Abstract
In the developed and developing countries, corrosive injury to the gastrointestinal system as a consequence of either accidental ingestion or as a result of self-harm has become a less common phenomenon compared to decades ago. This could partly be attributed to the tighter legislation imposed by the government in these countries on detergents and other corrosive products and general public awareness. Most busy upper gastrointestinal surgical units in these countries, especially in the developed countries will only encounter a small number of cases per year. Up to date knowledge on the best management approach is lacking. In this article, we present our experience of two contrasting cases of corrosive injury to the upper gastrointestinal tract in our thoracic unit in the last 2 years and an up-to-date Medline literature search has been carried out to highlight the areas of controversies in the management of corrosive injuries of the upper gastrointestinal tract. We concluded that the main principle in managing such patients requires a good understanding of the pathophysiology of corrosive injury in order to plan both acute and future management. Each patient must be evaluated individually as the clinical picture varies widely. Signs and symptoms alone are an unreliable guide to injury.
Keywords: Acid, Alkali, Oesophageal stricture, Endoscopy, Steroids, Oesophageal and gastric carcinoma
INTRODUCTION
In the developed and developing countries, corrosive injury to the gastrointestinal system as a consequence of either accidental ingestion or as a result of self-harm has become less of a common phenomenon. This could partly be attributed to the tighter legislation imposed by the government on detergents and other corrosive products and general public awareness[1,2]. Most busy upper gastrointestinal (GIT) surgical units, especially in the developed countries will only encounter a small number of cases per year. Up to date knowledge on the best management approach may therefore be lacking.
We present two contrasting cases of corrosive injury to the upper GIT, which presented to our thoracic unit in the last 2 years to highlight the contrasting aspects of chemical burn injury to the upper GIT. A Medline search has been carried out to extract relevant articles to enable us to perform a literature review to discuss the areas of controversies in the management of corrosive injuries of the upper GIT.
CASE REPORTS
Case 1
A 22-year-old male with learning disability attended the Casualty Department following accidental ingestion of a cupful of 30% caustic soda and had vomited immediately after it. On presentation, his voice was hoarse. He was also short of breath and drooling his saliva. On examination, he had a red, swollen tongue and his oropharynx was oedematous and inflamed. He was intubated to secure his airway and transferred to the intensive care unit. Other supportive treatments received included intravenous proton pump inhibitor (PPI) and total parental nutrition (TPN). He was extubated 2 d later. Early oesophagogastroscopy (Figure 1) revealed generally inflamed oropharynx and Savary grade 3 oesophagitis from 20 cm. Examination beyond this point was not attempted. Barium meal (Figure 2) carried out two weeks later showed a long stricture segment from just distal to the hypopharynx to the oesophago-gastric junction. The patient did not receive steroid therapy during any stage of his treatment. He was successfully managed with repeated progressively time spaced dilatation using a guide wire under fluoroscopy. He currently attends Day Procedure Unit every 6-12 wk for oesophageal dilatation.
Figure 1.
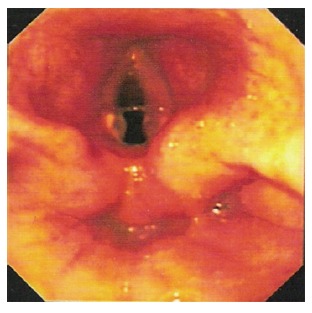
Endoscopic picture showing gross laryngeal edema with inflamed adjacent structures (case 1).
Figure 2.

Barium meal showing a long stricture segment from just distal to the hypopharynx to the oesophago-gastric junction (case 1).
