Abstract
AIM: To immunohistochemically examine micrometastasis and VEGF-C expression in hilar bile duct carcinoma (HBDC) and to evaluate the clinical significance of the results.
METHODS: A total of 361 regional lymph nodes from 25 patients with node-negative HBDC were immunostained with an antibody against cytokeratins 8 and 18 (CAM 5.2), and immunohistochemical staining of VEGF-C was performed in 34 primary resected tumors.
RESULTS: Lymph node micrometastasis was detected in 6 (24%) of the 25 patients and 10 (2.8%) of the 361 lymph nodes. Patients with micrometastasis showed significantly poorer survival rates than those without (P = 0.025). VEGF-C expression was positive in 17 (50%) of 34 HBDC, and significantly correlated with lymph node metastasis (P = 0.042) and microscopic venous invasion (P = 0.035).
CONCLUSIONS: It is suggested that immunohistochemically detected lymph node micrometastasis has an impact on the outcome of HBDC. VEGF-C expression is highly correlated with lymph node metastasis in HBDC and might therefore be a useful predictor.
Keywords: Hilar bile duct carcinoma, Lymph node metastasis, Micrometastasis, Vascular endothelial growth factor-C
INTRODUCTION
Hilar bile duct carcinomas (HBDC) are one of the most difficult to cure malignant gastroenterological tumors[1-4] and curative resection is essential for long-term survival. Because hilar bile duct tumors are in close proximity to vital structures in the hepatic hilum, such as the hepatic artery and portal vein, and since they tend to spread to the proximal biliary tract and perineural and perilymphatic spaces, hepatectomy with thorough systematic extended lymph node dissection is frequently required for curative resection. However, even with margin-negative resection, the prognosis after curative resection remains poor. One possible reason for the poor outcome is existence of occult lymph node metastasis that cannot be detected by conventional hematoxylin and eosin (HE) staining at the time of surgical resection. Immunohistochemical and molecular techniques have, however, made it possible to identify lymph node micrometastasis missed by traditional methods. Recently, immunohistochemical and/or genetic detection of lymph node micrometastases of various tumors, including carcinomas of the breast[5,6], lung[7,8], esophagus[9,10], stomach[11-14], colorectum[15,16] and gallbladder[17-19], has been reported. However, we were able to find only one report documenting this in HBDC[20].
Vascular endothelial growth factor C (VEGF-C) is a member of the highly glycosylated vascular endothelial growth factor (VEGF) family that regulates vasculogenesis, hematopoiesis, angiogenesis, lymphangiogenesis and vascular permeability, and has been implicated in many physiological and pathological processes[21,22]. Overexpression of VEGF-C cDNA in the skin of transgenic mice has been shown to selectively induce lymphatic endothelial cell proliferation and hyperplasia of the lymphatic vasculature[23]. It was also recently reported that a VEGF-C-transfected tumor cell line implanted into the stomach of nude mice gave rise to numerous lymph node metastases[24]. The most prominent VEGF-C expression has been detected in the human heart, placenta, muscle, ovary, and small intestine[25], and a positive correlation between expression and various clinicopathological factors, especially lymph node metastasis, has been reported in a number of tumors, including carcinomas of the thyroid[26], breast[27], lung[28], esophagus[29], stomach[30,31], colorectum[32], prostate[33] and pancreas[34]. However, no investigations have been conducted with regard to VEGF-C expression in HBDC and possible clinicopathological associations. In this study, we examined lymph node micrometastasis and VEGF-C expression in patients with HBDC and evaluated the clinical significance of the results.
MATERIALS AND METHODS
Patients and specimens
From January 1981 to August 2000, 61 patients with HBDC underwent surgical resection plus systematic lymph node dissection in the First Department of Surgery, Mie University School of Medicine. Of these patients, 34 underwent macroscopic and microscopic margin-negative resection. Patients consisted of 21 males and 13 females with a mean age of 64.4 ± 11.0 years (range: 37-89 years). The median follow-up period was 31.8 mo (minimum: 1.0 mon). No lymph node metastases were detected in 25 (73.5%) of the 34 patients by traditional pathologic examinations with HE staining.
