Abstract
AIM: To evaluate the immunohistochemical localization of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) on tumor tissue specimens from patients with hepatocellular carcinoma (HCC) and the serum levels of IL-6 and sIL-6R in a group of patients with HCC as well as liver cirrhosis (LC) in a group of patients with LC alone and in a control group.
METHODS: Three groups of subjects were studied: group I (n = 83) suffering from HCC and LC, group II (n = 72) suffering from LC alone and group III (n = 42) as healthy controls. All patients had hepatitis C virus infection. Serum IL-6 and IL-6R levels were determined using a commercially available ELISA kit. Immunohistochemistry was performed using the streptavidin-biotin complex and rabbit polyclonal antibodies against IL-6 and IL-6R.
RESULTS: Immunohistochemistry analysis showed a medium to strong cytoplasmic and membrane reactivity for IL-6 and IL-6R respectively, in at least 40% of cases of HCC, whereas liver cirrhosis patients and controls were negative for IL-6 or showed a very mild and focal dot-like cytoplasmic reaction for IL-6R. Serum IL-6 levels in HCC group were significantly higher than those in LC and control groups (P < 0.0001). There was no significant difference in sIL-6R concentrations among 3 groups. When the patients with HCC were divided into groups according to Okuda’s classification, a significant serum increase of IL-6 and sIL-6R level was observed from stage I to stage III (P < 0.02, P < 0.0005). When HCC and LC patients were divided into 3 classes of cirrhosis severity according to Child-Pugh, values in HCC patients were significantly higher than those in LC patients for each corresponding class (P < 0.01).
CONCLUSION: IL-6 serum levels in HCC patients are higher than those in LC patients and controls, suggesting an increased production of this cytokine by neoplastic cells. sIL-6R values are similar in all groups, increasing only in stage III HCC patients. These data suggest that they have a closer relationship with the neoplastic mass rather than with the residual functioning hepatic mass.
Keywords: Interleukin-6, Cytokine, Chronic liver disease, Immunohistochemistry
INTRODUCTION
Interleukin-6 (IL-6) is a proinflammatory cytokine which plays an important role in the host defence mechanism. Serum IL-6 levels are low in physiological conditions, but increase considerably in pathological conditions such as trauma, inflammation and neoplasia. In tumors, IL-6 may be involved in promoting the differentiation and growth of target cells. In fact, several neoplastic cell lines (such as esophageal cancer, renal cell carcinoma, multiple myeloma, prostate and ovarian cancer) have been shown to produce high in vitro levels of IL-6[1-5], and high concentrations of this cytokine are associated in vivo with a poor outcome of the disease in many types of tumours[6-12]. It has also been hypothesized that activation of the IL-6 gene is responsible for the derangement of some events which can lead to neoplastic degeneration[13].
IL-6 activity is mediated through the binding to its membrane receptor (IL-6R), which in turn promotes the interaction with another receptor component, gp130, able to transduce IL-6 signalling at the intracellular level[14]. High concentrations of soluble IL-6R, like IL-6, are present in serum and other biological fluids in different pathological conditions, because it is released from cells expressing it on their surface[15].
Many works have reported high serum levels of IL-6 in various liver diseases, such as acute hepatitis[16], alcoholic cirrhosis[17], HBV-associated chronic hepatitis, primary biliary cirrhosis (PBC)[18], chronic hepatitis and HCV-correlated liver cirrhosis[19,20] and in hepatocellular carcinoma (HCC)[21-24].
Studies on animal models have shown that transgenic mice expressing high levels of IL-6 and sIL-6R develop hepatic nodular hyperplasia and signs of sustained hepatocyte proliferation, suggesting that IL-6 and sIL-6R could provide the primary stimulus to cell proliferation and are involved in development of HCC[25].
This study aimed to evaluate the immunohistochemical expression and localization of IL-6 and sIL-6R on tissue specimens from patients with HCC-associated liver cirrhosis and liver cirrhosis alone, and the serum levels of IL-6 and sIL-6R in patients with HCC- associated liver cirrhosis (LC) and to compare them in patients with LC alone and healthy controls.