Case 2
A 33-year-old male with a history of overdose and assaults presented to the Casualty Department with hoarseness and stridor following ingestion of about 40 mL of battery acid (hydrochloric acid) secondary to deliberate self-harm. He was intubated to secure his airway and transferred to ITU. A CT scan of his chest and abdomen showed thickened distal oesophagus and stomach, small bilateral pleural effusion and no obvious sign of perforation. Early endoscopy showed inflammation and ulceration of the pharynx and oesophagus with contact bleeding and circumferential ulceration of the oesophageal mucosa at 25 cm (Figure 3). His stomach was filled with blood. The immediate supportive treatments included intravenous PPI, TPN for nutrition, steroids and broad-spectrum antibiotics because of gross laryngeal oedema and positive blood culture. He improved on conservative management and following extubation was allowed oral feeding. Endoscopy was repeated 10 d later and it showed small ulcers at the level of the vocal cords. Upper oesophagus was relatively spared. A tight cricopharyngeus was noted and his lower oesophagus showed a circumferential burn with slough. Similar findings were noted on the mid-body of the stomach and the antrum but the duodenum was spared.
Figure 3.
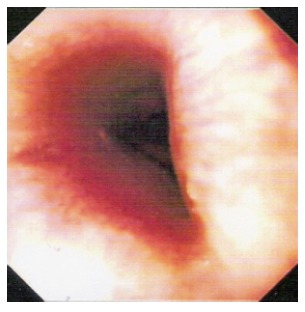
Oesophagogastric dissociation (OGD) of the patient after ingestion of battery acid showing circumferential burn to the lower oesophagus (case 2).
He was discharged home three weeks later only to be readmitted a week and a half post-discharge with symptoms and signs of gastric outlet obstruction. Endoscopy at this stage showed a normal oesophagus with an ulcerated and scarred gastric pylorus. The endoscope failed to advance beyond this point. These findings were confirmed on a dilute barium swallow (Figure 4). Roux-en-Y gastrojejunostomy was performed subsequently in order to bypass the stricture. He remained well post-operatively.
Figure 4.
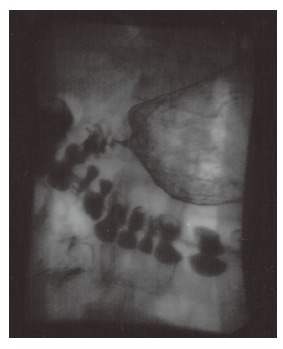
Barium study of patient showing partial gastric outlet obstruction with marked gastric dilatation with an irregular stricture of the pylorus and proximal duodenum (case 2).
DISCUSSION
There are a vast variety of chemicals commonly available in a modern western household that can be ingested either inadvertently or intentionally. Failure to recognize the seriousness of the accident and to provide adequate therapy could result in serious morbidity and mortality. Children account for more than 80% of accidental corrosive ingestion but ingestion in adult is more often of suicidal intent, and, therefore, tend to be more serious[3]. The mortality rate is between 10% to 20% and rises to 78% in cases of attempted suicide[4]. The extent of the injury depends on the type of agent, its concentration, quantity and physical state, the duration of exposure and the presence of food particles in the stomach[5-7].
Pathophysiology
The dichotomy of oesophageal versus gastric injury in cases of acid and alkali ingestion has long been recognized by surgeons and gastroenterologists[8]. Whilst acid is said to “lick the oesophagus and bite the pyloric antrum”, alkaline tends to cause a more uniformly severe mucosal injury to the oesophagus[3,6,9]. Although acid injury is usually limited to the stomach, 6%-20% of patients have other associated oesophageal and small intestinal injuries[6]. Our two cases clearly illustrated this with the caustic injury in case 1 causing extensive oesophageal injury whilst the acid resulting in gastric injury and sparing the oesophagus.
Acid injuries cause “coagulation necrosis” on tissue contact; the coagulum formed hinders any further tissue penetration[3,4,9,10]. On the other hand, caustic injuries induce “liquefaction necrosis”, a process that leads to the dissolution of protein and collagen, saponification of fats, dehydration of tissues and thrombosis of blood vessels, resulting in deeper tissue injury[3,4,9,10]. Zargar et al noted that acute gastric injury was present in 85.4% of their patients who had ingested acid, involving mainly the distal half of the stomach with 44.4% having late complications in the form of pyloric or antral stenosis and linitis plastica-like deformity[11]. The relative sparing of the duodenum is thought to be due to pyloric spasm induced by the irritant acid in the antrum and the alkaline pH of the duodenum[11]. Our patient who ingested battery acid developed partial gastric outlet obstruction. Contrast study performed documented duodenal rigidity and lack of normal mucosal pattern. However, the distinction between the expected sites of gastrointestinal injury following acid versus alkali ingestion is not always clear.
