Abstract
AIM: To investigate the expression of gastrin-releasing peptide (GRP) and GRP-receptor mRNA in non-tumor tissues of the human esophagus, gastrointestinal tract, pancreas and gallbladder using molecular biology techniques.
METHODS: Poly A+ mRNA was isolated from total RNA extracts using an automated nucleic acid extractor and, subsequently, converted into single-stranded cDNA (ss-cDNA). PCR amplifications were carried out using gene-specific GRP and GRP-receptor primers. The specificity of the PCR amplicons was further confirmed by Southern blot analyses using gene-specific GRP and GRP-receptor hybridization probes.
RESULTS: Expression of GRP and GRP-receptor mRNA was detected at various levels in nearly all segments of the non-tumor specimens analysed, except the gallbladder. In most of the biopsy specimens, co-expression of both GRP and GRP-receptor mRNA appeared to take place. However, expression of GRP mRNA was more prominent than was GRP-receptor mRNA.
CONCLUSION: GRP and GRP-receptor mRNAs are expressed throughout the gastrointestinal tract and provides information for the future mapping and determination of its physiological importance in normal and tumor cells.
Keywords: Gastrin releasing peptide (GRP), Gastrin-releasing peptide receptor (GRPR), mRNA expression, Morphogenesis, Gastrointestinal tract
INTRODUCTION
Gastrin-releasing peptide (GRP) is a member of the bombesin family of neuropeptides. Bombesin was originally isolated from the skin of the amphibian Bombina bombina, whereas GRP is the homologous peptide in mammals, including humans[1]. GRP and GRP-receptor are widely expressed in the central and enteric nervous systems (ENS). GRP is known to stimulate secretion of gastrin, gastric[2] and pancreatic juice[3] and hormones[4,5] to regulate the immune system[6], and to modulate smooth muscle contractility[7,8]. The direct expression of GRP and its receptor, and thus the exact mechanism behind its actions in gastrointestinal tissues, are only sparsely examined. Immunocyochemistry revealed the expression of GRP in submucosal cells of the ileum[9]. In colon, in vitro autoradiography showed the GRP-receptor expression in the myenteric, but not submucosal, plexus as well as on smooth muscle cells[10]. Examination of mucosal biopsies revealed GRP-receptor mRNA in cells lining the gastric antrum, but not in any other epithelial cells of the gastrointestinal tract[11].
GRP and GRP-receptor are frequently expressed in the gastrointestinal cancer cells, such as gastric adenocarcinoma[12,13], duodenal cancer[14], and colorectal cancer[15,16,17]. Cuttitta et al[18] have demonstrated that human cell lines derived from small-cell lung carcinomas of the lung (SCLC) proliferate in response to autocrine release of GRP. In gastrointestinal tumors, GRP-receptor activation only modestly increased tumor cell proliferation, but regulated tumor cell appearances or differentiation and, therefore, should be considered to act as a morphogen[1,16].
Based on these findings, it appears important to establish whether or not GRP and GRP-receptor mRNAs are expressed in non-tumor gastrointestinal tissues. In this study, we analyzed GRP and GRP-receptor mRNA expressions in the human esophagus, gastrointestinal tract, pancreas, and gallbladder by means of a reverse-transcription polymerase chain reaction (RT-PCR) technique and Southern blot analysis of the PCR amplicons.
MATERIALS AND METHODS
mRNA isolation and PCR amplification
The collection, origin and status of full thickness biopsies from the human gastrointestinal tract and surrounding tissues from surgically removed biopsies from patients undergoing surgery for gastric diseases has been described elsewhere in detail[19]. In all, 24 biopsy specimens and two control cDNAs (Table 1) were processed and analyzed for the expression of GRP and GRP-receptor mRNA. In subsequent PCR amplification experiments, mRNA and ss-cDNA preparations used were from a previous study and prepared as described recently[19].
Table 1.