MATERIALS AND METHODS
Patients
The study was performed in 207 subjects divided into three groups. Group I included 93 patients with HCC (61 males, 32 females, mean age 62.2 years, range 43-76 years). Diagnosis was made in 41 cases based on biopsy or cytological findings, diagnosis of the remaining cases was made on the basis of multiple, concordant imaging techniques (ultrasound, helicoidal computed tomography (CT), lipiodol-CT, selective angiography) and biochemical examination (AFP > 400 ng/mL). Some of the patients known as cirrhotics were enrolled in a prospective study for HCC screening, and others were referred to our center diagnosed as HCC. HCC was associated with the presence of serum HCV antibodies in all cases. The patients were then divided into the 3 stages of Okuda’s classification[26] which as well as neoplasia size were also taken into account of serum values of bilirubin and albumin and the presence of ascites. The last three parameters were used to evaluate the hepatic functioning mass and the severity of the underlying cirrhosis. In brief, stage I was an initial stage in which the neoplasm (or the sum of the nodules) measured less than 50% of the whole liver section on CT scan. There was no ascites, albumin levels were over 3 g/dL and bilirubin levels were below 3 mg/dL. Stage II was moderately advanced, with two or more indices of advanced disease. Stage III was very advanced, with three or all indices of advanced disease. Group II included 72 patients (48 males, 24 females, mean age 56.5 years, range 36-75 years) suffering from liver cirrhosis, consecutively selected from out- or in-patients examined at our hospital. Diagnosis was made in 46 cases based on biopsy findings and diagnosis of the remaining cases was made on the basis of unequivocal clinical, biochemical and instrumental data. A post-study follow-up for at least 6 mo excluded the existence of neoplasia. The disease was associated with hepatitis C virus infection in all cases. The control group was composed of 42 healthy asymptomatic subjects (31 males, 11 females, mean age of 54.9 years, range 45-61 years recruited from donors at the blood bank of our hospital. Liver disease was excluded on the basis of anamnestic, biochemical and instrumental data. There were no cases of neoplastic disease, evaluated by a follow-up for at least six months. Daily alcohol consumption of above 30 g/d was found in none of the three groups.
Methods
Blood samples were taken after overnight fasting. After centrifugation part of the sera was used to assay the main parameters of liver function by routine methods. The remainder was frozen at - 40 °C for IL-6 and sIL-6R assay. Serological testing for anti-HCV was performed using the third generation of enzyme-linked immunosorbent assay (ELISA) (Orthodiagnostic System, Raritan, New Jersey, USA) in accordance with the manufacturer’s instructions. Anti-HCV reacting samples were confirmed using the third generation of anti-HCV recombinant immunoblot assay (RIBA III, Chiron Corporation, Emeryville, CA, USA). Markers of HBV were tested using the Abbott RIA kit.
Liver biopsy samples were obtained for diagnostic purposes percutaneously according to the Menghini technique using 1.0-1.2 mm diameter needles (Surecut, Hospital Service, Rome, Italy). In some cases HCC was diagnosed with a thin needle (20 gauge, Surecut) guided by ultrascan using a Toshiba SSA 240 A apparatus with a 3.5 MHz probe.
Serum IL-6 and sIL-6R levels were determined using a commercially available ELISA kit (Quantikine, human IL-6 and sIL-6R, R & D Systems, Minneapolis, USA) in accordance with the manufacturer’s instructions.
Immunohistochemistry analysis was performed on ten different HCC samples, five cirrhotic and two normal liver samples. Histologically normal liver tissues were obtained from patients during surgery for cholelithiasis. Written informed consent was obtained. The specimens were fixed in formalin and embedded in paraffin. Five- µm thick sections were cut and dewaxed, hydrated and incubated in 3% hydrogen peroxide for 20 min. Sections were then heated in microwave oven and non-specific binding was blocked by incubation with 3% rabbit normal serum for 20 min. Immunohistochemistry was performed using the streptavidin-biotin complex (StreptABC) with the following antibodies: rabbit polyclonal antibody against IL-6 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antibody against IL6R (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:100 overnight in a 4 °C moist chamber and mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA, Dako, Santa Barbara, CA) at a dilution of 1:100 for 30 min at room temperature. Sections were then incubated for 30 min at room temperature with biotinylated anti-rabbit and anti-mouse immunoglobulin diluted in phosphate-buffered saline (PBS), with streptavidin-biotin complex for 50 min at room temperature and colour was developed with diaminobenzidine (DAB) for 40 min at room temperature, counterstained with Mayer haematoxylin for 1 min. The expression of IL-6 and IL-6R was considered positive when >30% of cells showed cytoplasmic or membrane staining. The percentage of PCNA- stained nuclei was calculated by counting the number of stained nuclei out of 1000 cells per high-power field for 10 different tumor sections.