Burn classification
Injuries secondary to chemical burns of the upper gastrointestinal tract are classified in similar fashion to thermal burn of the skin. They are classified into three degrees based only on the extent and severity of the superficial lesions[12]. An appreciation of the depth of the involvement may improve our treatment, but at present, no definite measurements of the depth can be made, and the grading at best is subjective. Endoscopic ultrasound may provide an answer. A third-degree burn can easily be mistaken for a second-degree burn[9].
Oropharyngeal burns and clinical symptoms have a low predictive value for severity of oesophageal injury[13,14]. Haller et al observed that 70% of their patients with oropharyngeal burns did not have significant damage to the oesophagus[15]. Both our patients had severe oropharyngeal burns requiring immediate intubation in order to protect their airway but only one developed severe oesophageal injury.
Early versus late endoscopy
Early endoscopy is regarded as the most appropriate measure based on which clinical decisions are made in people who have ingested corrosive substances[3,8,12,16-18]. The majority of physicians and surgeons now favour early endoscopy. Nevertheless, early endoscopy in the hands of a less-experienced endoscopist could be hazardous[2,10]. It is important to minimize force and air insufflation when passing the endoscope in this group of patients[10]. The risk of oesophageal perforation is low if the procedure is performed under general anaesthesia and the endoscope is passed to the first burned area but not beyond it[16]. Hawkins et al recommend diagnostic oesophagoscopy under general anaesthesia within the first 36 h of corrosive ingestion. In severe oropharyngeal burns, endoscopy may be deferred up to seven days to allow the acute oedema to subside, thereby reducing the risk of airway complication[8]. Zargar et al performed endoscopies on 88 patients within 96 h following corrosive ingestion and found no complications related to the procedure[19]. Others have also documented the safety of early endoscopy[3]. Both our patients had endoscopy carried out within 72 h following corrosive ingestion. Early endoscopy is essential in the continuing management of patients with corrosive injury as it affords an opportunity to verify directly the healing state of the mucosa and may be of value in predicting which patients require further early intervention[1,2,8,19,20].
Endoscopy is not without its limitations[1]. It is difficult to assess the depth of any burn with absolute certainty by observing superficial epithelial necrosis[2]. If a severe burn is encountered in the upper third of the oesophagus, the scope is not passed beyond this point. In this case, it will be difficult to ascertain the degree of injury to the rest of the oesophagus[3]. The area of burn may not be visualised, thus delaying the diagnosis[2]. Others have attempted the use of endoscopic ultrasound to improve the accuracy of diagnosis. However, Chiu et al did not find concomitant use of endoscopic ultrasound (EUS) useful in improving the accuracy of predicting early or late complication of stricture[21].
Manometric study
Genc and Mutaf investigated the use of manometric studies and suggested that it could give important data about the severity of the initial oesophageal injury[22]. Dantas et al showed that a majority of their patients with caustic oesophageal injury exhibit alterations in oesophageal motility, ranging from low amplitude non-peristaltic contractions to some degree of alteration of lower oesophageal sphincter pressure[5]. Thus, it could play a role in determining the prognosis. PPI is generally prescribed on the basis of associated reflux. This data provide supporting evidence for its use.
Complications of corrosive ingestion
Severe complications, often life threatening are common following corrosive injury to the upper gastrointestinal tract. These include tracheobronchial fistula in 3%, severe haemorrhage secondary to gastric involvement, aortoenteric fistula or gastrocolic fistula, strictures and perforation in 10% of cases[3,5,13,20]. Stricture formation, by far, remains the main long-term complication of this injury. Over 90% of patients with third-degree burns go on to develop stricture and 15%-30% if they have second-degree burns [3]. Mamede et al observed an 89.3% incidence of oesophagitis in their 37-year historical series; 72.6% of the cases involved progression to stenosis and 1% died during acute phase[7]. A lumen >10 mm in diameter is thought not to impede normal life and should be left alone[13].