Biopsy specimens, sex, age, and β-actin, GRP and GRP-receptor PCR amplicons detected after exposure to X–ray films for one or five days
| Experimental number | Tissue origin | Sex | Age | β-actin 1 d | GRP 1 d |
GRPR |
|
| 1 d | 5 d | ||||||
| 1 | Esophagus | M | 64 | + | + | + | + |
| 2 | Ventricle | M | 64 | + | - | + | + |
| 3 | Ventricle | M | 84 | + | + | weak | weak |
| 4 | Duodenum | M | 75 | + | - | - | weak |
| 5 | Duodenum | M | 63 | + | + | - | weak |
| 6 | Ileum | F | 78 | + | + | + | + |
| 7 | Caecum | M | 72 | + | + | + | + |
| 8 | Colon ascendens | F | 68 | + | - | - | weak |
| 9 | Colon ascendens | M | 83 | + | - | - | weak |
| 10 | Colon ascendens | F | 74 | + | + | - | weak |
| 11 | Colon ascendens | M | 72 | + | + | - | weak |
| 12 | Colon transversum | M | 79 | + | + | + | + |
| 13 | Colon transversum | F | 79 | + | + | + | + |
| 14 | Colon transversum | F | 79 | + | + | + | + |
| 15 | Colon transversum | F | 74 | + | + | - | - |
| 16 | Colon descendent | M | 79 | + | + | + | + |
| 17 | Colon sigmoideum | M | 69 | + | + | - | - |
| 18 | Colon sigmoideum | M | 86 | + | + | - | weak |
| 19 | Colon sigmoideum | M | 48 | + | + | + | + |
| 20 | Colon sigmoideum | M | 83 | + | + | + | + |
| 21 | Rectum | M | 81 | + | + | - | weak |
| 22 | Rectum | F | 54 | + | - | - | - |
| 23 | Rectum | M | 83 | + | + | + | + |
| 24 | Gallbladder | M | 58 | + | - | - | - |
| 25 | Pancreas | Control | + | + | + | + | |
| 26 | Stomach | Contro | + | + | + | + | |
β-actin amplification was performed in two rounds of PCR (30 and 25 cycles each time, respectively) with the same primers under cycle conditions as described above. Due to the positioning of the primers, cDNA and genomic DNA will yield β-actin fragments of different sizes (288 bp for cDNA and 400 bp for gDNA PCR amplicons, respectively). This allows monitoring for DNA contamination in ss-cDNA preparations and to assess for the integrity of the ss-cDNA used[19]. Control cDNA derived from pancreas and stomach mRNA was purchased from Clontech (BD-Biosciences, Clontech, Stockholm, Sweden).
PCR- amplification of GRP and GRP-receptor ss-cDNA
PCR was performed using a HotStarTaq Master mix kit (Qiagen, Hilden, Germany) in a final reaction volume of 25 μL. Each reaction contained 2 μL of the cDNA synthesis reaction as template. Quick-clone human pancreas and stomach cDNA (Clontech, BD Biosciences Stockholm, Sweden) were used as positive PCR amplification controls, whereas HotStar PCR amplification mix without ss-cDNA addition was used as a negative control. PCR amplification conditions, annealing temperature and primers used are shown in Table 2 and were taken from the study by Uchida et al[20]. For increased sensitivity, nested PCR amplifications were carried out for the detection of GRP and GRP-receptor cDNA. First round PCR amplicons were purified using a GFX PCR and Gel Band DNA Purification Kit (Amersham Biosciences, Uppsala, Sweden) and, subsequently, 1 μL was used in a nested PCR amplification. As positive PCR amplification controls, commercially available human stomach and pancreas cDNAs (Clontech, BD Biosciences Stockholm, Sweden) were included in the study. Negative PCR amplification controls (PCR mix without DNA template addition) were included to monitor possible contaminations.
Table 2.
PCR-primers, expected fragment sizes and PCR amplification conditions
| Primer | Sequence, 5’ to 3’ orientation | Size in bp |
PCR conditions |
|
| 1Cycles | Tannealing | |||
| hGRP-SE/1 | AGTCTCTGCTCTTCCCAGCCTCT | |||
| hGRP-AS/1 | GCAGAACTCAGTCTCTTAGGGGT | 558 | 30 | 55 °C |
| hGRP-SE/2 | CGTGCTGACCAAGATGTACC | |||
| hGRP-AS/2 | TCATTGCTGGTTCAGCTGGG | 349 | 30 | 62 °C |
| hGRPR-SE/1 | AGCCCGGCATAGATCTTATCTTC | |||
| hGRPR-AS/1 | AGGGGGCAAAATCAAGGGTCAAT | 1477 | 30 | 55 °C |
| hGRPR-SE/2 | CTCCCCGTGAACGATGACTGG | |||
| hGRPR-AS/2 | ATCTTCATCAGGGCATGGGAG | 388 | 30 | 62 °C |
| β-actin-SE | GCATGGAGTCCTGTCGCATCCACG | |||
| β-actin-AS | CGTCATACTCCTGCTTGCTGATCCA | 2288/400 | 30/25 | 55 °C |
1Numbers of cycles in first and second round PCR amplifications;
cDNA and gDNA PCR amplicon sizes, respectively.