Hepatectomy was performed in 29 (85.3%) of the 34 patients: extended right hepatectomy in 9 patients, left hepatectomy in 8, resection of segments 4a and 5 in 5, hilar resection in 4, extended left hepatectomy in 2, and caudate lobectomy only in 1. All 29 patients underwent combined resection of the caudate lobe. Two patients underwent combined resection of the portal vein, and 3 underwent pancreatoduodenectomy (PD) or pylorus-preserving pancreatoduodenectomy. Another 5 patients were treated with bile duct resection alone, including 2 patients who underwent combined PD.
A total of 361 lymph nodes dissected from 25 node-negative patients were examined immunohistochemically by staining with an antibody against cytokeratins 8 and 18, and all 34 primary tumors were immunohistochemically stained for VEGF-C. Tumor specimens and lymph nodes were collected from pathology files after obtaining informed consent from all patients in accordance with institutional guidelines.
Lymph node groups and resected margin status
Identification of the sites of lymph node metastasis were performed in accordance with the TNM Classification of Malignant Tumors proposed by the International Union Against Cancer (UICC)[35], which defines regional lymph nodes as the cystic duct, pericholedochal, hilar and peripancreatic (head only), periduodenal, periportal, celiac and superior mesenteric nodes, N0 as no regional lymph node metastasis and N1 as regional lymph node metastasis.
Evaluation of resected margin status was performed in accordance with the General Rules for Surgical and Pathological Studies on Cancer of the Biliary Tract (The 5th Edition) proposed by the Japanese Society of Biliary Surgery (JSBS)[36], which defines pEM0 as no tumor invasion within 5 mm of the resected margin, pEM1 as tumor invasion within 5 mm of the resected margin and pEM2 as distinct tumor invasion of the resected margin. pEM0 and pEM1 resections were defined as margin-negative in this study.
Immunohistochemical staining
Tissue samples were fixed in 10% formaldehyde with phosphate-buffered saline (PBS) and embedded in paraffin. Lymph node tissue was cut into six 5-µm thick sections, and primary tumor tissue was cut into a single 5-µm thick section. Briefly, the sections were deparaffinized with xylene and rehydrated through graded concentrations of ethanol. For antigen retrieval, sections were placed in 0.1mol/L citrate buffer (pH 6.0) and heated three times for 3 min each in a microwave oven (500 W). Lymph node sections were then incubated with a mouse monoclonal antibody (CAM 5.2; Becton Dickinson, San Jose, CA) specific for cytokeratins 8 and 18, and tumor sections were incubated with affinity-purified goat polyclonal antibodies (IBL, Fujioka, Japan) to VEGF-C at 1:30 dilution. Immunohistochemical detection of CAM 5.2 and VEGF-C was performed by a standard avidin-biotin method on an automated Ventana ES immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer’s instructions[37].
We examined 6 sections per lymph node and diagnosed micrometastasis when tumor cells were detected immunohistochemically, after being missed by routine histologic examinations with HE staining. VEGF-C immunoreactivity was mainly present in the cytoplasm of cancer cells and/or in the connective tissue around cancer cells. For evaluation of VEGF-C immunostaining, we examined at least 200 cancer cells per case. Cases in which at least 10% of the cancer cells were immunoreactive were defined as VEGF-C positive. All immunohistochemical evaluations were performed by an experienced histopathologist unaware of the clinicopathological features of the patients.
Statistical analysis
All statistical calculations were carried out using StatView-J 5.0 statistical software (SAS Institute, USA). Results are expressed as the means ± SD. Statistical analysis for comparisons of VEGF-C expression and clinicopathological factors (age, gender, lymphatic vessel invasion, microscopic venous invasion, perineural invasion and lymph node metastasis) were performed using the chi-square test and Fisher's exact probability test. Analysis for comparisons of VEGF-C expression and other factors (pT classification and histopathological grading) was performed using the Mann-Whitney U-test. The Kaplan-Meier method was used to estimate postoperative survival rates, and the generalized log-rank test was used to compare differences in survival rates. All P values were two-sided and P < 0.05 was considered statistically significant.