Early use of steroids and antibiotic: Prevention of stricture formation
Corticosteroids inhibit the transcription of certain matrix protein genes, procollagen, fibronectin, TGF-β and many cytokines. They also reduce the synthesis of α2-macroglobulin, an inhibitor of collagenase activity[23]. Animal experiments have shown that if antibiotics and steroids are given early following ingestion of a corrosive substance, the likelihood of stricture formation is reduced[2,24]. Bautista et al found dexamethasone more effective than prednisolone in preventing stricture formation following experimental oesophageal burns[24]. Mamade et al concluded from their clinical experience of 239 cases over 38 years that lower doses of steroid have little effect on the prophylaxis of stricture[25]. Higher doses only seem to contribute to the onset of complications such as increased vulnerability to infection and gastrointestinal bleeding[16,25]. Several authors have found corticosteroids ineffective in preventing oesophageal stricture[10,12,14,16,18]. This has also been shown in a more recent randomised controlled clinical trial in children.
Intra-lesional corticosteroid therapy has shown beneficial effects for refractory oesophageal strictures caused by corrosive burns. A report by Kochhar et al concluded that patients treated in this way experience a longer dilatation-free interval, thus requiring fewer dilations[26]. However, the number of patients involved in the study is small.
In our two contrasting cases, steroid was given to the patient with acid burns for the first 24 h in view of the severity of laryngeal oedema at presentation to avoid casualty. In another patient who suffered from caustic burns, steroid was not given because the literature suggests that the complication risk outweighs the efficacy in preventing stricture formation.
To date, there is no convincing evidence supporting the use of antibiotics in reducing stricture formation[18]. An animal study has shown that it could decrease infection in steroid treated burns[3]. Kirsh et al recommended the use of antibiotics for 7 d to 2 wk as a means to both decrease the risk of pulmonary infection and bacterial invasion through the injured oesophagus into the mediastinum[2]. Our general consensus when treating a patient with such injury is that antibiotic treatment should only be commenced when the patient is treated with steroids or there are signs of infection with source of infection and infecting organism identified. Prophylactic use of antibiotics without steroid treatment is unjustified[18].
Routine use of nasogastric (NG) tube
Mamede et al reported a significant lower incidence of stricture formation with routine use of NG tube for 15 d following the injury[25]. However the NG tube could not act as an oesophageal ‘mould’ because one could expect the stenosing effect to continue longer than 15 d. Wijburg et al also reported a decline in stricture formation in a patient with long-term nasogastric tube placement[27].
However, contrasting results were obtained from other literatures stating that long-term indwelling nasogastric insertion is known to cause long strictures of the oesophagus even in patients without oesophageal burns[3,18]. We do not advocate the use of a NG tube as we have experienced a number of patients who developed complex stricture following nasogastric insertion. Furthermore, the presence of a NG tube will aggravate reflux by making the lower oesophageal sphincter incompetent. We used TPN in both patients and would have proceeded to feeding jejunostomy if oral feeding was not soon established.
Experimental studies to prevent stenosis
In a recent experimental study, cytokines have been used successfully in preventing stricture formation by Berthet and colleagues[20]. The theory was based on the rationale of the inflammatory process and cascade of events. Epidermal growth factor (EGF) was used because of its properties of fibroblast stimulation and improvement of local vascular conditions. Interferon-γ (IFN-γ) was also used to reduce fibrosis as it inhibits collagen I and III formation and fibronectin synthesis[20]. Hydroxyproline was used as an indirect measurement of collagen production as it is the ultimate product of collagen degradation[20]. Stenosis was not observed in treated animals[20]. There was a lower level of hydroxyproline in combined treatment compared to EGF alone[20].