Southern blot analysis of PCR amplicons
PCR amplicons were electrophoretically separated on a
15 g/L agarose gel and Southern blot analysis was performed using 10 pmoles of [32P]-5’-end-labelled nested primers hGRP-SE/2 and hGRP-AS/2 or hGRPR-1/SE and hGRPR-2/AS primers as hybridisation probes (Table 2) under the conditions described elsewhere[19].
RESULTS
Integrity of ss-cDNA
All analyzed ss-cDNA yielded fragments of the expected length (288 bp) after two rounds of β-actin PCR amplification and Southern blot analysis, indicating that the ss-cDNA used was essentially free of DNA contamination[19].
Differential tissue expression of GRP and GRP-receptor mRNA
Initially, first round and nested GRP and GRP–receptor PCR amplification conditions were optimized by means of annealing temperature and cycle conditions using two established, premade PCR amplification mixes (puReTaq Ready-To-Go PCR beads, Amersham Biosciences and HotStarTaq Master mix kit, Qiagen). Essentially, the HotStarTaq Master mix kit was used under the conditions described in Table 2.
Southern blot analysis (one-day exposure) of nested GRP-PCR amplicons derived from ss-cDNA revealed PCR bands of the expected size in 18 of 24 (75%) biopsy specimens and in the human stomach and pancreas control cDNA’s. It appeared that GRP-PCR amplicons of two distinct sizes were present. These GRP-PCR amplicons were similar in sizes to earlier described GRP-PCR amplicons, derived from alternatively spliced GRP-mRNA[20]. However, no attempts were made to further investigate this point. Furthermore, 11 of 24 (46%) biopsy specimens and the human stomach and pancreas control cDNA yielded GRP-receptor PCR amplicons of the expected size (Figure 1, Table 1). After five-day exposure, an additional 9 of 24 (37%) biopsies yielded weak GRP-receptor PCR amplicons as judged by Southern blot analysis (Table 1), indicating a low level expression of GRP-receptor mRNA. Similarly, two tissues revealed the presence of an extra and larger GRP-receptor PCR amplicon. Its nature has not been further investigated.
Figure 1.
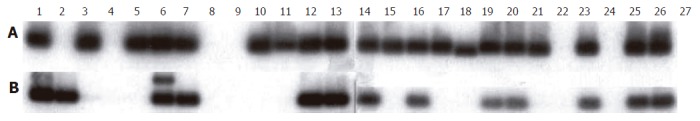
Southern blot hybridization analysis of nested PCR amplicons derived from ss-cDNA 1 to 26 as specified in Table 1. Lane 27 represents a negative PCR control (no ss-cDNA template added to the PCR master mix). Exposure to X-ray films was for 1 d using an intensifier screen at -70 °C. A: hGRP-PCR amplicons; B: hGRP receptor PCR amplicons.
No GRP and GRP-receptor PCR amplicons could be detected in the gallbladder tissue, indicating a lack of expression of these mRNAs. However, only one gallbladder biopsy was analyzed and, therefore, the result may not be conclusive since variable GRP and GRP-receptor mRNA expressions were observed in ventricle, duodenum, colon ascendens and rectum biopsies (Figure 1, Table 1). More specifically, GRP mRNA appeared to be expressed in 2 of 4 colon ascendens biopsies. In contrast, a weak band corresponding to GRP-receptor expression was detected in 4 of 4 colon ascendens biopsies after 5-d exposure, indicating a low level of GRP-receptor mRNA expression in these tissues (data not shown). Remarkably, PCR amplicons corresponding to GRP mRNA expression were detected in 4 of 4 transverse colon and 4 of 4 sigmoid colon biopsies (Figure 1). Similarly, GRP-receptor mRNA seemed to be co-expressed in 3 of 4 transverse colon and 3 of 4 sigmoid colon (Table 1).