RESULTS
Patient outcomes
Of the 34 patients with margin-free resected HBDC, 4 died of other causes; three of multiple organ failure including 1 postoperative death (within 1 mo), and 1 of unknown causes. In addition, 15 (50.0%) of the remaining 30 patients died of disease. Recurrence sites were the liver in 2 patients including 1 patient with combined lung recurrence, the peritoneum in 1 patient, and local regions in 11 patients. Of these 11 patients, 3 showed combined recurrence in other sites; 1 showed combined liver metastasis, 1 showed combined lung metastasis, and 1 showed combined peritoneum recurrence.
Detection of lymph node micrometastasis
Micrometastasis was detected in 6 (24.0%) of the 25 node-negative patients and 10 (2.8%) of the 361 lymph nodes by immunohistochemical examination with CAM5.2. Lymph node micrometastasis was present in the form of a single-cell metastasis (Figure 1A) or a small cluster of tumor cells (Figure 1B). Of the 6 patients with lymph node micrometastasis, 5 had regional lymph node micrometastasis and 1 had regional lymph node with para-aortic lymph node micrometastases.
Figure 1.
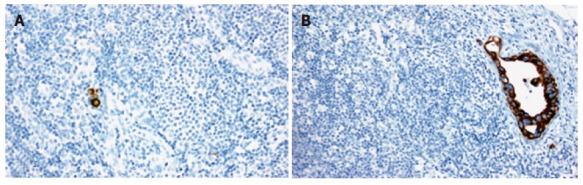
Immunohistochemical staining of lymph node micrometastasis with the monoclonal antibody CAM 5.2. A: Micrometastasis consisting of a single cell (original magnification, × 200). B: Micrometastasis consisting of a small cluster of tumor cells (original magnification, × 100).
Impact of lymph node micrometastasis on survival
Cumulative survival rates were compared according to nodal status: the without lymph node metastasis group versus lymph node micrometastasis group versus HE diagnosed (overt) lymph node metastasis group (Figure 2). The 3- and 5-year survival rates of the 19 patients without lymph node metastasis were 81.6 and 72.5%, respectively, as opposed to 20.8 and 20.8%, respectively, in the 6 patients with micrometastasis and 29.6% and 0.0%, respectively, in the 9 patients with overt lymph node metastasis. Patients with lymph node micrometastasis showed significantly worse survival rates than those without (P = 0.025), and moreover, patients with overt lymph node metastasis showed worse survival rates than those without (P = 0.0003). There were no statistical differences between patients with lymph node micrometastasis and those with overt lymph node metastasis (P = 0.469). Five patients died of disease without overt lymph node or micrometastasis. Of these, 4 died of local recurrence, including 1 patient with combined liver metastasis. The remaining patient died of liver and lung metastasis. Follow-up revealed that 3 patients with lymph node micrometastasis survived with no evidence of disease for 11.7 and 36.7 and 60.3 mon after surgical resection, respectively.
Figure 2.
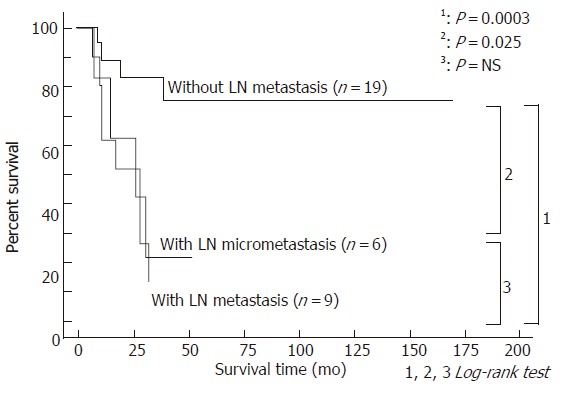
Survival curves after resection for hilar bile duct carcinoma according to the presence of lymph node metastasis, including micrometastasis.
To further evaluate the impact of lymph node micrometastasis on survival, survival rates were compared according to two groups: patients without lymph node metastasis versus those with overt lymph node and micrometastasis (Figure 3), and patients without lymph node metastasis and those with lymph node micrometastasis versus those with overt lymph node metastasis (Figure 4). The 3- and 5- year survival rates of the 19 patients without lymph node metastasis were 81.6 and 72.5%, respectively, as opposed to 25.5 and 8.5%, respectively, in the 15 patients with overt lymph node and micro metastasis (P = 0.0004). On the other hand, the 3- and 5- year survival rates of the 25 patients without lymph node metastasis and those with lymph node micrometastasis were 66.9 and 60.2%, respectively, as opposed to 14.8 and 0.0%, respectively, in the 9 patients with overt lymph node metastasis (P = 0.0015).
