibited at the dose studied though some hydropic changes were observed. This was accompanied by inhibition of cellular infiltration. The DL might be producing chemopreventive effects through inhibition of different mediators of inflammation[15]. The pro-inflammatory cytokines and reactive oxygen and nitrogen species are known to activate signaling molecules involved in inflammation and carcinogenesis. These include nuclear transcription factor NF-κB, inducible nitric oxide synthetase (iNOS) and cyclooxygenase-2 (COX-2)[22]. The elevated levels of tumor COX-2 have been reported to correlate with invasiveness and elevated VEGF levels that brings about neovascularization and progression of HCC[23,24]. In fact, a recent study has revealed that COX-2 expression correlates with VEGF expression and microvessel density in HCC caused by hepatitis B virus[25]. It is interesting to note that DL not only inhibited the inflammatory changes, but also produced a significant decrease in VEGF levels as observed with other anticancer drugs[26-28].
We further evaluated the cytotoxic effects of DL on hepatoma (Huh-7), non-hepatoma (COS-1) and non-cancerous (AML12) cell lines and observed that the cytotoxic activity was associated with one of the polar fractions of DL, i.e., fraction 8. Like total methanolic extract, fraction 8 also showed a strong cytotoxic effect on both transformed cell lines used here. However, a marginal effect on the killing of AML12 cells suggested a high degree of selectivity for transformed cells. Such differential killing of cancerous cells could relate to their altered metabolic status and/or membrane properties. In fact selective killing of cancerous cells by chemotherapeutic drugs like methotrexate and polyunsaturated fatty acids has been reported earlier as well[29,30]. Thus, it would be interesting to investigate the mechanism of such target selectivity by DL. The cytotoxic effect of DL was accompanied by intracellular fragmentation of target cell DNA. We observed a dramatic increase in the fragmentation of Huh-7 cells upon incubation with DL fraction 8. Though the action was rapid, it was not accompanied by expression of the common markers of intrinsic pathway of apoptosis such as Bcl-2 or caspase 3. Since Bcl-2 and caspases are markers of mitochondria-mediated apoptotic death, the genomic DNA can still undergo fragmentation by cellular DNase activity independent of mitochondrial pathway[31]. Hence a possible role of hypoxia and free radical-dependent activation of DNase activity or direct DNA damage and consequent degradation of cellular DNA[32] as evident here, cannot be ruled out. Thus, our study suggests that the latex of C. procera possesses an activity that has a chemopreventive action in vivo and cytotoxic action on cancer cell lines. Further study would be necessary to demonstrate its efficacy in HCC treatment.
ACKNOWLEDGMENTS
The senior research fellowships to Ganeshan Mathan and Soneera Arya from the Council of Scientific and Industrial Research, New Delhi during the study period are acknowledged.
Footnotes
Supported by the core grant of International Centre for Genetic Engineering and Biotechnology. New Delhi
S- Editor Guo SY L- Editor Zhang JZ E- Editor Liu WF
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 3.Downey RJ. Surgical management of lung cancer. J Thorac Imaging. 1999;14:266–269. doi: 10.1097/00005382-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kinne DW. The surgical management of primary breast cancer. CA Cancer J Clin. 1991;41:71–84. doi: 10.3322/canjclin.41.2.71. [DOI] [PubMed] [Google Scholar]
- 5.Chay CH, Cooper CC, Hellerstedt BA, Pienta KJ. Antimetastatic drugs in prostate cancer. Clin Prostate Cancer. 2002;1:14–19. doi: 10.3816/cgc.2002.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Hudis CA. Current status and future directions in breast cancer therapy. Clin Breast Cancer. 2003;4 Suppl 2:S70–S75. doi: 10.3816/cbc.2003.s.018. [DOI] [PubMed] [Google Scholar]
- 7.Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885–1891. doi: 10.3748/wjg.v9.i9.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 9.Yates JS, Mustian KM, Morrow GR, Gillies LJ, Padmanaban D, Atkins JN, Issell B, Kirshner JJ, Colman LK. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]