Kaygusuz et al investigated the effect of interferon-α-2b and octreotide in the treatment of corrosive burns of the oesophagus[28]. A histopathological examination of the exposed oesophagus demonstrated that octreotide and interferon-α-2b distinctively depressed the fibrotic activity in the second phase of wound healing that occurred in the oesophageal wall after a corrosive burn[28]. Gunel et al showed in their animal experiment that treatment with an antioxidant, such as vitamin E and methylprednisolone decreased tissue hydroxyproline and thus, inhibiting new collagen synthesis and stricture formation following corrosive injury[29]. However, all these studies are only carried out on animals and these treatments have not been tested on humans.
Management
The acute management is based on the acute trauma life support (ATLS) guidelines for burn injury. This includes securing the airway, pain relief and attending to adequate intravenous fluid replacement. Tracheostomy may be necessary in cases of severe laryngeal oedema, whereby tracheal intubation fails and there is a danger of completely closing over of the airway due to the edema[2,30]. The aim of treatment at this stage is to stabilize vital parameters. The patient is kept strictly nil by mouth in acute phase. A plain chest radiograph is advisable and might reveal signs of perforation, i.e. pneumomediastinum and free air under the diaphragm[3,19,28,30]. However the sensitivity is low and if perforation is suspected, diluted barium swallow should be carried out. It is crucial that the attending medical officers are aware of the severity of such injury and able to identify life-threatening complications associated with the injury. The use of antidote such as water or milk does not seem to prevent stenosis[25]. Endoscopy is the diagnostic procedure of choice in the absence of known perforation[3]. Patients with perforation require immediate surgery[3]. Gastric acid suppression with PPIs and H2-antagonists are often used in corrosive burn injury as oesophagitis and gastritis are common and patients have been kept fasting[18]. This treatment has been employed in both our patients in order to suppress gastric acid production and to prevent stress ulcers in the stomach.
Our first patient with the alkaline burns of the gastrointestinal tract who later developed oesophageal strictures was managed with frequently repeated dilatation. Hawkins et al reported a relatively high success rate with dilatation[16]. Dilatation could be antegrade or retrograde or a combination of both[1]. Early dilatation is not recommended due to associated high incidence of perforation and associated morbidity[3]. Most authors advised waiting 3 to 6 wk after the initial injury before attempting oesophageal dilatation[3,30]. Overall, oesophageal dilatation has proved to give good results in short strictures but might be dangerous for long and narrow oesophageal strictures[14,20]. Complex strictures are refractory to dilation therapy and fluoroscopic guidance has a valuable role in managing these types of strictures[23]. Repeat dilation sessions are needed in most cases with a goal of achieving a luminal diameter of 12 mm or larger in order to alleviate symptoms of solid dysphagia[23]. We suspect that the strategy of intense PPI therapy and repeated dilatation will reduce the number of impassable stricture that otherwise would have required oesophageal resection and reconstructive surgery.
The second patient with acid burns of the gastrointestinal tract developed gastric outlet obstruction within 3 wk of injury. The use of a steroid is of questionable value, and overall evidence from the literatures is not in favour of routine use. This is because it could mask the clinical signs of free perforation and infection[12,13,16]. Therefore, its use is limited mainly to patients with severe laryngeal oedema. Antibiotics have been used in this case for the treatment of an obvious chest infection. Gastric outlet obstruction has been found in association with oesophageal stricture in the region in 20% of cases[17]. In some cases gastric outlet obstruction can yield balloon dilatation but our patient required surgical bypass because of the complex nature of his stenosis. Alternative surgical reconstruction would be hemigastrectomy and resection of the first part of the duodenum with Bilroth I reconstruction. At the time of presentation, our patient was unfit for such a major operation.