DISCUSSION
Biopsy specimens were collected from various sites of the human gastrointestinal tract and surrounding tissues. Efforts were made to collect the biopsies from fresh, histologically normal tissues (Table 1). Our results showed that GRP and GRP-receptor mRNAs were widely expressed in the human gastrointestinal tract and surrounding tissues. It is tempting to speculate that the variation in GRP and GRP-receptor mRNA levels observed (Figure 1) may reflect a real-time mRNA expression situation. However, we can not exclude the possibility that artifacts based on sample selection (site of collecting and biopsy sizes) may contribute to the observed mRNA level variations.
Co-expression of GRP and GRP-receptor mRNA in the same tissue as observed could lend support to speculations about the existence of an autocrine and/or paracrine loop. For paracrine signaling, the communicating cells need to be in close proximity in order to establish such loops. Expressions of GRP and its receptor mRNA could be originating from different cell types in opposing parts of the tissue collected. To verify the existence of such an autocrine and/or paracrine loop, cellular co-expression of GRP and GRP-receptor mRNA and its subsequent translation into biologically active proteins must be confirmed. This could be achieved either by in situ hybridization or histochemistry techniques, using GRP and GRP-receptor specific hybridization probes or antibodies, respectively.
In lack of the results of such studies, the physiological importance of GRP and GRP-receptor mRNA expression in gastrointestinal tissues can only be speculated upon. However, based on earlier studies, it seems likely that GRP could act on the human colon via receptors on smooth muscle cells and gastric epithelial cells as well as on cells of the ENS[11,12]. GRP has been shown to be the primary transmitter of motor neurones to gastrin cells[2]. Pharmacological doses of GRP showed a concentration-dependent increase in the rhythmic activity of the ileocecum region[21] and evoked contractions of isolated muscle cells from jejunum[7]. Accordingly, inhibition of endogenous GRP delayed gastric emptying and gallbladder contraction[22]. In contrast, small bowel transit was prolonged by the same antagonist[22]. Thus, the effect on the small intestine may be important for mixing movements and not so much for the peristalsis. In our study, the presence of GRP and its receptor throughout the gastrointestinal tract, in addition to earlier studies that showed no expression of the peptides in the epithelial layer except gastric antrum[12] but on colonic smooth muscle cells and ENS[11], raises the hypothesis that the peptide may affect the motility along the entire GI tract. This is further underlined by the effects on smooth muscle cells[7,21]. Disturbed tissue levels of GRP have been described in patients with idiopathic intestinal pseudo-obstruction[23]. The physiology and pathophysiology behind intestinal motility and dysmotility are in many aspects unknown. It is difficult to study the physiological effect of one single peptide alone, as the ENS contains many different peptides with an important balance between them. However, GRP seems to be one of the interesting peptides in the regulation of gastrointestinal motility.
To best of our knowledge, this is the first study that describes the presence of GRP and its receptor in the human pancreas. It is in accordance with the observed effect of GRP on the secretion of pancreatic juice and pancreatic hormones[3-5]. Earlier animal studies have described that GRP is released from vagal, pancreatic nerves after stimulation[4,24]. GRP then acts by binding to a specific member of the 7 transmembrane spanning, G protein-coupled receptor superfamily where activation by GRP-receptors is coupled to phospholipase C and phospholipase D[24,25].
We were not able to detect GRP or GRP-receptor mRNA expression in the gallbladder. This may be explained by the fact that only one patient was examined. The earlier described effect of a GRP antagonist to inhibit gallbladder contraction suggests that GRP receptors are expressed in the gallbladder[22]. However, antagonists may antagonise more than one receptor, and the PCR technique in the present study was specifically examining the GRP-receptor, not similar receptors in the same family.
The effect of GRP in gastrointestinal carcinogenesis is unclear. Most of resected colon cancers aberrantly express GRP receptor mRNA[26], whereas immunohistochemically, less than three-quarters of human tumors express this protein[16]. Furthermore, not all of these receptors are functional when expressed, as only a minor amount of resected human colon cancers have been found to bind (125I-Tyr4) bombesin when studied pharmacologically[27]. The discrepancy between GRP-receptor mRNA and protein expression may be due to the frequency with which the coding sequence for this receptor is mutated[17]. Some authors suggest that GRP acts as a mitogen and increases tumor cell proliferation[18], while others have found that GRP/GRP-receptor co-expression in cancer promotes the development of a well-differentiated phenotype and is therefore more a morphogen than a mitogen[1]. Multiple studies suggest that the presence of these two peptides confers a survival advantage[14,16].