- 10.Kumar VL, Arya S. Medicinal uses and pharmacological properties of Calotropis procera. In: Govil JN, ed , editors. Recent Progress in Medicinal Plants. Vol. 11. Texas: Studium Press; 2006. pp. 373–388. [Google Scholar]
- 11.Smit HF, Woerdenbag HJ, Singh RH, Meulenbeld GJ, Labadie RP, Zwaving JH. Ayurvedic herbal drugs with possible cytostatic activity. J Ethnopharmacol. 1995;47:75–84. doi: 10.1016/0378-8741(95)01255-c. [DOI] [PubMed] [Google Scholar]
- 12.Van Quaquebeke E, Simon G, André A, Dewelle J, El Yazidi M, Bruyneel F, Tuti J, Nacoulma O, Guissou P, Decaestecker C, et al. Identification of a novel cardenolide (2''-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem. 2005;48:849–856. doi: 10.1021/jm049405a. [DOI] [PubMed] [Google Scholar]
- 13.Basu A, Chaudhuri AK. Preliminary studies on the antiinflammatory and analgesic activities of Calotropis procera root extract. J Ethnopharmacol. 1991;31:319–324. doi: 10.1016/0378-8741(91)90017-8. [DOI] [PubMed] [Google Scholar]
- 14.Mascolo N, Sharma R, Jain SC, Capasso F. Ethnopharmacology of Calotropis procera flowers. J Ethnopharmacol. 1988;22:211–221. doi: 10.1016/0378-8741(88)90129-8. [DOI] [PubMed] [Google Scholar]
- 15.Arya S, Kumar VL. Antiinflammatory efficacy of extracts of latex of Calotropis procera against different mediators of inflammation. Mediators Inflamm. 2005;2005:228–232. doi: 10.1155/MI.2005.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 17.Kumar VL, Kumar V, inventors. Extraction of latex of Calotropis procera and a process for the preparation thereof. # 737/DEL/2004 and PCT/IN 2005/000106 Indian Patent Application.
- 18.Lakhtakia R, Kumar V, Reddi H, Mathur M, Dattagupta S, Panda SK. Hepatocellular carcinoma in a hepatitis B 'x' transgenic mouse model: A sequential pathological evaluation. J Gastroenterol Hepatol. 2003;18:80–91. doi: 10.1046/j.1440-1746.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 20.van de Loosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Russell DW. Molecular Cloning: A laboratory Manual 3rded. New York: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 22.Ohshima H, Tazawa H, Sylla BS, Sawa T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res. 2005;591:110–122. doi: 10.1016/j.mrfmmm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 24.Tang TC, Poon RT, Lau CP, Xie D, Fan ST. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:1896–1902. doi: 10.3748/wjg.v11.i13.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng AS, Chan HL, To KF, Leung WK, Chan KK, Liew CT, Sung JJ. Cyclooxygenase-2 pathway correlates with vascular endothelial growth factor expression and tumor angiogenesis in hepatitis B virus-associated hepatocellular carcinoma. Int J Oncol. 2004;24:853–860. [PubMed] [Google Scholar]
- 26.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nolè F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 27.Deplanque G, Madhusudan S, Jones PH, Wellmann S, Christodoulos K, Talbot DC, Ganesan TS, Blann A, Harris AL. Phase II trial of the antiangiogenic agent IM862 in metastatic renal cell carcinoma. Br J Cancer. 2004;91:1645–1650. doi: 10.1038/sj.bjc.6602126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincenzi B, Santini D, Dicuonzo G, Battistoni F, Gavasci M, La Cesa A, Grilli C, Virzì V, Gasparro S, Rocci L, et al. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 2005;25:144–151. doi: 10.1089/jir.2005.25.144. [DOI] [PubMed] [Google Scholar]
- 29.Bégin ME, Ells G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986;77:1053–1062. [PubMed] [Google Scholar]
- 30.Chow M, Rubin H. Selective killing of preneoplastic and neoplastic cells by methotrexate with leucovorin. Proc Natl Acad Sci U S A. 1998;95:4550–4555. doi: 10.1073/pnas.95.8.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 32.Rollet-Labelle E, Grange MJ, Elbim C, Marquetty C, Gougerot-Pocidalo MA, Pasquier C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic Biol Med. 1998;24:563–572. doi: 10.1016/s0891-5849(97)00292-x. [DOI] [PubMed] [Google Scholar]