Understanding the pathophysiology of corrosive injury is important in planning both acute and on-going management. Scar retraction begins as early as the end of the second week and lasts for 6 mo. Six to twelve months is considered the average time before full fibrosis is achieved after the injury[31]. Oesophagectomy carried out too early prior to the scar tissue maturation might increase the risk of anastomostic stenosis[32]. Han et al advocate delaying major reconstructive surgery in patients with caustic burns for at least 6 mo from time of injury provided that emergency surgery is not indicated[32]. Emergency oesophagectomy plus exteriorisation or immediate reconstruction is however indicated in cases of perforation and contamination of the mediastinum[9].
Risk of carcinoma
The association of lye stricture and carcinoma of the oesophagus has been known since 1896[2]. Kiviranta believed that the incidence of oesophageal cancer among victims of lye stricture is at least 1000 times greater than that in the normal population[33]. The interval between lye ingestion and the development of carcinoma ranges between 25 to 40 years. However, this risk may be overestimated. Marchand did not encounter a single case in 135 patients with caustic strictures of the oesophagus over a period of 6 years[34]. Carver and colleague had 2 patients out of 233 patients with lye strictures over a period of 25 years[35]. Mamede and colleague found 4 (1.6%) out of the 239 patients from their 37-year historical series developed oesophageal cancer after caustic soda ingestion[7]. In these cases, operative risk may exceed the potential risk of cancer.
The risk of gastric cancer is less known[8]. Gray and Holmes first reported in 1948 findings of squamous metaplasia in the stomach of a patient who had ingested acid[36]. Similar findings were subsequently reported by O’Donnell and colleagues[37] and later by Eaton and Tennekoon[38]. Some surgeons are more aggressive in resection of the involved stomach because of the danger of subsequent gastric metaplasia[6,9]. The predisposition to cancer justifies regular follow-up and surveillance endoscopy. However, the patient should be warned of the cumulative dangers of other risk factors for oesophageal cancer, such as alcohol abuse and smoking[30].
CONCLUSION
The literature on treatment of patients with corrosive injuries to the upper gastrointestinal tract is both controversial and inconclusive. The main principle in managing such patients is that each patient must be evaluated individually as the clinical picture varies widely. Signs and symptoms alone are an unreliable guide to injury. Both the acute and the chronic phases of the clinical presentation require different management. Psychiatric support is sometimes needed during both the acute and chronic phases. The general consensus is that the initial treatment is supportive; ensuring the airway is patent and to establish haemodynamic stability. Early endoscopy has a crucial role in both diagnosing the severity of the injury, as well as, in managing the patient. Total parenteral nutrition is a useful adjunct. Operation is generally reserved for patients who have ingested large amounts of corrosive substance and in whom tissue necrosis is highly likely. Extensive necrosis noted on endoscopy and patients with evidence of perforation are indications for immediate surgical intervention. As for intractable oesophageal strictures when dilatation is dangerous or impossible, surgical intervention may be unavoidable. However, this must be balanced against the mortality and sometimes considerable morbidity following surgery[2,30]. Follow up endoscopy should be carried out within 6 wk following discharge from the hospital[30]. Diligent follow-up is advised to ensure patient has satisfactory gastrointestinal function restored and to correct late onset complications.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