Statistical analysis
The data were expressed as mean ± SD. Groups were compared using the Mann-Whitney U test. χ2 test was used for the frequency analyses. Simple linear regression test and Spearman’s rank correlation test were used where appropriate. The cut-off values IL-6 and sIL-6R were calculated as the value of the maximized likelihood ratio (LR) obtained using the following formula: LR = probability of true positives + probability of true negatives/probability of false positives + probability of false negatives. P < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the median, range and ratio of serum IL-6 and sIL-6R values in the 3 study groups, and also in the HCC group divided according to Okuda’s classification. Analysis performed with the Mann-Withney U test showed that the HCC group had significantly higher IL-6 values than the LC (z = 3.1, P < 0.002) and control group (z = 5.4, P < 0.0001). However, the LC patients had significantly higher IL-6 serum levels than controls (z = 2.3, P < 0.03). A significant difference was found in sIL-6R serum levels between the HCC group and controls (z = 2.1, P < 0.04), but not between the HCC and LC groups.
Table 1.
Median and range of serum values of IL-6 (pg/mL) and sIL-6R (ng/mL) in controls, liver cirrhotoc (LC) and hepatocellular carcinoma (HCC) patients and divided according to stage of disease
| Controls | LC | HCC | HCC Stage I | HCC Stage II | HCC Stage III | Z= | P | |
| (n = 42) | (n = 72) | (n = 93) | (n = 28) | (n = 46) | (n = 19) | |||
| median | median | median | median | median | median | |||
| (range) | (range) | (range) | (range) | (range) | (range) | |||
| (a) | (b) | (c) | (d) | (e) | (f) | |||
| IL-6 | 3.6 | 6.4 | 14 | 6.5 | 12 | 32 | b-c: z = 3.1 | 0.002 |
| (3-17) | (3-155) | (3-301) | (3-270) | (3-301) | (3-110) | a-c : z = 5.4 | 0.0001 | |
| a-b : z = 2.3 | 0.03 | |||||||
| d-f : z = 2.8 | 0.005 | |||||||
| e-f : z = 2.7 | 0.01 | |||||||
| b-d : z = 1.9 | 0.05 | |||||||
| sIL-6R | 34.8 | 39.2 | 42.4 | 34.2 | 39.6 | 56 | a-c : z = 2.1 | 0.04 |
| (7.2-80) | (4.8-80) | (17.6-80) | (17.6-65.6) | (18-80) | (30-80) | b-f : z = 2.7 | 0.01 | |
| d-f : z = 3.2 | 0.002 | |||||||
| e-f : z = 2.9 | 0.004 |
Analysis of the values after division of the HCC group according to Okuda’s classification showed that serum values of IL-6 were significantly higher in stage III patients than in stage II and stage I patients (z = 2.7, P < 0.01 and z = 2.8, P < 0.005, respectively). Moreover, IL-6 values in stage I HCC patients were also significantly higher than those in the LC patients (z = 1.9, P < 0.05). There was no significant difference in sIL-6R values between stage I and stage II patients and the LC patients. The only significant difference was found between the stage III HCC patients and the LC patients, stage I and stage II HCC patients (P < 0.004).
Spearman’s rank correlation test showed a significant increase in IL-6 and sIL-6R levels from stage I to stage III (r = 0.28, P < 0.02; r = 0.39, P < 0.0005, respectively).
Table 2 shows the median and range of IL-6 and sIL-6R in the HCC and LC groups divided according to Child-Pugh classes. The median IL-6 value was higher in HCC group than in the LC group, but the difference was significant only in classes A and C (P < 0.0005 and < 0.04, respectively) when compared to the corresponding LC groups. The median sIL-6R value decreased from class A to C in the cirrhotic group, but increased in the HCC group. Consequently, in the LC group the median sIL-6R value was significantly higher in class A (P < 0.04), while in the HCC group it was higher in class C (P < 0.03).
Table 2.
Median and range of serum values of IL-6 (pg/mL) and sIL-6R (ng/ml) in HCC and LC patients divided into groups according to Child-Pugh classes
|
Class A |
Class B |
Class C |
||||
| LC | HCC | LC | HCC | LC | HCC | |
| (n = 20) | (n = 44) | (n = 33) | (n = 30) | (n = 19) | (n = 19) | |
| median | median | median | median | median | median | |
| (range) | (range) | (range) | (range) | (range) | (range) | |
| IL-6 | 3a | 6.5a | 8.5 | 14 | 8c | 28c |
| (3-52) | (3-270) | (3-155) | (3-301) | (3-132) | (3-301) | |
| sIL-6R | 49.8b | 36.8b | 37.2 | 42.8 | 6.8d | 56.2d |
| (21.2-80) | (17.6-67.2) | (4.8-72) | (18-72.8) | (24.4-57.2) | (24-80) | |
P < 0.04 vs sIL-6R ;
P < 0.04,
P < 0.03 vs IL-6.