Figure 3.
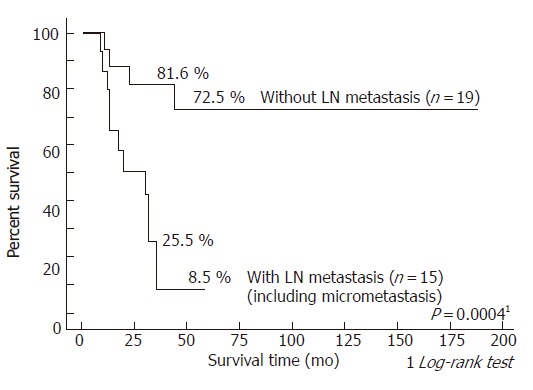
Survival curves after resection for hilar bile duct carcinoma according to the presence of lymph node metastasis: patients without lymph node metastasis versus those with overt lymph node and micro metastasis.
Figure 4.
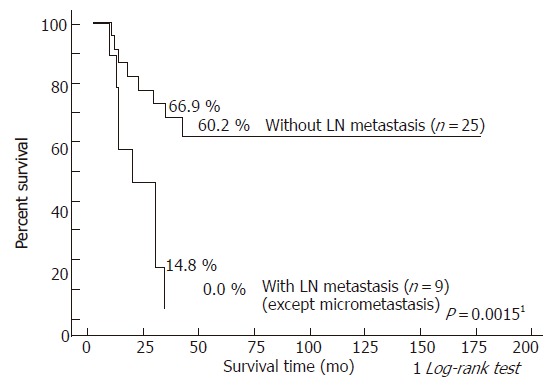
Survival curves after resection for hilar bile duct carcinoma according to the presence of lymph node metastasis: patients without lymph node metastasis and those with lymph node micrometastasis versus those with overt lymph node metastasis.
VEGF-C expression and clinicopathological factors
VEGF-C expression was observed in 17 (50.0%) of the 34 primary tumors (Figure 5). The correlations between VEGF-C expression and clinicopathological factors are shown in Table 1. Microscopic venous invasion (P = 0.035) and lymph node metastasis (P = 0.042) were significantly correlated with VEGF-C expression.
Figure 5.
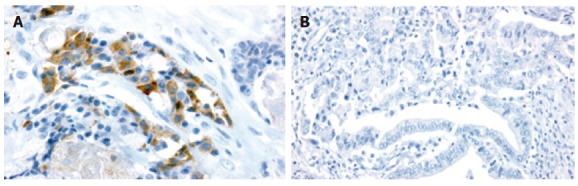
Immunohistochemical staining of primary tumors with VEGF-C polyclonal antibody. A: VEGF-C positive (original magnification, × 400). B: VEGF-C-negative (original magnification, × 200).
Table 1.
Clinicopathological factors and VEGF-C expression
| VEGF-C expression | ||||
| Positive (n = 16) | Negative (n = 18) | P Value | ||
| Age | 64.7 ± 11.6 | 64.1 ± 10.8 | 0.870 | |
| Gender ( M / F ) | 10 / 6 | 11 / 17 | 0.999 | |
| pT classification1 | pT1 | 1 | 4 | 0.56 |
| pT2 | 8 | 11 | ||
| pT3 | 7 | 3 | ||
| Histopathological Grading1 | G1 | 10 | 15 | 0.225 |
| G2 | 6 | 2 | ||
| G3 | 0 | 1 | ||
| Lymphatic vessel invasion | (presence) | 14 (87.5 %) | 13 (72.2 %) | 0.405 |
| Venous invasion | (presence) | 10 (62.5 %) | 4 (22.2 %) | 0.035 |
| Perineural invasion | (presence) | 14 (87.5 %) | 10 (55.6 %) | 0.063 |
| Lymph nodes metastasis | Metastasis (-) | 6 | 13 | 0.042 |
| Metastasis (+) | 10 | 5 | ||
| (including micrometastasis) | ||||
According to the TNM staging system. pT classification: pT1: Tumor confined the bile duct; pT2: Tumor invades beyond the wall of the bile duct; pT3: Tumor invades the liver, gallbladder, pancreas, and/or unilateral tributaries of the portal vein (right or left) or hepatic artery (right or left); pT4: Tumor invades any of follwing: main portalvein or its tributaries bilatellary, common hepatic artery, or other adjacent structures, e.g., colon, stomach, duodenum, abdominal wall. Histopathological Grading: G1: Well differentiated; G2: Moderately differentiated; G3: Poorly differentiated.