In conclusion, GRP and GRP-receptor mRNA appear to be expressed throughout the human gastrointestinal tract. This provides information for the future mapping of GRP and GRP-receptor expression at the cellular level, and thereby further determination of its physiological importance in normal and tumor cells.
Footnotes
Supported by the Molecular Biology Program (Grant No. 21407), Laboratory Medicine Center-LMC, University Hospital Linköping, Sweden, and the Development Foundation of Region Skåne, Sweden
S- Editor Wang J L- Editor Kumar M E- Editor Bai SH
References
- 1.Jensen JA, Carroll RE, Benya RV. The case for gastrin-releasing peptide acting as a morphogen when it and its receptor are aberrantly expressed in cancer. Peptides. 2001;22:689–699. doi: 10.1016/s0196-9781(01)00380-1. [DOI] [PubMed] [Google Scholar]
- 2.McConalogue K, Furness JB. Gastrointestinal neurotransmitters. Baillieres Clin Endocrinol Metab. 1994;8:51–76. doi: 10.1016/s0950-351x(05)80226-5. [DOI] [PubMed] [Google Scholar]
- 3.Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481:1–14. doi: 10.1016/j.ejphar.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Knuhtsen S, Holst JJ, Baldissera FG, Skak-Nielsen T, Poulsen SS, Jensen SL, Nielsen OV. Gastrin-releasing peptide in the porcine pancreas. Gastroenterology. 1987;92:1153–1158. doi: 10.1016/s0016-5085(87)91071-7. [DOI] [PubMed] [Google Scholar]
- 5.Wood SM, Jung RT, Webster JD, Ghatei MA, Adrian TE, Yanaihara N, Yanaihara C, Bloom SR. The effect of the mammalian neuropeptide, gastrin-releasing peptide (GRP), on gastrointestinal and pancreatic hormone secretion in man. Clin Sci (Lond) 1983;65:365–371. doi: 10.1042/cs0650365. [DOI] [PubMed] [Google Scholar]
- 6.De la Fuente M, Del Rio M, Hernanz A. Stimulation of natural killer and antibody-dependent cellular cytotoxicity activities in mouse leukocytes by bombesin, gastrin-releasing peptide and neuromedin C: involvement of cyclic AMP, inositol 1,4,5-trisphosphate and protein kinase C. J Neuroimmunol. 1993;48:143–150. doi: 10.1016/0165-5728(93)90186-3. [DOI] [PubMed] [Google Scholar]
- 7.Micheletti R, Grider JR, Makhlouf GM. Identification of bombesin receptors on isolated muscle cells from human intestine. Regul Pept. 1988;21:219–226. doi: 10.1016/0167-0115(88)90004-3. [DOI] [PubMed] [Google Scholar]
- 8.Bitar KN, Zhu XX. Expression of bombesin-receptor subtypes and their differential regulation of colonic smooth muscle contraction. Gastroenterology. 1993;105:1672–1680. doi: 10.1016/0016-5085(93)91062-m. [DOI] [PubMed] [Google Scholar]
- 9.Dhatt N, Buchan AM. Colocalization of neuropeptides with calbindin D28k and NADPH diaphorase in the enteric nerve plexuses of normal human ileum. Gastroenterology. 1994;107:680–690. doi: 10.1016/0016-5085(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 10.Rettenbacher M, Reubi JC. Localization and characterization of neuropeptide receptors in human colon. Naunyn Schmiedebergs. Arch Pharmacol. 2001;364:291–304. doi: 10.1007/s002100100454. [DOI] [PubMed] [Google Scholar]
- 11.Ferris HA, Carroll RE, Lorimer DL, Benya RV. Location and characterization of the human GRP receptor expressed by gastrointestinal epithelial cells. Peptides. 1997;18:663–672. doi: 10.1016/s0196-9781(97)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.Preston SR, Woodhouse LF, Jones-Blackett S, Wyatt JI, Primrose JN. High affinity binding sites for gastrin releasing peptide on human gastric cancer and Ménétrier's mucosa. Cancer Res. 1993;53:5090–5092. [PubMed] [Google Scholar]