Figure 1 shows the results of the imunohistochemistry analysis. In the HCC group (ten cases), moderate to strong cytoplasmic and membrane reactivity for IL-6 and IL-6R respectively, was observed in at least 40% of cases (Figures 1A, 1B), whereas control cases of normal liver (two cases) were negative (Figure 1C) or showed a very mild and focal dot-like cytoplasmic reaction for IL-6R (Figure 1D). In liver cirrhosis group (ten cases) immunohistochemistry was negative on the whole or similar to control cases. PCNA immunoreactivity was observed in some nuclei of neoplastic cases with a mean value of 50% in the evaluated areas (Figure 1E).
Figure 1.
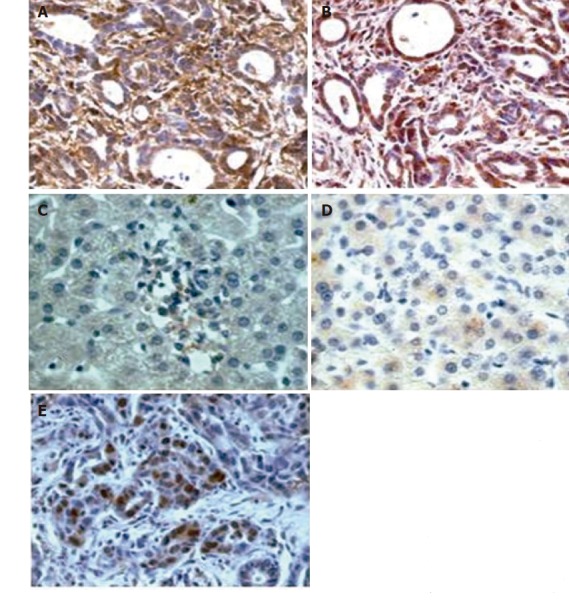
Results of immunohistochemical analysis of cytoplasmic and membrane reaction of IL-6 and IL-6R in HCC (A, B), normal liver (C, D), and cirrhotic (E) patients.
DISCUSSION
Circulating IL-6 levels are elevated in patients with chronic viral[18-20] and alcoholic hepatitis[17]. Increased IL-6 is correlated with the stage of disease in liver cirrhosis [27]. Higher levels of IL-6 correlated with tumor size and cancer aggressiveness in patients with HCC[22].
At present, there are no data about the behaviour of circulating sIL-6R in patients with HCC and very few for other neoplasms. However, in multiple myeloma higher sIL-6R values correlate with a poor outcome of the disease[28]. Therefore, the aim of this study was to evaluate the expression and localization of IL-6 and sIL-6R in pathological liver tissue specimens as well as the serum concentrations of these two cytokines and their ratio in patients with LC-associated HCC and to compare them with patients with LC alone and healthy controls.
Our results showed that the median IL-6 levels in HCC patients were higher than those in LC patients and the controls, indicating that production of the cytokine is increased by the tumor cells. This is supported by the immunohistochemistry result and the fact that the highest IL-6 values were found in Okuda’s stage III, in which the neoplastic mass is the most extensive. However, LC patients also had higher median IL-6 values than controls, but only for Child’s classes B and C, while values in class A were close to control levels. In classes B and C, increased IL-6 serum levels compared to control values might reflect a response of the residual hepatic cells to cytolysis and to the attempt to recover the liver mass[25] associated with a clear impairment. In contrast, in HCC patients, whatever class of the disease, the median values were higher than those in LC patients, indicating that IL-6 serum levels increase when patients pass from cirrhosis to HCC. This was not true in the sIL-6R values, because the median value in HCC patients was significantly higher than that in controls (P < 0.04), and similar to that of LC patients. When the patients with HCC and LC were divided according to Child-Pugh’s classification, the median serum values of sIL-6R tended to decrease from class A to class C in LC patients, while the opposite occurred in HCC patients, indicating that in cirrhotic patients sIL-6R production and release decrease as the disease progresses owing to the reduction of the liver parenchymal mass. On the other hand, in HCC patients, increased sIL-6R serum concentration might be due to the increasing tumor mass.