Prognostic factors for hilar bile duct carcinoma
To identify useful prognostic factors, we performed univariate analysis of the following possible independent prognostic factors: age (above 60 years versus 60 years or less), gender, operative procedure (hepatectomy versus bile duct resection), histopathological grading (well differentiated versus moderately or poorly differentiated), lymphatic vessel invasion, microscopic venous invasion, perineural invasion, microscopic resection margin (em0 versus em1), VEGF-C expression, lymph node metastasis (including micrometastasis) and lymph node metastasis (excluding micrometastasis) (Table 2). Ultimately, 4 independent variables (microscopic resection margin (P = 0.040), VEGF-C expression (P = 0.036), lymph node metastasis (including micrometastasis) (P = 0.0004) and lymph node metastasis (excluding micrometastasis) (P = 0.0017)) were identified as statistically significant predictors of survival.
Table 2.
Univariate analysis of survival
| Variable | 5-yr survival (%) | P Value | |
| Age | < 60 vs ≥ 60 | 40.0 vs 44.9 | 0.912 |
| Gender | male vs female | 42.3 vs 45.5 | 0.872 |
| Operative procedure | hepatectomy vs bile duct resection | 42.2 vs 60.0 | 0.430 |
| Histopathologic Grading1 | G2, G3 vs G1 | 37.5 vs 46.0 | 0.393 |
| Lymphatic vessel invasion | present vs absent | 37.4 vs 75.0 | 0.076 |
| Venous invasion | present vs absent | 36.4 vs 49.7 | 0.185 |
| Perineural invasion | present vs absent | 36.3 vs 68.6 | 0.064 |
| Microscopic resection margin2 | em1 vs em0 | 31.7 vs 83.3 | 0.040 |
| VEGF-C expression | positive vs negative | 23.3 vs 70.5 | 0.036 |
| Lymph node metastasis (Including micrometastasis) | positive vs negative | 8.5 vs 72.5 | 0.0004 |
| Lymph node metastasis (except micrometastasis) | positive vs negative | 0.0 vs 60.2 | 0.0017 |
According to the TNM staging system. Histopathological Grading: G1 Well differentiated, G2 Moderately differentiated, G3 Poorly differentiated.
According to the Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of the Biliary Tract. em0: no tumor invades within 5 mm from resected margin; em1: tumor invades within 5mm from resected margin.
DISCUSSION
Lymph node metastasis is a well known important predictor of prognosis with a wide variety of malignant tumors, and some studies have reported a significant relationship between lymph node metastasis and prognosis of HBDC patients[38-40]. However, patients with early stage carcinoma and no apparent lymph node metastasis sometimes die of metastasis after surgery despite complete resection of the primary lesion. One of the possible reason for the poor outcome in these patients is occult lymph node metastasis not identified by conventional HE staining at the time of surgical resection.
Numerous studies on the incidence and significance of lymph node micrometastasis in cancer patients have been conducted in recent years. A number of investigators have proposed the prognostic significance of lymph node micrometastasis for various tumors including lesions of the lung, esophagus, stomach and colon, while others have suggested that lymph node micrometastasis is not significant for patient outcome. Thus, there is no consensus on the clinical significance of lymph node micrometastasis. However, we were able to find only one report documenting this in HBDC. Tojima et al[20] investigated 954 nodes from 45 patients with pN0 hilar cholangiocarcinoma after curative resection, and found micrometastasis in 13 (1.4%) nodes from 11 (24.4%) patients. Their data yielded similar survival curves for patients with and without lymph node micrometastasis (5-year survival rates: 43.6% vs 42.1%, respectively).