References
- 1.Holinger PH. Management of esophageal lesions caused by chemical burns. Ann Otol Rhinol Laryngol. 1968;77:819–829. doi: 10.1177/000348946807700501. [DOI] [PubMed] [Google Scholar]
- 2.Kirsh MM, Ritter F. Caustic ingestion and subsequent damage to the oropharyngeal and digestive passages. Ann Thorac Surg. 1976;21:74–82. doi: 10.1016/s0003-4975(10)64894-1. [DOI] [PubMed] [Google Scholar]
- 3.Gumaste VV, Dave PB. Ingestion of corrosive substances by adults. Am J Gastroenterol. 1992;87:1–5. [PubMed] [Google Scholar]
- 4.Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology. 2004;51:1397–1400. [PubMed] [Google Scholar]
- 5.Dantas RO, Mamede RC. Esophageal motility in patients with esophageal caustic injury. Am J Gastroenterol. 1996;91:1157–1161. [PubMed] [Google Scholar]
- 6.McAuley CE, Steed DL, Webster MW. Late sequelae of gastric acid injury. Am J Surg. 1985;149:412–415. doi: 10.1016/s0002-9610(85)80121-5. [DOI] [PubMed] [Google Scholar]
- 7.Mamede RC, de Mello Filho FV. Ingestion of caustic substances and its complications. Sao Paulo Med J. 2001;119:10–15. doi: 10.1590/S1516-31802001000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maull KI, Scher LA, Greenfield LJ. Surgical implications of acid ingestion. Surg Gynecol Obstet. 1979;148:895–898. [PubMed] [Google Scholar]
- 9.Estrera A, Taylor W, Mills LJ, Platt MR. Corrosive burns of the esophagus and stomach: a recommendation for an aggressive surgical approach. Ann Thorac Surg. 1986;41:276–283. doi: 10.1016/s0003-4975(10)62769-5. [DOI] [PubMed] [Google Scholar]
- 10.Sugawa C, Lucas CE. Caustic injury of the upper gastrointestinal tract in adults: a clinical and endoscopic study. Surgery. 1989;106:802–806; discussion 806-807. [PubMed] [Google Scholar]
- 11.Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702–707. [PubMed] [Google Scholar]
- 12.Di Costanzo J, Noirclerc M, Jouglard J, Escoffier JM, Cano N, Martin J, Gauthier A. New therapeutic approach to corrosive burns of the upper gastrointestinal tract. Gut. 1980;21:370–375. doi: 10.1136/gut.21.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarfati E, Gossot D, Assens P, Celerier M. Management of caustic ingestion in adults. Br J Surg. 1987;74:146–148. doi: 10.1002/bjs.1800740225. [DOI] [PubMed] [Google Scholar]
- 14.Nuutinen M, Uhari M, Karvali T, Kouvalainen K. Consequences of caustic ingestions in children. Acta Paediatr. 1994;83:1200–1205. doi: 10.1111/j.1651-2227.1994.tb18281.x. [DOI] [PubMed] [Google Scholar]
- 15.Haller JA Jr, Andrews HG, White JJ, Tamer MA, Cleveland WW. Pathophysiology and management of acute corrosive burns of the esophagus: results of treatment in 285 children. J Pediatr Surg. 1971;6:578–584. doi: 10.1016/0022-3468(71)90382-4. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins DB, Demeter MJ, Barnett TE. Caustic ingestion: controversies in management. A review of 214 cases. Laryngoscope. 1980;90:98–109. doi: 10.1288/00005537-198001000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Tekant G, Eroğlu E, Erdoğan E, Yeşildağ E, Emir H, Büyükünal C, Yeker D. Corrosive injury-induced gastric outlet obstruction: a changing spectrum of agents and treatment. J Pediatr Surg. 2001;36:1004–1007. doi: 10.1053/jpsu.2001.24725. [DOI] [PubMed] [Google Scholar]
- 18.Ramasamy K, Gumaste VV. Corrosive ingestion in adults. J Clin Gastroenterol. 2003;37:119–124. doi: 10.1097/00004836-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Zargar SA, Kochhar R, Mehta S, Mehta SK. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37:165–169. doi: 10.1016/s0016-5107(91)70678-0. [DOI] [PubMed] [Google Scholar]
- 20.Berthet B, Castellani P, Brioche MI, Assadourian R, Gauthier A. Early operation for severe corrosive injury of the upper gastrointestinal tract. Eur J Surg. 1996;162:951–955. [PubMed] [Google Scholar]