When the patients with HCC were divided according to Okuda’s classification, IL-6 values significantly increased as the disease worsened. At all the stages the median IL-6 values were significantly elevated compared to the cirrhotic patients, indicating that neoplastic degeneration even in its initial stages, causes variations in IL-6 levels, which could enable us to discriminate cirrhotic from HCC patients. Interestingly, sIL-6R levels were elevated only in patients with a more severe disease (stage III).
The high serum levels of IL-6 and sIL-6R were corroborated by the immunohistochemistry analyses, which showed the marked expression of both cytokines and their receptor in the tumor. This expression correlates to cell proliferation, as demonstrated by the concomitant presence of a high percentage of PCNA positive nuclei. The existence of such a relationship has also been reported for colorectal cancer[29]. On the whole, our results suggest that HCC cells, especially in advanced stages of the disease, may produce and secrete IL-6 and sIL-6R to stimulate their growth by an autocrine/paracrine mechanism as suggested by previous reports concerning cells derived from hepatomas or other types of cancer[30-34].
IL-6 production by tumor cells might also contribute to systemic complications such as induction of cachexia in the host[11] and local immunosuppression rather than immunopotentiation[35]. Thus, the present study might highlight the potential therapeutic benefits of approaches such as use of anti-sense nucleotides or IL-6R super antagonists, which can overcome the adverse effects of IL-6 in HCC, as in hepatoma cells, multiple myeloma, prostate cancer and other tumors[31,34-36].
In conclusion, the IL-6 levels in patients with LC-associated HCC are higher than in patients with LC alone and controls, indicating that production of this cytokine is increased by tumor cells. This has been confirmed by the higher IL-6 values in stage III than in stages I and II of the disease. These results might be of help in differentiating cirrhosis from LC-associated HCC suggest that measures aimed at blocking adverse effects of IL-6 may be of potential clinical utility in HCC.
Footnotes
Supported by: grant from Ministero dell’Istruzione, dell’Università e della Ricerca year 2004 (to GM)
S- Editor Guo SY L- Editor Wang XL E- Editor Cao L
References
- 1.Oka M, Iizuka N, Yamamoto K, Gondo T, Abe T, Hazama S, Akitomi Y, Koishihara Y, Ohsugi Y, Ooba Y, et al. The influence of interleukin-6 on the growth of human esophageal cancer cell lines. J Interferon Cytokine Res. 1996;16:1001–1006. doi: 10.1089/jir.1996.16.1001. [DOI] [PubMed] [Google Scholar]
- 2.Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, Sonoda T, Hirano T, Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 3.Lee JD, Sievers TM, Skotzko M, Chandler CF, Morton DL, McBride WH, Economou JS. Interleukin-6 production by human melanoma cell lines. Lymphokine Cytokine Res. 1992;11:161–166. [PubMed] [Google Scholar]
- 4.Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res. 1990;50:7786–7788. [PubMed] [Google Scholar]
- 5.Watson JM, Sensintaffar JL, Berek JS, Martínez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990;50:6959–6965. [PubMed] [Google Scholar]
- 6.Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukamoto T, Kumamoto Y, Miyao N, Masumori N, Takahashi A, Yanase M. Interleukin-6 in renal cell carcinoma. J Urol. 1992;148:1778–1781; discussion 1781-1782;. doi: 10.1016/s0022-5347(17)37026-x. [DOI] [PubMed] [Google Scholar]
- 8.Seguchi T, Yokokawa K, Sugao H, Nakano E, Sonoda T, Okuyama A. Interleukin-6 activity in urine and serum in patients with bladder carcinoma. J Urol. 1992;148:791–794. doi: 10.1016/s0022-5347(17)36721-6. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa H, Sone S, Takahashi Y, Haku T, Yano S, Shinohara T, Ogura T. Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer. 1995;71:1095–1098. doi: 10.1038/bjc.1995.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martínez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164:1038–1042; discussion 1042-1043;. doi: 10.1016/0002-9378(91)90582-c. [DOI] [PubMed] [Google Scholar]
- 11.Oka M, Yamamoto K, Takahashi M, Hakozaki M, Abe T, Iizuka N, Hazama S, Hirazawa K, Hayashi H, Tangoku A, et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996;56:2776–2780. [PubMed] [Google Scholar]
- 12.Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P'eng FK, Chi CW. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996;91:1417–1422. [PubMed] [Google Scholar]