In this study, we demonstrated significant differences between outcomes of HBDC patients with and without lymph node micrometastases. Interestingly, a stronger correlation was recognized when patients with lymph node micrometastasis were treated as lymph node metastasis positive, compared to when they were treated as lymph node metastasis negative (P = 0.0004 versus P = 0.0017) (Figures 3 and 4). This might suggest the need to consider lymph node micrometastasis as overt lymph node metastasis.
One possible reason for the above-mentioned differing results is the number of sections examined. The number of sections immunohistochemically stained is considered an important factor in the diagnosis of lymph node micrometastasis. Many investigators examine lymph node micrometastasis using various sections of different thickness for immunohistochemical staining; however, the total thickness examined tends to range from 3 to 30 µm[6,8,10,13-20]. Sasaki et al examined the correlation between the number of CAM 5.2 sections and cumulative positive rate of lymph node metastasis[41]. They found that positive metastasis detection reached a plateau when over 9 sections (total thickness 27 µm) were examined. In this study, to identify lymph node micrometastasis, we examined six 5-µm sections (total thickness 30 µm) per lymph node by immunohistochemical staining. When we examined only one to four sections per lymph node, we found fewer lymph node micrometastases (data not shown).
Another possible reason for the differing results is the criteria of lymph node micrometastasis. In many studies, including ours, micrometastasis is defined as tumor cells detected only by immunohistochemical staining. However, some authors set size criteria for micrometastasis, such as deposits less than 2[42] or 0.5 mm in diameter[20,43]. Recent progress in molecular biological techniques has led to the development of genetic methods for detecting micrometastasis, including RT-PCR. RT-PCR is capable of detecting more micrometastasis foci than immunohistochemical staining[44]. Five patients without overt lymph node or micro metastasis died of disease recurrence in this study. If we use RT-PCR to detect lymph node micrometastasis, we will be able to evaluate lymph node micrometastasis in more detail, and the significance of lymph node micrometastasis will potentially increase. Therefore, further examinations using RT-PCR appear necessary.
VEGF-C is a specific ligand of VEGFR-3 and VEGFR-2, and has been shown to stimulate lymphangiogenesis and angiogenesis both in vitro and in vivo. Nakashima et al[45] investigated VEGF-C expression in 52 patients with gallbladder carcinoma and found that expression was significantly stronger (P < 0.001) in patients with lymph node metastasis than those without, and that the VEGF-C-positive group showed poorer outcomes than the negative group (P < 0.001).
Our study revealed a significant correlation between VEGF-C expression and both the presence of lymph node metastasis (HE detected and micrometastasis) and outcome of HBDC. These results suggest that VEGF-C expression might play an important role in causing lymph node metastasis in HBDC, consistent with the findings of previous studies regarding other malignant tumors.
In conclusion, our findings suggest that immunohistochemical detection of lymph node micrometastasis provides very useful information of survival rates after surgery for HBDC. However, considering that 1 patient with lymph node micrometastasis survived for more than 5-years with no evidence of tumor recurrence, long-term survival is thus possible for some patients with lymph node micrometastasis; therefore, extended lymph node dissection is necessary in HBDC patients. Although further study is needed, VEGF-C seems to be a useful predictor of overt and micro lymph node metastasis.