- 21.Chiu HM, Lin JT, Huang SP, Chen CH, Yang CS, Wang HP. Prediction of bleeding and stricture formation after corrosive ingestion by EUS concurrent with upper endoscopy. Gastrointest Endosc. 2004;60:827–833. doi: 10.1016/s0016-5107(04)02031-0. [DOI] [PubMed] [Google Scholar]
- 22.Genç A, Mutaf O. Esophageal motility changes in acute and late periods of caustic esophageal burns and their relation to prognosis in children. J Pediatr Surg. 2002;37:1526–1528. doi: 10.1053/jpsu.2002.36177. [DOI] [PubMed] [Google Scholar]
- 23.Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol. 2002;35:117–126. doi: 10.1097/00004836-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Bautista A, Tojo R, Varela R, Estevez E, Villanueva A, Cadranel S. Effects of prednisolone and dexamethasone on alkali burns of the esophagus in rabbit. J Pediatr Gastroenterol Nutr. 1996;22:275–283. doi: 10.1097/00005176-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mamede RC, De Mello Filho FV. Treatment of caustic ingestion: an analysis of 239 cases. Dis Esophagus. 2002;15:210–213. doi: 10.1046/j.1442-2050.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 26.Kochhar R, Ray JD, Sriram PV, Kumar S, Singh K. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509–513. doi: 10.1016/s0016-5107(99)70052-0. [DOI] [PubMed] [Google Scholar]
- 27.Wijburg FA, Beukers MM, Heymans HS, Bartelsman JF, den Hartog Jager FC. Nasogastric intubation as sole treatment of caustic esophageal lesions. Ann Otol Rhinol Laryngol. 1985;94:337–341. [PubMed] [Google Scholar]
- 28.Kaygusuz I, Celik O, Ozkaya O O, Yalçin S, Keleş E, Cetinkaya T. Effects of interferon-alpha-2b and octreotide on healing of esophageal corrosive burns. Laryngoscope. 2001;111:1999–2004. doi: 10.1097/00005537-200111000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Günel E, Cağlayan F, Cağlayan O, Canbilen A, Tosun M. Effect of antioxidant therapy on collagen synthesis in corrosive esophageal burns. Pediatr Surg Int. 2002;18:24–27. doi: 10.1007/s003830200005. [DOI] [PubMed] [Google Scholar]
- 30.Hugh TB, Kelly MD. Corrosive ingestion and the surgeon. J Am Coll Surg. 1999;189:508–522. doi: 10.1016/s1072-7515(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 31.Demirbilek S, Aydin G, Yücesan S, Vural H, Bitiren M. Polyunsaturated phosphatidylcholine lowers collagen deposition in a rat model of corrosive esophageal burn. Eur J Pediatr Surg. 2002;12:8–12. doi: 10.1055/s-2002-25082. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Cheng QS, Li XF, Wang XP. Surgical management of esophageal strictures after caustic burns: a 30 years of experience. World J Gastroenterol. 2004;10:2846–2849. doi: 10.3748/wjg.v10.i19.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiviranta NK. Corrosive carcinoma of the esophagus. Acta Otolaryngology (Supplement) 1952;102:1. [Google Scholar]
- 34.MARCHAND P. Caustic strictures of the oesophagus. Thorax. 1955;10:171–181. doi: 10.1136/thx.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CARVER GM Jr, SEALY WC, DILLON ML Jr. Management of alkali burns of the esophagus. J Am Med Assoc. 1956;160:1447–1450. doi: 10.1001/jama.1956.02960520009003. [DOI] [PubMed] [Google Scholar]
- 36.GRAY HK, HOLMES CL. Pyloric stenosis caused by ingestion of corrosive substances; report of case. Surg Clin North Am. 1948;Aug; 28(Mayo Clinic Number):1041–1056. doi: 10.1016/s0039-6109(16)32491-4. [DOI] [PubMed] [Google Scholar]
- 37.O'DONNELL CH, ABBOTT WE, HIRSHFELD JW. Surgical treatment of corrosive gastritis. Am J Surg. 1949;78:251–255. doi: 10.1016/0002-9610(49)90339-6. [DOI] [PubMed] [Google Scholar]
- 38.Eaton H, Tennekoon GE. Squamous carcinoma of the stomach following corrosive acid burns. Br J Surg. 1972;59:382–387. doi: 10.1002/bjs.1800590514. [DOI] [PubMed] [Google Scholar]