- 13.Kishimoto T, Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 15.Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, Osugi Y, Tokunaga T, Kishimoto T. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148:2175–2180. [PubMed] [Google Scholar]
- 16.Sun Y, Tokushige K, Isono E, Yamauchi K, Obata H. Elevated serum interleukin-6 levels in patients with acute hepatitis. J Clin Immunol. 1992;12:197–200. doi: 10.1007/BF00918089. [DOI] [PubMed] [Google Scholar]
- 17.Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221–225. [PMC free article] [PubMed] [Google Scholar]
- 18.Kakumu S, Shinagawa T, Ishikawa T, Yoshioka K, Wakita T, Ida N. Interleukin 6 production by peripheral blood mononuclear cells in patients with chronic hepatitis B virus infection and primary biliary cirrhosis. Gastroenterol Jpn. 1993;28:18–24. doi: 10.1007/BF02774999. [DOI] [PubMed] [Google Scholar]
- 19.Huang YS, Hwang SJ, Chan CY, Wu JC, Chao Y, Chang FY, Lee SD. Serum levels of cytokines in hepatitis C-related liver disease: a longitudinal study. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62:327–333. [PubMed] [Google Scholar]
- 20.Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211–215. doi: 10.1007/BF02936370. [DOI] [PubMed] [Google Scholar]
- 21.Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, King KL, Loong CC, Hsia CY, Chi CW. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaguarnera M, Di Fazio I, Laurino A, Romeo MA, Giugno I, Trovato BA. [Role of interleukin 6 in hepatocellular carcinoma] Bull Cancer. 1996;83:379–384. [PubMed] [Google Scholar]
- 23.Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannitrapani L, Cervello M, Soresi M, Notarbartolo M, La Rosa M, Virruso L, D'Alessandro N, Montalto G. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:46–52. doi: 10.1111/j.1749-6632.2002.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 25.Maione D, Di Carlo E, Li W, Musiani P, Modesti A, Peters M, Rose-John S, Della Rocca C, Tripodi M, Lazzaro D, et al. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 1998;17:5588–5597. doi: 10.1093/emboj/17.19.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Genesca J, Gonzalez A, Segura R, Catalan R, Marti R, Varela E, Cadelina G, Martinez M, Lopez-Talavera JC, Esteban R, et al. Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 1999;94:169–177. doi: 10.1111/j.1572-0241.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 28.Wierzbowska A, Urbańska-Ryś H, Robak T. Circulating IL-6-type cytokines and sIL-6R in patients with multiple myeloma. Br J Haematol. 1999;105:412–419. [PubMed] [Google Scholar]
- 29.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–2531. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Matsuguchi T, Okamura S, Kawasaki C, Niho Y. Production of interleukin 6 from human liver cell lines: production of interleukin 6 is not concurrent with the production of alpha-fetoprotein. Cancer Res. 1990;50:7457–7459. [PubMed] [Google Scholar]
- 31.Kumagai N, Tsuchimoto K, Tsunematsu S, Toda K, Takeuchi O, Saito H, Morizane T, Tsuchiya M, Ishii H. Inhibition of growth of human hepatoma cells by dual-function antisense IL-6 oligonucleotides. Hepatol Res. 2002;22:119–126. doi: 10.1016/s1386-6346(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 32.Cervello M, Notarbartolo M, Landino M, Cusimano A, Virruso L, Montalto G, D'Alessandro N. Downregulation of wild-type beta-catenin expression by interleukin 6 in human hepatocarcinoma HepG2 cells: a possible role in the growth-regulatory effects of the cytokine. Eur J Cancer. 2001;37:512–519. doi: 10.1016/s0959-8049(00)00421-4. [DOI] [PubMed] [Google Scholar]
- 33.Borsellino N, Bonavida B, Ciliberto G, Toniatti C, Travali S, D'Alessandro N. Blocking signaling through the Gp130 receptor chain by interleukin-6 and oncostatin M inhibits PC-3 cell growth and sensitizes the tumor cells to etoposide and cisplatin-mediated cytotoxicity. Cancer. 1999;85:134–144. [PubMed] [Google Scholar]
- 34.Lu C, Kerbel RS. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol. 1993;120:1281–1288. doi: 10.1083/jcb.120.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanner J, Tosato G. Impairment of natural killer functions by interleukin 6 increases lymphoblastoid cell tumorigenicity in athymic mice. J Clin Invest. 1991;88:239–247. doi: 10.1172/JCI115283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demartis A, Bernassola F, Savino R, Melino G, Ciliberto G. Interleukin 6 receptor superantagonists are potent inducers of human multiple myeloma cell death. Cancer Res. 1996;56:4213–4218. [PubMed] [Google Scholar]