Footnotes
S- Editor Wang J L- Editor Zhang JZ E- Editor Bi L
References
- 1.Tabata M, Kawarada Y, Yokoi H, Higashiguchi T, Isaji S. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:148–154. doi: 10.1007/s005340050169. [DOI] [PubMed] [Google Scholar]
- 2.Kawarada Y, Das BC, Naganuma T, Tabata M, Taoka H. Surgical treatment of hilar bile duct carcinoma: experience with 25 consecutive hepatectomies. J Gastrointest Surg. 2002;6:617–624. doi: 10.1016/s1091-255x(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millis RR, Springall R, Lee AH, Ryder K, Rytina ER, Fentiman IS. Occult axillary lymph node metastases are of no prognostic significance in breast cancer. Br J Cancer. 2002;86:396–401. doi: 10.1038/sj.bjc.6600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer. 2003;98:2105–2113. doi: 10.1002/cncr.11761. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Kobayashi Y, Ishikawa Y, Tsuchiya S, Okumura S, Nakagawa K, Tokuchi Y, Hayashi M, Nishida K, Hayashi S, et al. Prognostic value of genetically diagnosed lymph node micrometastasis in non-small cell lung carcinoma cases. Cancer Res. 2000;60:6472–6478. [PubMed] [Google Scholar]
- 8.Gu C, Oyama T, Osaki T, Kohno K, Yasumoto K. Expression of Y box-binding protein-1 correlates with DNA topoisomerase IIalpha and proliferating cell nuclear antigen expression in lung cancer. Anticancer Res. 2001;21:2357–2362. [PubMed] [Google Scholar]
- 9.Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. doi: 10.1056/NEJM199710233371702. [DOI] [PubMed] [Google Scholar]
- 10.Komukai S, Nishimaki T, Suzuki T, Kanda T, Kuwabara S, Hatakeyama K. Significance of immunohistochemical nodal micrometastasis as a prognostic indicator in potentially curable oesophageal carcinoma. Br J Surg. 2002;89:213–219. doi: 10.1046/j.0007-1323.2001.01981.x. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H, Mafune K, Tange T, Tatematsu M, Kaminishi M. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer. 2003;105:136–143. doi: 10.1002/ijc.11031. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Natsugoe S, Ishigami S, Uenosono Y, Takao S, Aikou T. Rapid immunohistochemical detection of lymph node micrometastasis during operation for upper gastrointestinal carcinoma. Br J Surg. 2003;90:563–566. doi: 10.1002/bjs.4083. [DOI] [PubMed] [Google Scholar]
- 13.Morgagni P, Saragoni L, Scarpi E, Zattini PS, Zaccaroni A, Morgagni D, Bazzocchi F. Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg. 2003;27:558–561. doi: 10.1007/s00268-003-6797-y. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–774. doi: 10.1007/BF02574499. [DOI] [PubMed] [Google Scholar]
- 15.Noura S, Yamamoto H, Miyake Y, Kim Bn, Takayama O, Seshimo I, Ikenaga M, Ikeda M, Sekimoto M, Matsuura N, et al. Immunohistochemical assessment of localization and frequency of micrometastases in lymph nodes of colorectal cancer. Clin Cancer Res. 2002;8:759–767. [PubMed] [Google Scholar]
- 16.Yasuda K, Adachi Y, Shiraishi N, Yamaguchi K, Hirabayashi Y, Kitano S. Pattern of lymph node micrometastasis and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2001;8:300–304. doi: 10.1007/s10434-001-0300-5. [DOI] [PubMed] [Google Scholar]
- 17.Nagakura S, Shirai Y, Yokoyama N, Hatakeyama K. Clinical significance of lymph node micrometastasis in gallbladder carcinoma. Surgery. 2001;129:704–713. doi: 10.1067/msy.2001.114764. [DOI] [PubMed] [Google Scholar]
- 18.Tajima Y, Tomioka T, Ikematsu Y, Ichinose K, Inoue K, Kanematsu T. Immunohistochemical demonstration of cytokeratin is useful for detecting micrometastatic foci from gallbladder carcinoma in regional lymph nodes. Jpn J Clin Oncol. 1999;29:425–428. doi: 10.1093/jjco/29.9.425. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama N, Shirai Y, Hatakeyama K. Immunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibody. Cancer. 1999;85:1465–1469. doi: 10.1002/(sici)1097-0142(19990401)85:7<1465::aid-cncr6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Tojima Y, Nagino M, Ebata T, Uesaka K, Kamiya J, Nimura Y. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg. 2003;237:201–207. doi: 10.1097/01.SLA.0000048446.18118.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 22.Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- 23.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 24.Yanai Y, Furuhata T, Kimura Y, Yamaguchi K, Yasoshima T, Mitaka T, Mochizuki Y, Hirata K. Vascular endothelial growth factor C promotes human gastric carcinoma lymph node metastasis in mice. J Exp Clin Cancer Res. 2001;20:419–428. [PubMed] [Google Scholar]