- 13.Carroll RE, Carroll R, Benya RV. Characterization of gastrin-releasing peptide receptors aberrantly expressed by non-antral gastric adenocarcinomas. Peptides. 1999;20:229–237. doi: 10.1016/s0196-9781(98)00164-8. [DOI] [PubMed] [Google Scholar]
- 14.Williams BY, Schonbrunn A. Bombesin receptors in a human duodenal tumor cell line: binding properties and function. Cancer Res. 1994;54:818–824. [PubMed] [Google Scholar]
- 15.Chave HS, Gough AC, Palmer K, Preston SR, Primrose JN. Bombesin family receptor and ligand gene expression in human colorectal cancer and normal mucosa. Br J Cancer. 2000;82:124–130. doi: 10.1054/bjoc.1998.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. Aberrant expression of gastrin-releasing peptide and its receptor by well-differentiated colon cancers in humans. Am J Physiol. 1999;276:G655–G665. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- 17.Carroll RE, Ostrovskiy D, Lee S, Danilkovich A, Benya RV. Characterization of gastrin-releasing peptide and its receptor aberrantly expressed by human colon cancer cell lines. Mol Pharmacol. 2000;58:601–607. doi: 10.1124/mol.58.3.601. [DOI] [PubMed] [Google Scholar]
- 18.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 19.Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept. 2004;119:39–44. doi: 10.1016/j.regpep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Uchida K, Kojima A, Morokawa N, Tanabe O, Anzai C, Kawakami M, Eto Y, Yoshimura K. Expression of progastrin-releasing peptide and gastrin-releasing peptide receptor mRNA transcripts in tumor cells of patients with small cell lung cancer. J Cancer Res Clin Oncol. 2002;128:633–640. doi: 10.1007/s00432-002-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vadokas B, Ludtke FE, Lepsien G, Golenhofen K, Mandrek K. Effects of gastrin-releasing peptide (GRP) on the mechanical activity of the human ileocaecal region in vitro. Neurogastroenterol. Motil. 1997;9:265–270. doi: 10.1046/j.1365-2982.1997.d01-59.x. [DOI] [PubMed] [Google Scholar]
- 22.Degen LP, Peng F, Collet A, Rossi L, Ketterer S, Serrano Y, Larsen F, Beglinger C, Hildebrand P. Blockade of GRP receptors inhibits gastric emptying and gallbladder contraction but accelerates small intestinal transit. Gastroenterology. 2001;120:361–368. doi: 10.1053/gast.2001.21174. [DOI] [PubMed] [Google Scholar]
- 23.el-Salhy M, Norrgård O. Colonic neuroendocrine peptide levels in patients with chronic idiopathic slow transit constipation. Ups J Med Sci. 1998;103:223–230. doi: 10.3109/03009739809178951. [DOI] [PubMed] [Google Scholar]
- 24.Gregersen S, Ahrén B. Studies on the mechanisms by which gastrin releasing peptide potentiates glucose-induced insulin secretion from mouse islets. Pancreas. 1996;12:48–57. doi: 10.1097/00006676-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Corjay MH, Dobrzanski DJ, Way JM, Viallet J, Shapira H, Worland P, Sausville EA, Battey JF. Two distinct bombesin receptor subtypes are expressed and functional in human lung carcinoma cells. J Biol Chem. 1991;266:18771–18779. [PubMed] [Google Scholar]
- 26.Saurin JC, Rouault JP, Abello J, Berger F, Remy L, Chayvialle JA. High gastrin releasing peptide receptor mRNA level is related to tumour dedifferentiation and lymphatic vessel invasion in human colon cancer. Eur J Cancer. 1999;35:125–132. doi: 10.1016/s0959-8049(98)00276-7. [DOI] [PubMed] [Google Scholar]
- 27.Preston SR, Woodhouse LF, Jones-Blackett S, Miller GV, Primrose JN. High-affinity binding sites for gastrin-releasing peptide on human colorectal cancer tissue but not uninvolved mucosa. Br J Cancer. 1995;71:1087–1089. doi: 10.1038/bjc.1995.210. [DOI] [PMC free article] [PubMed] [Google Scholar]