- 25.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 26.Fellmer PT, Sato K, Tanaka R, Okamoto T, Kato Y, Kobayashi M, Shibuya M, Obara T. Vascular endothelial growth factor-C gene expression in papillary and follicular thyroid carcinomas. Surgery. 1999;126:1056–1061; discussion 1061-1062. doi: 10.1067/msy.2099.101432. [DOI] [PubMed] [Google Scholar]
- 27.Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, Fox SB. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res. 2000;6:4278–4286. [PubMed] [Google Scholar]
- 28.Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S. Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res. 2000;6:2431–2439. [PubMed] [Google Scholar]
- 29.Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, Matsutani N, Yasui W, Chayama K. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93:662–666. doi: 10.1002/ijc.1379. [DOI] [PubMed] [Google Scholar]
- 30.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 31.Kabashima A, Maehara Y, Kakeji Y, Sugimachi K. Overexpression of vascular endothelial growth factor C is related to lymphogenous metastasis in early gastric carcinoma. Oncology. 2001;60:146–150. doi: 10.1159/000055312. [DOI] [PubMed] [Google Scholar]
- 32.Kawakami M, Furuhata T, Kimura Y, Yamaguchi K, Hata F, Sasaki K, Hirata K. Quantification of vascular endothelial growth factor-C and its receptor-3 messenger RNA with real-time quantitative polymerase chain reaction as a predictor of lymph node metastasis in human colorectal cancer. Surgery. 2003;133:300–308. doi: 10.1067/msy.2003.45. [DOI] [PubMed] [Google Scholar]
- 33.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer. 1999;80:309–313. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285–292. doi: 10.1097/00006676-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Sobiin SH, Wittekind LH. International Union Against Cancer (UICC). TNM classification of malignant tumors, 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 36.Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of the Biliary Tract, 5th ed. Tokyo: Kanehara; 2003. [Google Scholar]
- 37.Zemzoum I, Kates RE, Ross JS, Dettmar P, Dutta M, Henrichs C, Yurdseven S, Hofler H, Kiechle M, Schmitt M, et al. Invasion factors uPA/PAI-1 and HER2 status provide independent and complementary information on patient outcome in node-negative breast cancer. J Clin Oncol. 2003;21:1022–1028. doi: 10.1200/JCO.2003.04.170. [DOI] [PubMed] [Google Scholar]
- 38.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, Wex C, Lobeck H, Hintze R. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818; discussion 819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Nimura Y. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogura Y, Kawarada Y. Surgical strategies for carcinoma of the hepatic duct confluence. Br J Surg. 1998;85:20–24. doi: 10.1046/j.1365-2168.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki M, Watanabe H, Jass JR, Ajioka Y, Kobayashi M, Hatakeyama K. Immunoperoxidase staining for cytokeratins 8 and 18 is very sensitive for detection of occult node metastasis of colorectal cancer: a comparison with genetic analysis of K-ras. Histopathology. 1998;32:199–208. doi: 10.1046/j.1365-2559.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 42.Glickman JN, Torres C, Wang HH, Turner JR, Shahsafaei A, Richards WG, Sugarbaker DJ, Odze RD. The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer. 1999;85:769–778. [PubMed] [Google Scholar]
- 43.Natsugoe S, Mueller J, Stein HJ, Feith M, Höfler H, Siewert JR. Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer. 1998;83:858–866. [PubMed] [Google Scholar]
- 44.Okami J, Dohno K, Sakon M, Iwao K, Yamada T, Yamamoto H, Fujiwara Y, Nagano H, Umeshita K, Matsuura N, et al. Genetic detection for micrometastasis in lymph node of biliary tract carcinoma. Clin Cancer Res. 2000;6:2326–2332. [PubMed] [Google Scholar]
- 45.Nakashima T, Kondoh S, Kitoh H, Ozawa H, Okita S, Harada T, Shiraishi K, Ryozawa S, Okita K. Vascular endothelial growth factor-C expression in human gallbladder cancer and its relationship to lymph node metastasis. Int J Mol Med. 2003;11:33–39. [PubMed] [Google Scholar]


